Manganese(II) and Copper(I) Compounds Based on Two Derivatives of Imidazo[1,5-a]pyridine: Synthesis,Structures, Magnetic Properties, and Catalytic Activity①
2022-04-16ZHANGQinCUIYnFengZHANGXiMeiLIHongYAOJinLei
ZHANG Qin CUI Yn-Feng ZHANG Xi-Mei LI Y-Hong② YAO Jin-Lei
a (College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China)
b (Jiangsu Key Laboratory of Micro and Nano Heat Fluid Flow Technology and Energy Application,School of Physical Science and Technology, Suzhou University of Science and Technology, Suzhou 215009, China)
ABSTRACT The utilization of 2,6-bis(imidazo[1,5-a]pyridin-3-yl)pyridine (bipp) and 3-(pyridin-2-yl)imidazo[1,5-a]pyridine (pip) in the compounds of manganese(II) and copper(I) ions is presented. Two complexes,[Mn(bipp)(SO4)(H2O)]n (1) and [Cu(pip)2]ClO4 (2), were prepared with different characteristics in structure. Compound 1 exhibits a one-dimensional chain topology, and 2 is a homoleptic Cu(I) complex. The dc magnetic susceptibility investigations reveal the antiferromagnetic (AF) MnII···MnII interactions in complex 1. The catalytic activity of compound 2 toward ketalization reactions was studied. Complex 2 exhibits high activity for the ketalization transformations of aliphatic ketones.
Keywords: coordination complexes, chain configuration, magnetism, catalysis;
1 INTRODUCTION
The coordination complexes of manganese(II) and copper(I) ions have been the subject of intensive research owing to their aesthetically pleasing structures, and intriguing physicochemical properties which are closely relevant to the design of new magnetic[1-10]and catalytic[11-20]materials.Coordination compounds chelated by derivatives of imidazo-[1,5-a]pyridine were widely investigated in recent years[21-23].This series of ligands possess several nitrogen donors, and they are electron rich and highly conjugated. These features render them to be the suitable ligands for assembling versatile coordination complexes. Recent advances in this area revealed that the complexes supported by this series of ligands exhibited potential applications in DNA cleavage[24-25],catalysis[26-27], and photoluminescent materials[28-30]. Therefore, the development of efficient synthetic methodologies for both imidazo[1,5-a]pyridine scaffolds and their complexes is of great importance.
With the motivations for synthesizing manganese(II) and copper(I) coordination complexes, and comprehensively studying the magnetic properties and catalytic activity of these compounds, we carried out the reactions of manganese(II) and copper(II) salts with 2,6-bis(imidazo[1,5-a]-pyridin-3-yl)pyridine (bipp) and 3-(pyridin-2-yl)imidazo[1,5-a]pyridine (pip). Two complexes with formulae [Mn(bipp)-(SO4)(H2O)]n(1) and [Cu(pip)2]ClO4(2) were prepared(Scheme 1). The magnetic properties of 1 and catalytic activity of 2 toward ketalization reactions were investigated.Herein, the preparation and structures of compounds 1 and 2 were reported. The magnetic properties of 1 and catalytic behavior of 2 were also presented.
