Controllable Synthesis, Polar Behavior and Photoelectric Properties of BiOCl Microplates①
2022-04-16LILiXinCHENChenLIZiHoWANGFeiFeiLIUYunYIZhiGuo
LI Li-Xin CHEN Chen LI Zi-Ho WANG Fei-Fei② LIU Yun YI Zhi-Guo②
a (Key Laboratory of Optoelectronic Material and Device,Department of Physics, Shanghai Normal University, Shanghai 200444, China)
b (State Key Laboratory of High Performance Ceramics and Superfine Microstructure,Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 201800, China)
c (Research School of Chemistry, The Australian National University, Canberra, ACT 2600, Australia)
ABSTRACT Bismuth oxychloride (BiOCl) square microplates were prepared via a facile hydrothermal method.The X-ray diffraction patterns of the samples reveal a tetragonal BiOCl phase, and the scanning electron microscopy images show plate-like structures with large lateral size of 3~6 μm and thickness in the range of 100~300 nm. The effects of surfactant, reaction temperature and duration on the morphology of BiOCl powders are systematically investigated. The polar behavior of a BiOCl single-crystalline microplate is examined by using piezoresponse force microscopy evidenced over 80 pm displacement under 40 V bias voltage. In addition, the photoelectric performance of the BiOCl microplates is evaluated by using electrochemical workstation with three-electrode system, and large photocurrent densities (over 0.5 μA/cm2) and fast photoresponse (0.7~1.1 s) are detected by applying both 365 nm monochromatic light and sunlight illumination. The surface potential changes of BiOCl microplate under different light condition, characterized by in-situ Kelvin probe force microscopy, further verify the separation ability of the photo-induced charge carriers. These findings would be beneficial for further design photocatalytic and piezocatalytic materials.
Keywords: bismuth oxychloride, photoelectric, microplate, hydrothermal method;
1 INTRODUCTION
In recent years, two-dimensional (2D) layered materials,represented by graphene, are attracting increasing attention in a variety of areas due to the unique performance in comparison to their bulk counterparts[1,2]. In addition to graphene, Bi-based 2Dlayered materials exhibit great potentials in certain fields as well, such as photoelectric units, energy conversion systems and memory devices[3-5]. Among them,bismuth oxyhalides BiOX (X = Cl, Br, I) are currently a research hotspot particularly in the field of photocatalysis and photodetectors[6-10].
At room temperature, BiOX material exhibits a tetragonal structure, featured by alternating (Bi2O2)2+layers and double X-layers along thec-axis[11-14]. These layers are bonded by van der Waals force and provide a large space to induce electric dipoles along thec-axis. The resulted internal electric field was suggested to be the driving force for the efficient separation of the photo excitons in view of the good photocatalytic activities of BiOX compound[15,16]. Although the tetragonal structure of BiOX bulk crystal belongs to a non-polar point group 4/mmm, the point group can be reduced to 4mmpolar structure in the case of ultrathin 2Dstructures. Accordingly, piezoelectricity was assumed in 2Dstructured BiOX materials, and piezo-catalytic or piezo/photoelectric co-catalytic performance studies were carried out in several recent reports[17-21]. However, the piezoelectricity of BiOX 2Dnanomaterials hasn’t been sufficiently verified due to the small dimension and limited characterization approaches.
One easy approach to effectively characterize the piezoelectricity of BiOX 2Dmaterials is to synthesize plate-like structures with large plane size, which will benefit the existing testing methods. Many chemical methods, such as sonochemistry[22,23], solvothermal[24], hydrolysis[25,26], precipitation[27], etc., have been applied to synthesize BiOX nanomaterials. Hydrothermal process is the most practical method to control the morphology and dimensions of the nanostructures through simply adjusting the hydrothermal temperature and duration[28], pH value[29], and surfactant[30].
In this study, we aim to synthesize BiOCl powders with large size 2Dplate-like structures by using hydrothermal method. Through adjusting the synthesis temperature and duration, BiOCl square microplates were successfully obtained. Then, the polar response of a single BiOCl microplate was characterized by using piezoresponse force microscopy (PFM). In addition, the photoelectric performance of the BiOCl microplates was further investigated to evaluate the separation ability of the photo-induced charge carriers.
2 EXPERIMENTAL
2. 1 Materials and methods
Bismuth nitrate pentahydrate Bi(NO3)3·5H2O (analytical grade, Macklin, Shanghai, China) and potassium chloride KCl (analytical grade, Aladdin, Shanghai, China) were used as raw materials. For a standard procedure, 5 mmol Bi(NO3)3·5H2O and 5 mmol KCl were dissolved into 80 mL deionized water under magnetic stirring at room temperature.A predetermined amount of surfactants sodium citrate (analytical grade, Titan, Shanghai, China), varying from 0 to 1.5 mmol, were added into the solutions to control the morphology of the resultant powders. The well-dispersed solutions were moved into a 100 mL Teflon-lined stainless-steel hydrothermal reactor after stirring for 30 mins. The hydrothermal reaction was carried out at 160 and 180 ℃ for 6 to 48 h. The resultant solid products were separated from the solution and washed with deionized water by using centrifuge, then dried at 60 ℃ for 12 h for further characterization.
