Ultrastructural and enzymatic alterations in the ovary of Rhodnius prolixus infected with Trypanosoma rangeli
2022-04-22GuilhermeMachadoRosaneLopesSimoneOliveiraSimoneFreitasJacenirSantosMalletAndrSantosDeniseFederSuzeteGomes
Guilherme S. Machado, Rosane L. Lopes, Simone S. C. Oliveira, Simone P. C. Freitas, Jacenir R. Santos-Mallet,5,6, André L. S. Santos, Denise Feder, Suzete A. O. Gomes
1Programa de Pós-Graduação em Ciências e Biotecnologia, Instituto de Biologia, Universidade Federal Fluminense, Niterói, RJ, Brazil
2Laboratório de Biodiversidade de Insetos e Patógenos, Departamento de Biologia Geral, Instituto de Biologia, Universidade Federal Fluminense, Niterói, RJ,Brazil
3Laboratório de Estudos Avançados de Microrganismos Emergentes e Resistentes, Departamento de Microbiologia Geral, Instituto de Microbiologia Paulo de Góes, Universidade Federal do Rio de Janeiro, RJ, Brazil
4Fundação Oswaldo Cruz Piauí, Teresina, PI, Brazil
5Laboratório Interdisciplinar de Vigilância Entomológica em Díptera e Hemíptera, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz, Rio de Janeiro, RJ, Brazil 6Universidade Iguaçu, Nova Iguaçu, RJ, Brazil
7Laboratório de Biologia de Insetos, Departamento de Biologia Geral, Instituto de Biologia, Universidade Federal Fluminense, Niterói, RJ, Brazil
ABSTRACT
Objective: To investigate the morphological structure of ovarian follicular cells and biochemical parameters of both ovaries and fat bodies (sites of vitellogenesis) from Rhodnius (R.) prolixus infected with Trypanosoma (T.) rangeli.
Methods: Adult virgin females of R. prolixus were fed upon a membrane apparatus containing heat-inactivated citrated rabbit blood and a suspension of T. rangeli epimastigotes (Macias strain).Females from the control group and all the males received parasitefree blood. Transmission electron microscopy was used to reveal the morphological aspects of ovarian follicle cells in both control and parasite-infected groups. Protein profile, proteolytic activities and Western blotting analyses were performed in either ovary or fat body samples of control and parasite-infected groups.
Results: According to the ultrastructural data, T. rangeli infection elicited a degeneration process in the ovarian follicular cells of R.prolixus. Proteolytic assays indicated a reduction in the activity of aspartic peptidases in the ovary and fat body from parasite-infected group, while a significant increase in the cysteine peptidase activity was measured in both insect organs. Additionally, immunoblotting revealed that vitellogenin was overexpressed in the ovary of parasite-infected insects.
Conclusions: T. rangeli infection seems to elicit an early programmed cell death in the ovarian follicle cells as well as induces the modulation on the activities of different peptidase classes in either ovaries or fat bodies and the overexpression of the vitellogenin in the ovary of R. prolixus.
KEYWORDS: Trypanosoma rangeli; Rhodnius prolixus; Ovarian follicle; Vitellogenin; Aspartic protease; Cysteine protease
Significance
The pathogenic effects of Trypanosoma rangeli on Rhodnius prolixus leads to a delay in molting, influences the gut microbiota and increases mortality of this insect vector. However, the impacts of Trypanosoma rangeli infection in the reproductive tissues are poorly understood. Herein, we demonstrate that Trypanosoma rangeli induces morphological alterations in ovarian follicular cells, modulates the activities of peptidases in ovaries and fat body, as well increases the vitellogenin expression in the ovary,during the vitellogenesis, in Rhodnius prolixus.