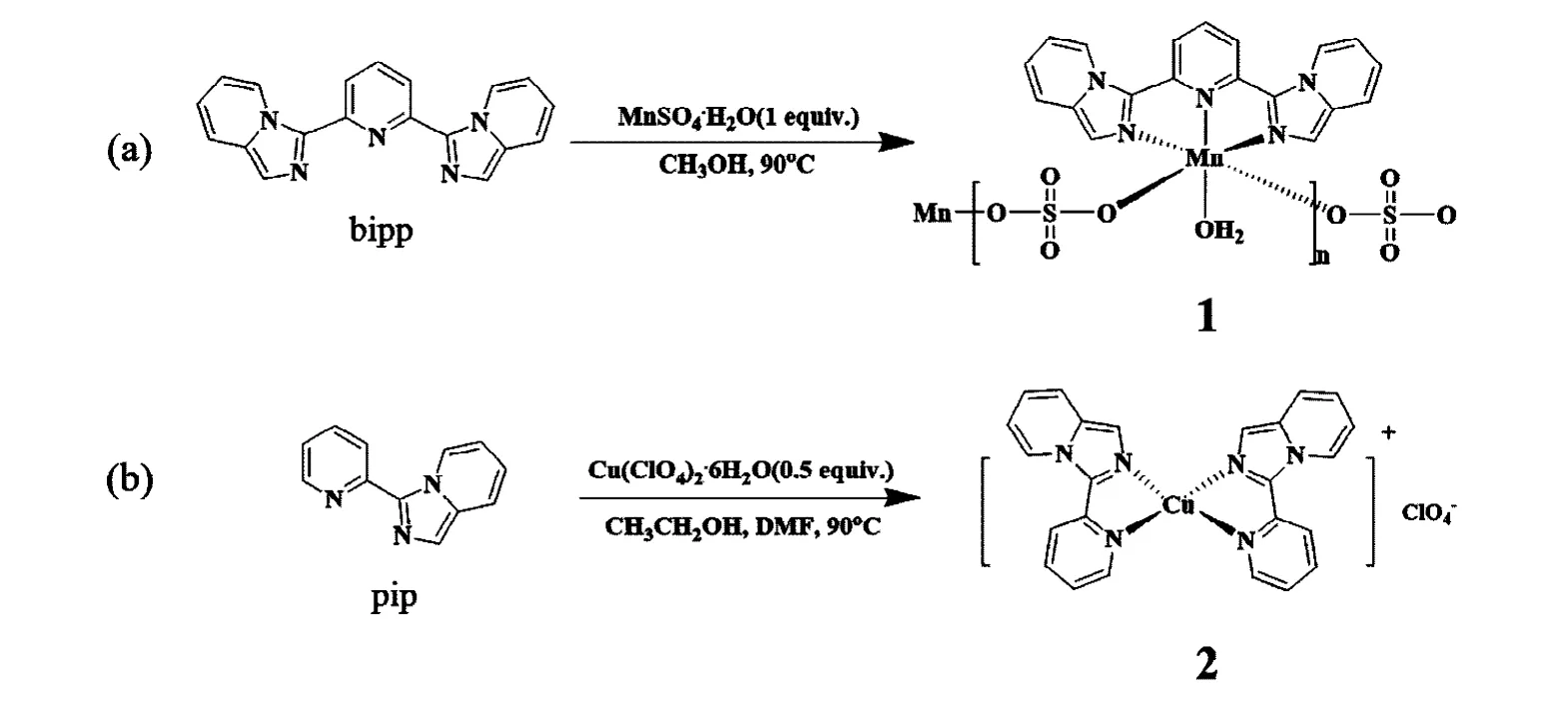
Scheme 1. Synthesis of compounds 1 )a) and 2 (b)
2 EXPERIMENTAL
2. 1 Physical measurements
All manipulations were conducted under solvothermal conditions in air. All solvents and reagents were available from chemical suppliers and used directly. 2,6-Bis(imidazo-[1,5-a]pyridin-3-yl)pyridine (bipp) and 3-(pyridin-2-yl)imidazo[1,5-a]pyridine (pip) were prepared by following the reported procedures[21-23]. FT-IR spectroscopy studies were carried out on a Nicolet MagNa-IR 500 spectrometer with samples prepared as KBr disks. Elemental analyses of carbon, hydrogen and nitrogen atoms were performed with a Carlo-Erba EA1110 CHNO-S analyser. The magnetic susceptibilities of 1 were determined on an MPMS XL-7 SQUID magnetometer.