2. 2 Characterization
The phase structure of the hydrothermal products was identified using a Bruker D8 advance X-ray diffractometer(CuKαradiation source, Bruker, Karlsruhe, Germany). The step size and dwell are 0.02° and 0.12 s, respectively. The micromorphology of the samples was observed by scanning electron microscope (SEM, JSM-6700F, 10 kV, Jeol, Japan).To prepare the samples for SEM tests, the BiOCl powders were ultrasonically dispersed with anhydrous ethanol, and then the suspension was dropped on the sample stage covered with conductive tape.
The PFM measurements were performed in the dual AC resonance tracking (DART) PFM modes (PFM, MFP-3D,Asylum Research, USA). This technique could enhance contrast in the amplitude and phase image and reduce the topographical crosstalk while mapping the local electromechanical properties. Conductive cantilevers ASYELEC-01 with tip coatings of Ti/Ir (5/20) were used. The nominal spring constants were 2 N/m with a fundamental resonance frequency of the free tip-vibration (non-contact resonance)of approximately 70 kHz. The local piezoelectric hysteresis loop was obtained in the switching spectroscopy PFM(SS-PFM) mode and the applied ac voltage was 1 V. Before each measurement of the local piezoelectric hysteresis loop,the calibration was performed through the combination of the force curve and thermal noise method. Kelvin probe force microscopy (KPFM, SPA400, Hitachi, Japan) was also applied to measure the surface potential distribution of the BiOCl nano/microplate. The BiOCl powders were dispersed in ethanol, and then the suspension was dropped on a cleaned silicon substrate for PFM tests or on a gold-coated silicon substrate for KPFM tests.
The light absorption ability of BiOCl powders was measured by using the diffuse reflectance spectra (DRS) mode of a UV-vis spectrophotometer (Cary 5000, Varian, USA). The photoelectric properties and electrochemical impedance spectra (EIS) of the BiOCl powders were carried out by using three-electrode system and an electrochemical workstation (CS310H, Kesite, Wuhan, China). For the threeelectrode system, an indium-tin oxide (ITO) sheet glass coated with BiOCl powders was equipped as the working electrode, a platinum wire as the counter electrode, and a saturated calomel electrode (SCE) as the reference electrode,and 0.1 M Na2SO4solution was used as electrolyte solution.365 and 405 nm monochromatic lights, and a 300 W Xenon lamp were employed as the light sources, and all the tests were performed at room temperature.
3 RESULTS AND DISCUSSION
3. 1 Controllable synthesis of BiOCl nano/microplates
Herein, aiming to obtain large size 2Dstructure BiOCl,the content of sodium citrate is first adjusted from 0 to 1.5 mmol. The XRD results given in Fig. 1 show that, by varying the sodium citrate content, all the resultant BiOCl powders exhibit a pure tetragonal phase indexed by JCPDS No.85-0861. The sharp and well-shaped diffraction peaks indicate good crystallization of the BiOCl powders. Besides,the intensity of the different characteristic peaks of BiOCl can be changed by adjusting the sodium citrate content,which is influenced by the powder size and morphology. The corresponding SEM images show that the particle size is significantly reduced by adding sodium citrate. Samples A to C obtained by adding different contents of sodium citrate all show irregular and highly agglomerated powders, while sample D obtained with no surfactants presents separated plate-like square particles with uniform size. When sodium citrate was added to the reaction solution, the citrate ion(C5H7O5COO-) would be adsorbed on the surface of[Bi2O2]2+layer, thus delaying the formation and growth of the nanoparticles[31]. Therefore, under the same reaction time,the size of BiOCl without sodium citrate is larger than that of the sample with sodium citrate. Consequently, sodium citrate was removed, and the holding temperature and duration of the hydrothermal process were further optimized to obtain larger size plate-like BiOCl powders.

Fig. 1. XRD patterns (a) and SEM images (b~e) of the BiOCl powders obtained by varying the sodium citrate content for the hydrothermal process at 160 ℃ for 12 h. Sample A: 1.5 mmol (b); Sample B: 1 mmol (c); Sample C: 0.5 mmol (d); Sample D: 0 mmol (e).The bottom XRD pattern is the standard one of JCPDS No.85-0861
Fig. 2 shows the SEM images of BiOCl powders obtained at different hydrothermal temperature and holding time.Without adding sodium citrate, all the obtained BiOCl powders show plate-like 2Dmorphology without agglomeration.With the increase of reaction temperature, the enhanced crystallization rate of nanoparticles leads to the increase of crystallinity and particle size[28,32]. Under the same hydrothermal temperature, the powder size gradually increases with the extension of reaction time. In same reaction time,the powders synthesized at 180 ℃ show better uniformity than that at 160 ℃. The powders obtained at 180 ℃ for 48 h show larger width, however, the thickness of the plate also increases to around 500~800 nm. Therefore, in view of the uniform square plate shape with thinner thickness, the powders obtained at 180 ℃ for 12 h was applied for the characterization of piezoelectric and photoelectric performance.