1. Introduction
The production of gametes in insects depends on both physiological and environmental mechanisms. In females, the gametes are developed during the oogenesis process, involving several tissues under hormonal control, mainly the ovary[1,2]. Oogenesis is divided in 3 distinct phases: previtellogenesis, vitellogenesis and choriogenesis[3]. During previtellogenesis, ovarian follicle cells secrete cytoplasmic content into the oocytes[4]. In hematophagous insects, vitellogenesis is elicited by the blood meal, then oocytes absorb, via ovarian follicle cells, yolk proteins (mainly vitellin) that were synthesized in the fat body as vitellogenin[5-7]. Ovarian follicle cells also produce vitellogenin in the Hemiptera Rhodnius (R.)prolixus, some Diptera and Coleoptera species[8,9]. In choriogenesis,the ovarian follicle cells deposit an eggshell on the oocyte surface[10].During vitellogenesis, the oocytes accumulate yolk components,such as vitellin, lipids, granules of glycogen and peptidases[8,11]. The proteolytic enzymes are involved in the yolk protein degradation during the embryo development[3,12]. Peptidases are also essential in ovarian follicle cells in order to cleave proteins after the oogenesis and in response to cell stress, leading to programmed cell death (PCD)and premature degradation of yolk proteins[13,14].
Parasite infections could modulate the oogenesis process, for example, in Anopheles stephensi, Plasmodium yoelii nigeriensis induces resorption of ovarian follicular cells, reducing the eggs production[15]. Infection of Aspergillus niger elicited atresia in the epithelium of ovarian follicles of R. prolixus[16].
Trypanosoma (T.) rangeli is a non-pathogenic protozoan for mammals, including humans, but it is relevant for the epidemiology of Chagas disease due to the serological cross-reactivity with T.cruzi that can interfere in the diagnosis of this relevant neglected disease[17]. In insects, T. rangeli causes pathology, mainly, in triatomines of the genus Rhodnius, leading to an inhibition, delay or defective in the molt process as well as inducing tissue damage,reduction in the symbiotic populations and increases the insect mortality[18].
In R. prolixus, an important vector of T. cruzi, it is also considered a host of T. rangeli. T. cruzi infection did not delay pre-oviposition time, while T. rangeli affects reproductive fitness and may cause a delay in egg laying, reduced fecundity and fertility[19]. However,the impacts of T. rangeli in the reproductive tissues are poorly understood. There is a lack of studies regarding the ovarian follicle profile, when females of R. prolixus are infected by T. rangeli. In this context, we investigated the morphological structure of ovarian follicle cells and biochemical parameters in ovaries and fat bodies(sites of vitellogenesis) from R. prolixus infected with T. rangeli.
2. Materials and methods
2.1. Parasite maintenance
The epimastigote forms of T. rangeli (Macias strain) were cultured at 28 ℃ using a biphasic medium containing the Novy, McNeal,and Nicolle (NNN) blood agar[20] and liver infusion tryptose,supplemented with 20% inactivated fetal bovine serum.
2.2. Insect oral infection and dissection
Adult virgin females of R. prolixus were fed upon a membrane apparatus containing heat-inactivated citrated rabbit blood and a suspension containing T. rangeli (1×106epimastigotes/mL).Females from the control group and all the males received parasitefree blood under the same conditions. The experimental protocols were conducted following the guidelines of the Ethics Committee on Animal Experimentation Use under the approved protocol number 57/14-5.
The insects were sorted into breeding pairs (one male and one female) and the copulation was conducted as described in Aguirre and co-workers[13]. After the copulation, on the 7th day after the blood feeding, the females from both control (n=15) and parasiteinfected (n=15) groups were anesthetized for 5 min in the freezer,then the ovaries and fat bodies were dissected using iris scissors in 6.6 mM phosphate-buffered saline (PBS), pH 7.4.
2.3. Transmission electron microscopy
To examine morphological changes in reproductive tissues caused by T. rangeli infection, ovaries of R. prolixus were carefully dissected, washed three times in 6.6 mM PBS, pH 7.4, and fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2, for 1 h at room temperature. The ovary samples were washed three times in the same buffer for 10 min and post-fixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer, pH 7.2, for 1 h in the dark, at room temperature. Then, the ovary samples were washed three times in the same buffer for 10 min and dehydrated in a graded concentration of acetone (50%, 70%, 90% and 100%)for 10 min. Subsequently, the ovary samples were transferred to PolyBed 812 resin. Ultra-thin sections were stained with 5% aqueous uranyl acetate for 5 min and 1% lead citrate for 1 min.The samples were analyzed with a JEOL JEM 1011 transmission electron microscope (Jeol Corp., Tokyo, Japan).