2. 2 Preparation of 1 and 2
2. 2. 1 Preparation of [Mn(bipp)(SO4)(H2O)]n (1)
To a 10 mL thick glass tube were added MeOH (4 mL),bipp (0.1 mmol, 31 mg), and MnSO4·H2O (0.1 mmol, 17 mg). The tube was then sealed and heated to 90 ºC for 72 hours. Red block-shaped crystals of the product were obtained upon gradually cooling the reaction mixture to ambient temperature. These crystals were isolated, rinsed with MeOH and dried. The yield of the product was 95% calculated by MnSO4·H2O. Elemental analysis calcd. (%) for C19H15MnN5O5S: C, 47.51; H, 3.15; N, 14.58. Found (%): C,47.59; H, 3.43; N, 14.63. Selected ATR IR data (cm-1): 3158(w), 2159 (w), 1631 (w), 1604 (w), 1591 (m), 1573 (m),1553 (m), 1482 (s), 1359 (m), 1308 (w), 1263 (m), 1256 (m),1173 (w), 1134 (w), 1114 (m), 1071 (s), 1037 (m), 921 (w),908 (m), 831 (m), 844 (w), 792 (m), 753 (m), 740 (m), 683(s), 670 (s), 663 (m), 619 (m).
2. 2. 2 Preparation of [Cu(pip)2]ClO4 (2)
To a 10 mL thick glass tube were added MeOH (4 mL),DMF (1 mL), Cu(ClO4)2·6H2O (0.1 mmol, 17 mg) and pip(0.1 mmol, 20 mg). The tube was then sealed and heated to 90 ºC for 72 hours. Red block-shaped crystals of the product were obtained upon gradually cooling the reaction mixture to ambient temperature. These crystals were isolated, rinsed with EtOH and dried. The yield of the product was 70%calculated by Cu(ClO4)2·6H2O. Elemental analysis calcd. (%)for C24H18ClCuN6O4: C, 52.09; H, 3.28; N, 15.19. Found: C,51.95; H, 3.21; N, 15.03. The main ATR IR data (cm-1):1597 (m), 1474 (m), 1447 (w), 1407 (s), 1365 (m), 1260 (m),1168 (m), 1084 (s), 1013 (w), 978 (w), 913 (w), 826 (w),778 (m), 767 (m), 731 (m), 719 (m), 678 (m), 619 (m).
2. 3 Ketalization reactions catalysed by 2
To a 10 mL Schlenk flask were added ketone (1.4 mmol), ethylene glycol (1.4 mmol), toluene (1 mL) and compound 2 (2 mg). The reaction mixture was heated to reflux with a Dean-Stark being connected to the bottom of a condenser for 24 h. Then compound 2 was removed by filtration. The yields of the products were calculated by1H NMR.
2. 4 General crystallographic details
Diffraction data measurements for these two compounds were carried out on a Bruker Smart Apex II diffractometer at 296.15 K. SHELXS package was employed to solve the structures of 1 and 2[31]. All ordered non-hydrogen elements were refined anisotropically by full-matrix least-squares calculations againstF2[32]. X-ray powder diffraction data were determined on a Rigaku D/Max-2500 diffractometer utilizing a graphite monochromator and a Cu-target tube.
3 RESULTS AND DISCUSSION
3. 1 Characterization and structure description of 1 and 2
These two compounds were characterized by IR (Fig. S1),X-ray powder diffraction analysis (Fig. S3), and elemental analysis. The existence of the coordinated water in compound 1 was confirmed by measuring the TG curve (Fig. S2).The PXRD patterns of these two compounds (experimental and simulated PXRD patterns, Fig. S3) indicated that the phases of compounds 1 and 2 were pure. Compound 1 underwent several step weight loss. The first step from 25 to 225 °C was assigned to the removal of the coordinated H2O molecule (calcd.: 3.74%; found:ca.3.75%), suggesting high thermal stability of 1.
The structure refinement details and crystal data are listed in Table S1. The main interatomic distances and angles are collected in Table 1. The structures of 1 and 2 are presented in Figs. 1 and 2. The main crystallographic parameters are summarized in the following descriptions.