Fig. 2. SEM images of BiOCl powders obtained at different synthesis temperature and holding time. (A~D) hydrothermal reaction at 160 ℃ for 6 h (a), 12 h (b), 24 h (c), 48 h (d); (e~f) hydrothermal reaction at 180 ℃ for 6 h (e), 12 h (f), 24 h (g), 48 h (h)
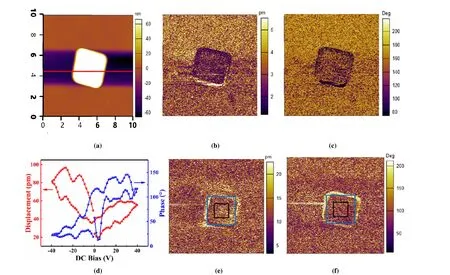
Fig. 3. PFM characterization of a single BiOCl microplate. (a) Topography image; (b) Amplitude image; (c) Phase image; (d) Local displacement and phase hysteresis loops; The amplitude (e) and phase (f) images of the microplate after polarization by ±50 V external electric fields:Negative voltages with an amplitude of 50 V were written in the center area of 1.5 × 1.5 μm2 and an equal amount of positive voltage were written in the outer square area of 3 × 3 μm2
3. 2 Polar behavior characterization
The polar behavior of a single BiOCl microplate was characterized by PFM (Fig. 3). The topography image (Fig. 3a)reveals the width ~3µm and thickness ~100 nm of the microplate, which is consistent with the SEM results (Fig. 2f).The dark PFM amplitude image (Fig. 3b) implies very weak piezoelectric response along direction perpendicular to the microplate surface, and the phase image (Fig. 3c) shows no obvious multi-domain patterns, which may be resulted by single domain structure or nano-polar regions. But the ferroelectric-like behavior of the BiOCl microplate is observed by the local displacement loop with typical butterfly shape and the phase hysteresis loop (Fig. 3d). The local effective piezoelectric coefficientd33,locis estimated to be around 39 pm/V by using the displacement under zero bias divided by AC voltage (1 V) applied to the PFM tip[33,34].
External field was applied to the BiOCl microplate to evaluate the polarization switching characteristics (Figs. 3e and 3f). Negative voltages with an amplitude of 50 V were written in the center area of 1.5 × 1.5μm2(the black box area) and an equal amount of positive voltage was written in the outer square area of 3 × 3μm2(the blue box area). In comparison with the amplitude and phase images before electric polarization (Figs. 3b and 3c), the amplitude and phase images after +50 V polarization (Figs. 3e and 3f) show stronger piezoelectric response, implying the domain switching. However, after applying -50 V to the small black box area, no significant opposite domain switching is detected.

Fig. 4. (a) UV-vis absorbance spectrum of BiOCl microplates. The inset shows the calculated bandgap (Eg); (b) Transient photocurrent responses of the BiOCl photoelectrode under periodic light on/off illumination by different light sources (200 mW/cm2). 1 V bias was applied;(c) Estimation of the photoresponse time of the BiOCl photoelectrode (extracted from Fig. 4b); (d) Electrochemical impedance spectra of BiOCl photoelectrode under dark and different illumination conditions (200 mW/cm2)
3. 3 Photoelectric performance
The UV-vis absorbance spectra in Fig. 4a indicate an absorption edge ~375 nm of the BiOCl microplates, revealing its UV response. The band gap (Eg) of BiOCl samples was estimated by Kubelka-Munk formula as (αhν) =A(hν-Eg)n/2,whereα,h,ν,A, andEgare the absorption coefficient,Planck constant, photon frequency, proportion constant, and band gap, respectively. The optical bandgap of BiOCl is calculated to be around 3.4 eV (inset of Fig. 4a), which is consistent with the previous reports[35-37].