2.4. Proteolytic activity
T. rangeli epimastigotes and the tissues (fat bodies and ovaries)obtained from R. prolixus were disrupted in a FastPrep equipment(Fisher scientific, Pennsylvania, United States), 3 min on ice and 20 s in the equipment for a total of 8 cycles, with beads and lysis buffer, containing 10 mM Tris-HCl, pH 7.2, and 1%(3-[(3-cholamidolpropyl) dimethylammonio]-1 propanesulfonate),pH 7.0. Then, all extracts were centrifuged separately at 15 000×g for 30 min at 4 ℃, and the supernatant was immediately used to determine the protein content and the aspartic and cysteine peptidase activities. The protein concentration was determined by the method described by Lowry and co-workers[21], using bovine serum albumin as standard.
Aspartic peptidase activity was determined using 7-methoxycoumarin-4-acetyl-Gly-Lys-Pro-Ile-Leu-Phe-Phe-Arg-Leu-Lys-D-Arg-amide (cathepsin D fluorogenic substrate,Sigma-Aldrich Chemical Co.). Briefly, the reaction was started by the addition of cathepsin D substrate (2 µM) to the parasite extract (30 µg protein) or insect tissues (ovaries and fat bodies from uninfected and parasite-infected insects) (30 µg protein) in a buffer containing 0.2 M sodium phosphate, pH 4.0, 0.1 M citric acid and 1 mM ethylenediamine tetraacetic acid. The system was treated with pepstatin A (10 µM), a classical aspartic peptidase inhibitor, and a non-treated system was used as control. After 1 h, the cleavage of the cathepsin D substrate was detected in a spectrofluorimeter(SpectraMax Gemini XPS, Molecular Devices, California, United States) with 328 nm excitation and 393 nm emission wavelengths.The assays were controlled for self-liberation of the fluorophore over the same time interval and the proteolytic activity was expressed as arbitrary fluorescence units (AFU)[22].
Cysteine peptidase activity was determined using Z-Phe-Arg-AMC(cathepsin B, L and cathepsin L-like cruzipain fluorogenic substrate,Sigma-Aldrich Chemical Co.). The reaction was started by the addition of substrate (10 µM) to the parasite extract (30 µg protein)or insect tissues (ovaries and fat bodies from uninfected and parasiteinfected insects) (30 µg protein) in a buffer containing 50 mM sodium phosphate, pH 5.0, supplemented with 2 mM dithiothreitol.The reactional systems were treated with E-64 (10 µM), a classical cysteine peptidase inhibitor, and a non-treated system was used as control. After 1 h, the cleavage of the cysteine substrate was detected in a spectrofluorimeter (SpectraMax Gemini XPS, Molecular Devices, California, United States) with 380 nm excitation and 460 nm emission wavelengths. The assays were controlled for selfliberation of the fluorophore over the same time interval and the proteolytic activity was expressed as arbitrary fluorescence units(AFU)[22].
2.5. SDS-PAGE and western blotting assays
The proteins present in the cell extracts of T. rangeli, fat bodies and ovaries (obtained from both uninfected and parasite-infected R. prolixus) were quantified by the Lowry method[21], as mentioned above. The extracts, equivalent to 30 µg of protein, were added to SDS-PAGE sample buffer (62 mM Tris-HCl, pH 6.8, 2% SDS, 25% glycerol, 0.01% bromophenol blue and 1 mM β-mercaptoethanol),followed by heating at 100 ℃ for 5 min and then polypeptides were separated in 12% SDS-PAGE[23]. Electrophoresis was processed at 200 V and 20 mA, for 2 h. Proteins were detected by silver staining.The molecular mass of each protein was calculated by comparing it to molecular weight standards (Thermo Scientific, Massachusetts,United Estates).
Alternatively, polypeptides separated in SDS-PAGE were transferred to a nitrocellulose membrane in buffer containing 25 mM Tris-HCl,200 mM glycine and 20% methanol at 100 V/300 mA for 2 h at 4 ℃. Then, the membranes were blocked with a blocking buffer(PBS, pH 7.2; 0.1% Tween-20 and 5% nonfat dry milk) overnight at 4 ℃. Subsequently, the membranes were washed three times for 10 min in 0.1% PBS/Tween-20 and incubated with the antivitellogenin rabbit polyclonal antibody (primary antibody) (dilution of 1: 5 000) for 1 h. The membranes were washed three times for 10 min in the same solution and a peroxidase-conjugated antirabbit IgG (secondary antibody) (dilution of 1: 5 000) for 1 h.Finally, membranes were washed, as described above, and exposed to electrochemiluminescence (ECL). Images were acquired using ImageQuant TL 8.1 software.