Compound 1 crystalizes in monoclinic space groupP21/c.Mr= 480.36,a= 10.6615(6),b= 15.4762(8),c= 12.6476(7)Å,β=113.3130(10)°,Dc= 1.665 g/cm3,V= 1916.47(18) Å3,μ=0.843 mm-1,F(000) = 980,R= 0.0380,wR= 0.0977, and goodness-of-fit is 1.094. Each asymmetric unit contains one MnIIion, one bipp ligand, one SO42-group and a coordination water molecule (Fig. 1). The MnIIcenter is coordinated by N1, N2 and N3 atoms from one bipp ligand, O1 and O3A atoms from two SO42-groups and O2 atom from coordinated H2O molecule, giving a [MnN3O3] core. Along theaaxis,the adjacent MnIIions are bridged by one O-S-O unit from one SO42-anion. The MnII···MnIIdistance is 6.476 Å. Within compound 1, two similar mononuclear [Mn1A(bipp)(μ3-O)]and [Mn1B(bipp)(μ3-O)] units are bridged by the [MnN3O3]core in a mirror mode, leading to a chain structure of[Mn(bipp)(SO4)(H2O)]n(Fig. 1). The average Mn-N length is 2.259 Å, and it is larger than that of Mn-O distance (2.158 Å). The bond angles of the MnIIion from the axial position range from 71.38(7)° to 142.90(7)°, and bond angles from the bridged plane fall in the scope of 83.84(7)°~168.53(7)°.A slight distortion from the ideal octahedral structure with a CShM (continuous symmetry measurement) value of 3.237 was calculated by using SHAPE 2.1 software[33].
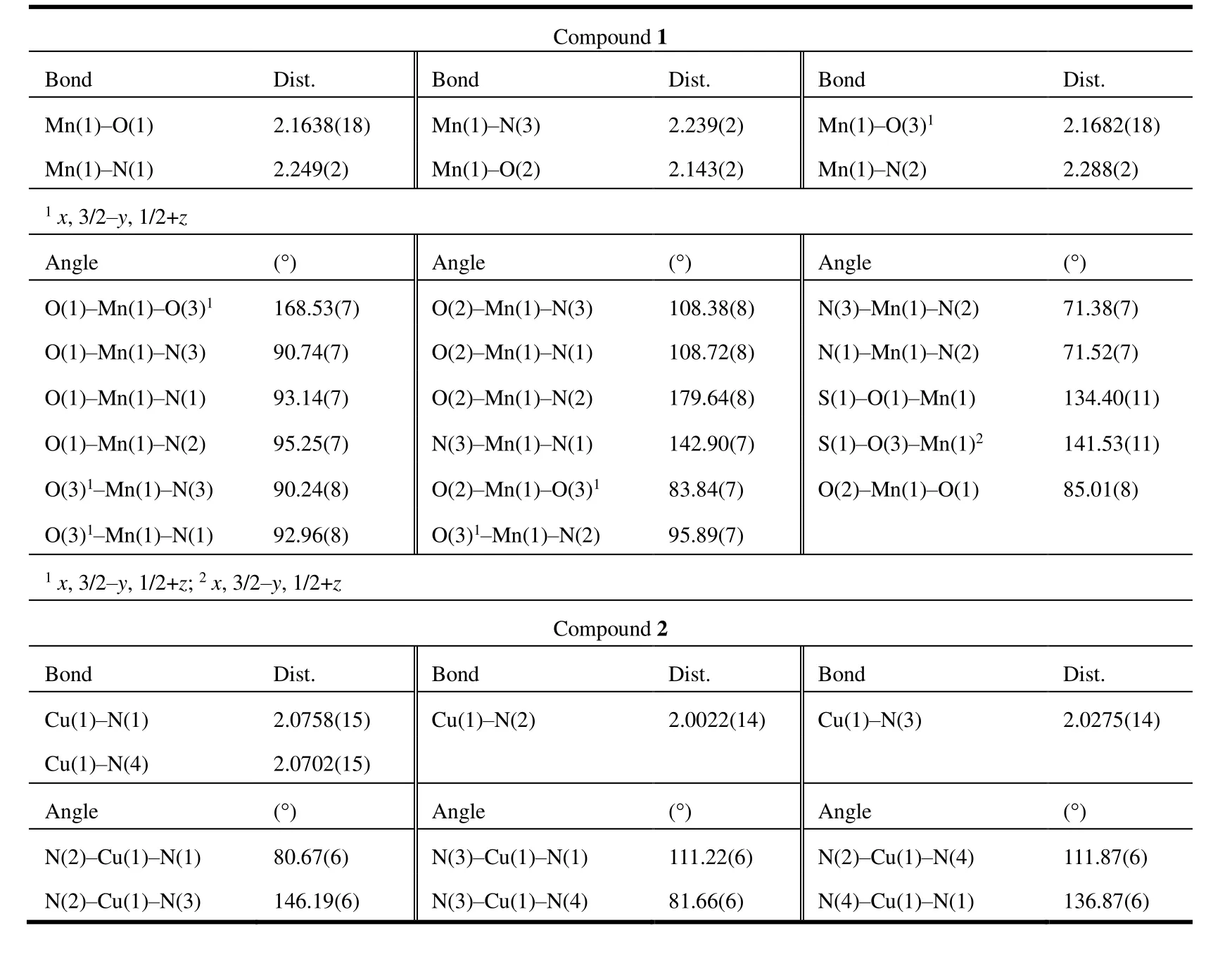
Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for 1 and 2