2.6. Statistical analysis
All experiments were performed in triplicate in three independent experimental sets. Data were expressed as mean±standard desviation. The graphics and data were constructed and analyzed statistically by means of Student’s t-test using GraphPad Prism 6 software (California, United States). P<0.05 was assumed as significant (P<0.05).
3. Results
3.1. Transmission electron microscopy observation
In the uninfected R. prolixus insects, the ovarian follicle cells showed a uniform nucleus with condensed chromatin and the mitochondria were normally distributed in the cytoplasm (Figure 1A and 1B). In the parasite-infected female insects, the ultrastructural images of the ovarian follicle cells revealed a degeneration process as judged by the chromatin fragmentation (Figure 1C),mitochondrial swelling with loss of internal crystal folding,electron-dense material inside cytoplasmic vacuoles and extensive areas of cytoplasm disorganization (Figure 1D).

Figure 1. Ovarian follicular cells of Rhodnius prolixus. A: chromatin profile of the control group; B: mitochondria profile in the control group; C:chromatin fragmentation of infected group; D: swollen mitochondria with loss of cristae internal folding and cytoplasmic material inside vacuoles of the infected group. N: nucleus; cc: chromatin; *chromatin fragmentation;M: mitochondria; Va: vacuoles.
3.2. Proteolytic activity
In this set of experiments, initially, we decided to evaluate the proteases expressed in T. rangeli when axenically grown in vitro in the NNN medium in order to evidence the proteolytic activity profile in this parasite before the infection in R. prolixus. Therefore,proteolytic activities were measured in the extracts obtained from T.rangeli epimastigote forms. In this context, a low proteolytic activity corresponding to the aspartic peptidase class was measured in the extracts of T. rangeli epimastigotes as judged by the cleavage of the aspartic peptidase fluorogenic substrate; however, the prototypal aspartic peptidase inhibitor pepstatin A only slightly reduced the substrate cleavage (Figure 2A). Contrarily, high amount of cysteine peptidases were measured in the epimastigotes’ extract, as well as a significant reduction (approximately 60%) on the peptide substrate degradation was observed when incubated in the presence of the classical cysteine peptidase inhibitor E-64 (Figure 2B).

Figure 2. Proteolytic activity in Trypanosoma rangeli. A: aspartic peptidase activity in the absence (Tr) and in the presence of pepstatin A (Tr+Pep A).B: cysteine peptidase activity in the absence (Tr) and in the presence of E-64(Tr+E-64). The proteolytic activity was expressed as arbitrary fluorescence units (AFU). n=15, data were expressed as mean±SD, and comparison were analyzed with Student’s t-test. *P<0.05.
Secondly, we evaluated the effects of T. rangeli infection on the peptidase activities in both ovaries and fat bodies extracted from R. prolixus. Pepstatin A-sensitive aspartic peptidase activity significantly (P<0.05) decreased (around 40%) in fat bodies’homogenates of the parasite-infected insects compared to the uninfected ones (Figure 3A). Contrarily, classical cysteine peptidase activities, inhibited by E-64, were drastically increased (by approximately 75%) in fat bodies’ homogenates in parasite-infected insects in comparison with uninfected ones (Figure 3B).

Figure 3. Proteolytic activity in Rhodnius prolixus fat body. A: aspartic peptidase activity in the absence and in the presence of PepA. B: cysteine peptidase activity in the absence and in the presence of E-64. The proteolytic activity was expressed as arbitrary fluorescence units (AFU). n=15, data were expressed as mean±SD, and comparison were analyzed with Student’s t-test. *P<0.05.