Compound 2 crystalizes in triclinic space groupP1.Mr=553.43,a= 7.3860(4),b= 10.5325(6),c= 14.5829(8) Å,α=88.959(2)°,β=78.193(2)°,γ=80.407(2)°,Dc= 1.679 g/cm3,V= 1094.77(10) Å3,μ=1.168 mm-1,F(000) = 564,R=0.0322,wR= 0.0852, and goodness-of-fit is 1.050. Compound 2 is composed of one [Cu(pip)2]+unit and one ClO4-counterion (Fig. 2). The CuIion of [Cu(pip)2]+unit is chelated by two pip ligands. Thus the CuIcentre displays tetrahedron geometry. The distances of Cu-Npyridinebonds are 2.0758(14) and 2.0275(14) Å, respectively. The Cu-Nimidazoledistances are 2.0702(15) and 2.0022(14) Å, respectively.
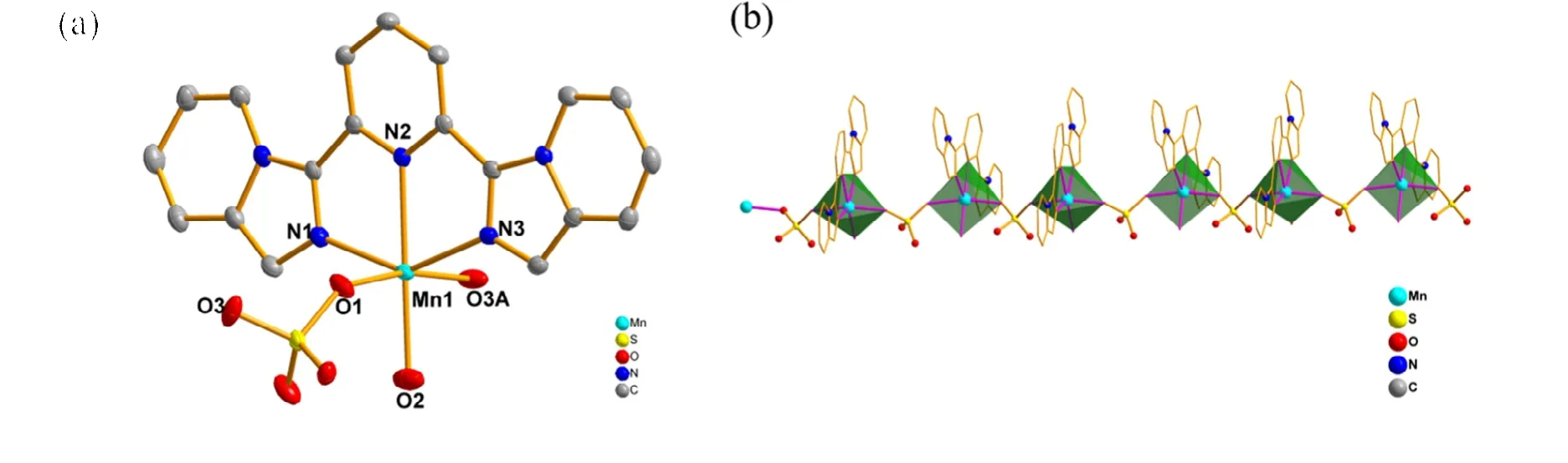
Fig. 1. (a) Asymmetric unit of compound 1. (b) Molecular structure of 1 showing the chain-like configuration and coordination polyhedrons of the MnII ions. Symmetry codes: A: x, 3/2-y, 1/2+z

Fig. 2. (a) Crystallographically determined structure of 2 with the ellipsoids of 30% probability level. (b) Coordination polyhedron of the CuI ion

Fig. 3. χMT products of 1 between 1.8 and 300 K at 1000 Oe. The solid line represents the fitted values based on the model[34]
3. 2 Dc magnetic susceptibility studies of 1
Dc magnetic susceptibility values of 1 were determined between 1.8 and 300 K. TheχmTproduct for two asymmetric units at 300 K is 8.04 cm3·K·mol-1(Fig. 3). This number is smaller than that of expected. As the theoretical value for two non-interacted MnIIions is 8.75 cm3·K·mol-1(g= 2.00,S= 5/2). Upon cooling from 300 to 70 K, theχmTvalue remains almost constant. With further cooling, the value ofχmTsharply decreases to 1.56 cm3·K·mol-1at 1.8 K, proving that MnIIcomplex is antiferromagnetically coupled.
To quantitatively assess the antiferromagnetical couplings in complex 1, we fitted the interactions of compound 1 by using the classical spin Heisenberg model (1-J) for a MnII2compound[34].
Fitting the magnetic susceptibilities of 1 using the reported model[34]gaveg= 1.95 andJ= -0.34 cm-1. The negativeJvalue indicated weakly antiferromagnetic MnII···MnIIcouplings.
3. 3 Catalytic properties of 2
Having the crystal structures of 1 and 2 in hand, we next studied the catalytic behavior of compound 2 for the ketalization reactions of various ketones. Ketalization reaction is an efficient methodology to protect carbonyl moiety of ketones[35].Typically, ketalization reaction is catalyzed by a Lewis acid.As the CuIion of 2 is four-coordinated, it may serve as a Lewis acid to activate the oxygen of the carbonyl group.
The protection of carbonyl groups of several ketones by ethylene glycol was conducted in toluene at 110 ℃. As can be seen in Table 2, when the substrates are aliphatic ketones,namely, acetone, 2-butanone, acetyl acetone and cyclohexanone (entries 1~3, 5), compound 2 can efficiently catalyze the reactions, generating the protected ketones with the yields of 88% (Fig. S6), 97% (Fig. S7), 97% (Fig. S8) and 99% (Fig. S10), respectively. The catalytic activity of complex 2 toward aromatic ketones is lower. It is found that a yield of only 5% (Fig. S9) was given for acetophenone.