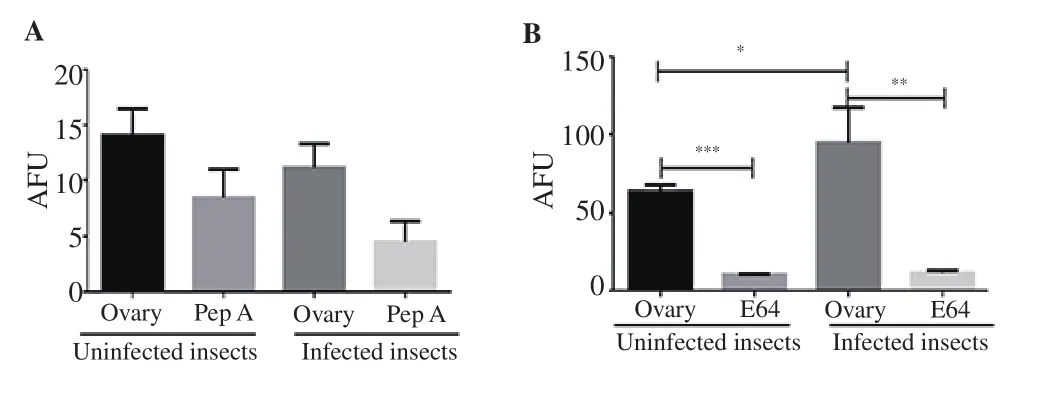
Figure 4. Proteolytic activity in Rhodnius prolixus ovary. A: aspartic peptidase activity in the absence and in the presence of PepA. B: cysteine peptidase activity in the absence and in the presence of E-64. The proteolytic activity was expressed as arbitrary fluorescence units (AFU). n=15, data were expressed as mean±SD, and comparison were analyzed with Student’s t-test. *P<0.05; **P<0.01; ***P<0.001.
In R. prolixus ovaries’ homogenates both aspartic and cysteine peptidases were measured under the employed experimental conditions. The comparison between uninfected and parasiteinfected insects revealed that aspartic peptidase activity was not modulated by T. rangeli infection (Figure 4A), while cysteine peptidase activity was considerably higher in infected insects (Figure 4B).
3.4. SDS-PAGE and Western blotting analyses
The protein profiles of T. rangeli epimastigotes as well as the fat bodies and ovaries of uninfected and parasite-infected R. prolixus were evidenced by SDS-PAGE (Figure 5). No significant differences were observed in the protein profiles of extracted fat bodies from both uninfected and parasite-infected insects. Regarding the ovaries from R. prolixus, the protein profiles were similar in both systems analyzed; however, ovaries from parasite-infected insects presented higher expression of some proteins, particularly those with molecular masses ranging from 150 to 120 kDa and 50 and 45 kDa(Figure 5).

Figure 5. Protein profiles of Trypanosoma rangeli and Rhodnius prolixus.MW: molecular weight; Tr: proteins profile of Trypanosoma rangeli; FB: fat body of control group; FB+Tr: fat body of infected group; OV: ovaries of control group; OV+Tr: ovaries of infected group.
Western blotting analysis confirmed the presence of different subunits of the vitellogenin protein in fat bodies and ovaries of either uninfected or parasite-infected insects (Figure 6). In this context, the anti-vitellogenin antibody recognized subunits(150 kDa and 50-45 kDa) of vitellogenin in fat bodies’ samples.Apparently, the levels of vitellogenin were the same in fat bodies in both uninfected and T. rangeli-infected insects. In the R. prolixus ovaries, vitellogenin was identified by the anti-vitellogenin antibodies as polypeptides of molecular masses of 150-120 kDa,60 kDa, 50-45 kDa and 35 kDa in higher levels in parasite-infected insects than the uninfected ones (Figure 6).

Figure 6. Vitellogenin expression in Rhodnius prolixus. Tr: vitellogenin was not detected in Trypanosoma rangeli; FB: vitellogenin in fat body of control group; FB+Tr: vitellogenin in fat body of infected group. OV: vitellogenin in ovaries of control group; OV+Tr: vitellogenin in ovaries of infected group.Molecular masses of the vitellogenin subunits are indicated at the left.
4. Discussion
Several studies demonstrated that T. rangeli infection can affect the triatomine R. prolixus in various ways, directly or indirectly,inducing morphological, biochemical and molecular alterations in different tissues[24,25]. In this regard and taking into consideration the possible impacts of this parasite in the reproductive tissues of R.prolixus, we investigated morphological and biochemical changes in ovary of this triatomine infected with T. rangeli. It is important to mention that in ours experimental design we choose copulated females since once during the copulation male can transfer to the female secretions released thought the accessory glands, as well vitellogenic steroid hormones[26,27].
In ovarian follicle cells of infected insects, mitochondrial morphological alterations might be occurred due to an increase of reactive oxygen species in the cells[28]. The imbalance of these molecules could elicit an autophagic cell death[29]. Additionally,cytoplasmic contents into vacuoles also indicates an autophagic mechanism[30]. Furthermore, chromatin fragmentation can be a clear evidence of apoptotic cell death[31]. Many stress pathways sequentially induce autophagy and apoptosis in the same cell[32].Therefore, these subcellular changes in the vitellogenesis phase appear to be a signal generated by the parasite, directly or indirectly and this signal, probably, induced an early PCD via autophagic and apoptotic mechanisms. As well as, in Aspergillus niger infection that elicited an ovarian follicular atresia due to an early PCD,pointing out the involvement of apoptosis and autophagy pathways in R. prolixus[16]. Furthermore, Fellet and co-workers[19] observed that when R. prolixus was infected with T. rangeli, in addition to a delay in egg laying, a decrease in insects’ capacity to convert ingested blood into eggs as well as egg viability. We suggest that these morphological alterations in the ovarian follicle cells are one of the reasons that could impair the insect reproductive cycle.Once, an early PCD in some ovarian follicle cells might increase pre-oviposition time and reduce the eggs production/viability.However, further analysis should be performed to confirm cell death mechanisms and if these processes could lead the ovarian follicles to an atretic stage.
Aspartic and cysteine peptidase activity detected in the ovaries and fat bodies of R. prolixus were altered when the insects were infected with T. rangeli. We also measured these proteolytic enzymes in extracts of T. rangeli epimastigotes as a positive control[33]. Aspartic peptidase activity in the fat bodies of parasite-infected insects was lower than that observed in uninfected insects. Previous analysis indicated that T. rangeli infection often results in severe damages in the fat body, decreasing the amounts of molecules stored in this tissue[34]. Our hypothesis is that the parasite infection mobilized some aspartic peptidases stored in the fat body to the hemolymph and also reduced their production. Additionally, only in the fat body of parasite-infected insects presented aspartic peptidases sensitive to the pepstatin A, such as cathepsins D and E. During the embryogenesis, cathepsin-like peptidases are involved in the vitellin degradation to provide nutrients to the developing embryo in different arthropods[35-37]. The aspartic peptidase cathepsin D is the major regulator of yolk protein degradation in R. prolixus[38].Most of these proteinaceous molecules are synthesized in the fat body, as well as in the ovary, then secreted in the hemolymph, as pro-enzymes and taken up by the growing oocytes[39,40]. In our finding, when the insects were infected with T. rangeli, the aspartic peptidase activity decreased in ovary compared to the uninfected insects. Probably, the parasite infection reduced the production of these proteolytic enzymes in the fat body, as mentioned above;therefore, they are not delivered to the ovary. Besides, the amounts of aspartic peptidases synthesized in the ovary might be reduced due to the parasite infection. Meanwhile, cysteine peptidases strongly increased in fat bodies and ovaries of T. rangeli infected insects.In Culex pipiens pallens, the cysteine peptidases, called cathepsin B and L-like peptidases, were activated under unfavorable condition(blood deprivation) to promote follicular atresia and resorption of oocytes[40]. Based on these data, we can assume two hypotheses:1) an indirect effect of T. rangeli infection leading to an apoptotic cell death, as evidenced by ultrastructural alterations of the ovarian follicular cells, probably due to the high activity of the measured cysteine peptidases in both tissues; 2) T. rangeli invaded and elicited a PDC, via apoptosis in both tissues. Then, cysteine peptidase activity refers to the parasite extracts and also the damaged tissues. We detected, in the hemolymph, epimastigotes of T. rangeli after 7 days of the oral infection (data not shown). Therefore, it is reasonable to predict that this parasite could colonize the fat body and ovary during this period.