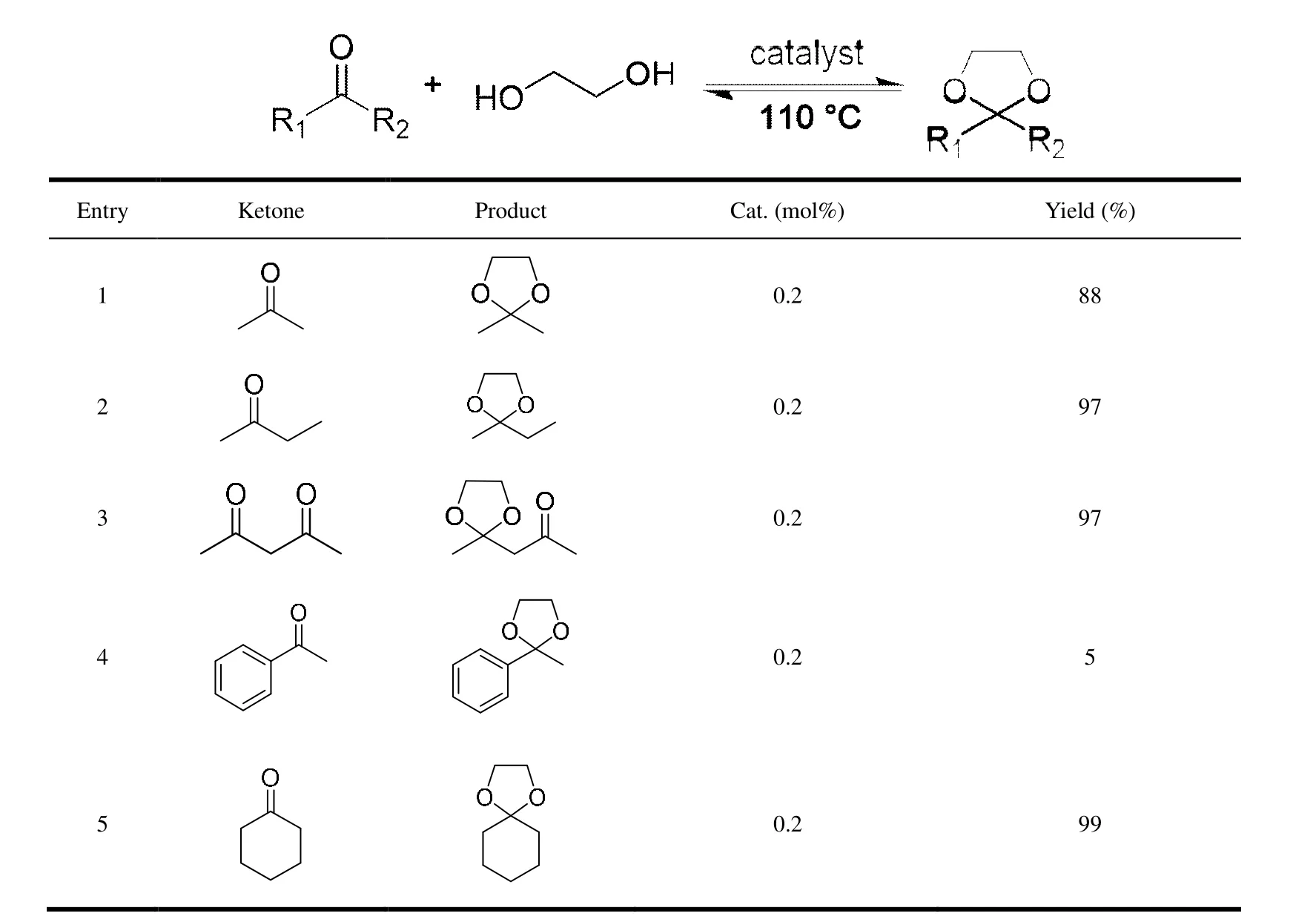
Table 2. Ketalization of Ketones Catalysed by 2
4 CONCLUSION
In conclusion, two complexes [Mn(bipp)(SO4)(H2O)]n(1)and [Cu(pip)2]ClO4(2) were synthesized and characterized.They display diverse structures. The magnetic properties of compound 1 were studied, revealing antiferromagnetic interactions between metal centers. Compound 2 was able to catalyze the ketalization reactions of aliphatic and aromatic ketones. It exhibited high activity towards the ketalization of aliphatic ketones.
杂志排行
结构化学的其它文章
- Structural and Electronic Properties of Lutetium Doped Germanium Clusters LuGen(+/0/-) (n = 6~19):A Density Functional Theory Investigation①
- Discovery of Benzimidazole Derivatives as Novel Aldosterone Synthase Inhibitors: QSAR, Docking Studies, and Molecular Dynamics Simulation①
- QSAR Models for Predicting Additive and Synergistic Toxicities of Binary Pesticide Mixtures on Scenedesmus Obliquus①
- Preparation, Crystal Structure and Fungicidal Activity of N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide Compounds①
- Antibiotic Silver Particles Coated Graphene Oxide/polyurethane Nanocomposites Foams and Its Mechanical Properties①
- Planar Tetracoordinate Carbon in 6σ + 2π Double Aromatic CBe42- Derivatives①