Additionally, we detected that the protein profiles were the same in fat body and ovary samples in both uninfected and parasite-infected insects. However, when the insects were infected with T. rangeli,some proteins were overexpressed in ovary and the molecular masses of these proteins (150-120 kDa and 50-45 kDa, approximately)which are close to those found in the vitellogenin subunits presented the fat body and ovarian follicular cells of R. prolixus[6,41]. In this sense, we tested if the overexpressed proteins could be subunits of vitellogenin and if these subunits would be detected in the other samples. Western blotting analysis confirmed the presence of vitellogenin in all the experimental groups, including the ovary samples of parasite-infected insects. The vitellogenin expression in the fat body seems to remain unaffected at 7 days after the infection.However, previous studies reveled that pathogenic infections reduced the vitellogenin biosynthesis in the fat body and the capacity of oocytes to uptake this molecule in different insects[42,43]. Therefore,further analysis should be performed to evaluate these parameters in order to confirm if T. rangeli infection are able or not to module the production and/or release of the vitellogenin. In the ovary samples of parasite-infected insects, we confirmed that the amounts of the vitellogenin were higher than in the uninfected insects. This result seems to evidence an example of adaptive insect response to the parasite infection. Once, during T. rangeli infection, some ovarian follicles might remain healthy and then they could produce/recruit more vitellogenin molecules, as compensatory mechanism.However, the aspartic peptidase activity is lower in ovary of parasiteinfected R. prolixus, as mentioned above, thus, probably, not all these vitellogenin molecules will be degraded in the embryogenesis phase.
The present study revealed that the infection of R. prolixus with T. rangeli induces morphological and biochemical alterations in the insect ovary, particularly during the vitellogenesis phase. We also detected some changes in the fat body of this insect during the infection with T. rangeli. The pathogenic effects could elicit an early PCD in the ovarian follicle cells as well as induce the modulation on the activities of different peptidases (particularly those belonging to the aspartic and cysteine-type peptidase classes) in either ovaries or fat bodies and the overexpression of the vitellogenin in the ovary of R.prolixus.
Among the limitations found in our analysis, we could not confirm cell death mechanisms and if these processes could lead the ovarian follicles to an atretic stage. Therefore, further investigations concerning comparative proteomic analysis could reveal molecular aspects associated with cells death mechanism and others protein expression in the ovary of R. prolixus infected and uninfected with T.rangeli and clarify the effects of this parasitism on the Ovogenesis of this insect-vector.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgements
The authors are grateful to the Plataforma de Microscopia Eletrônica from Universidade Federal Fluminense-Niterói, RJ,Brazil, Plataforma de Microscopia Eletrônica Rudolph Barth from Instituto Oswaldo Cruz-Fundação Oswaldo Cruz, Rio de Janeiro,RJ, Brazil; Laboratório Interdisciplinar de vigilância Entomológica em Diptera e Hemiptera, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz, Rio de Janeiro, RJ, Brazil and Laboratório de Estudos Avançados de Microrganismos Emergentes e Resistentes Universidade Federal do Rio de Janeiro, RJ, Brazil for technical assistance.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES,Finance Code 001).
Authors’ contributions
GSM, DF and SAOG conceptualized the study. GSM, SSCO and SPCF collected all data. These data were analyzed by GSM,RLL, SSCO, SPCF, JRSM, ASS, DF and SAOG. Manuscript was prepared by RLL and SAOG and edited by RLL, SSCO, SPCF,JRSM, ALSS, DF and SAOG. All the authors read the final version of the manuscript and approved for publication.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- The outbreak of dengue during the COVID-19 pandemic in Pakistan: The emergence of overlapping crises
- Patterns of adverse drug reaction reporting in Ethiopia: A database analysis of spontaneous reports from 2013 to 2018
- Systematic mutational analysis of epitope-grafted ED3 immunogenicity reveals a DENV3-DENV4 bi-serospecific ED3 mutant
- Evaluation of Cuban Bacillus thuringiensis (Berliner, 1911) (Bacillales: Bacillacea)isolates with larvicidal activity against Aedes aegypti (Linnaeus, 1762) (Diptera:Culicidae)
- Furuncular myiasis by Wohlfahrtia magnifica (Diptera: Sarcophagidae) in a healthy child
- Impact of vaccination on SARS-CoV-2 infection: Experience from a tertiary care hospital