Fig. 4b presents the photocurrent response of the BiOCl photoelectrode to evaluate the separation ability of photoinduced charge carriers. The 365 and 405 nm monochromatic lights and a Xenon lamp are employed as the light sources, and the light intensity is kept as 200 mW/cm2. The BiOCl photoelectrode presents a larger photocurrent density over 0.5μm/cm2under 365 nm than under 405 nm light.This result is consistent with the light response ability of the BiOCl microplates (Fig. 4a). Due to the negligible absorption of 405 nm light, the amount of photo-induced charge carriers is much less than that excited by 365 nm light,which results in the weak photocurrent densities. Although the photocurrent of BiOCl can be enhanced by sunlight, the main contribution is from the UV part, which accounts for only ~5% of the sunlight spectrum. Accordingly, it is no doubt an effective approach to optimize the photoelectric properties by reducing the bandgap of BiOCl.
One light on/off cycle of the photocurrent response of BiOCl is enlarged in Fig. 4c to reveal the response speed to different lights. The rising time of the photocurrent is around 1.1 s under both 365 nm and sunlight. The fast-rising time indicates a good separation efficiency of the photo-induced charge carriers for BiOCl. The decaying time for sunlight is around 0.7 s. As for 365 nm light, the photocurrent consists a fast decaying process 0.7 s. Fig. 4d gives the electrochemical impedance spectrum of BiOCl photoelectrode under different light conditions. The arc radius of BiOCl under 365 nm light illumination is much smaller than that under dark, implying that the resistance of BiOCl is effectively reduced due to the large amount of the photo-excited charge carriers.
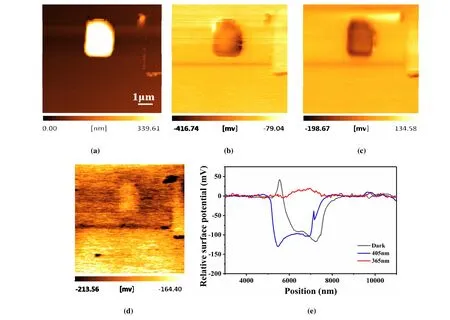
Fig. 5. Light-induced evolution of the surface potential distribution of a BiOCl microplate on gold-coated silicon substrate: Topography image(a) and surface potential image under dark (b), illuminated by 405 nm light (c) and 365 nm light (d) and corresponding line profiles (e)
The surface potential distribution of a BiOCl microplate was characterized by usingin-situKelvin probe force microscopy. A gold-coated silicon substrate was used for all the tests. Fig. 5a presents the topography image of a BiOCl microplate, and Fig. 5b to 5d presents the corresponding surface potential images under dark and illuminated by 405 and 365 nm monochromatic light, respectively. The surface potential distribution of the BiOCl microplate is relatively uniform. Since the surface potential of the gold-coated silicon also shows obvious changes under different light conditions,the evolution of the surface potential for BiOCl microplate is evaluated by using the relative potential between BiOCl and the substrate. Fig. 5e presents the line profiles of the relative potentials under different light conditions. The line profile under dark indicates that the surface potential of the BiOCl microplate is approximately 100 mV lower than that of the substrate, and no significant changes are detected when illuminated by 405 nm light, which is consistent with the negligible photocurrent response to 405 nm light (Fig. 4b). Under 365 nm light illumination, the surface potential of the BiOCl microplate is largely enhanced and becomes slightly higher than that of the substrate. The obvious surface potential changes indicate the increasement of the surface charges induced by the efficient separation of the photo-excited charge carriers. The noteworthy surface protentional change under 365 nm light illumination implies a good potential of photocatalytic application of the BiOCl microplates, but is limited to the UV range of the solar spectrum.
4 CONCLUSION
In conclusion, 2Dstructured BiOCl microplates were successfully prepared by hydrothermal method without adding surfactants. By adjusting the reaction temperature and holding time, BiOCl powders with uniform square plate shape were obtained at 180 ℃ for 12 h. The lateral size of the plates is around 3~6μm, and the thickness falls in the 100~300 nm range. The ferroelectric-like behavior of the BiOCl microplates is observed by the local displacement loop with typical butterfly shape and the phase hysteresis loop characterized by using PFM. By employing electrochemical workstation with three-electrode system, significant photocurrent of the BiOCl microplates was obtained when illuminated by both 365 nm monochromatic light and sunlight illumination. The surface potential changes under different light illumination show great potential of photocatalytic application of the BiOCl microplates by tuning the illumination conditions.
杂志排行
结构化学的其它文章
- Structural and Electronic Properties of Lutetium Doped Germanium Clusters LuGen(+/0/-) (n = 6~19):A Density Functional Theory Investigation①
- Discovery of Benzimidazole Derivatives as Novel Aldosterone Synthase Inhibitors: QSAR, Docking Studies, and Molecular Dynamics Simulation①
- QSAR Models for Predicting Additive and Synergistic Toxicities of Binary Pesticide Mixtures on Scenedesmus Obliquus①
- Preparation, Crystal Structure and Fungicidal Activity of N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide Compounds①
- Antibiotic Silver Particles Coated Graphene Oxide/polyurethane Nanocomposites Foams and Its Mechanical Properties①
- Planar Tetracoordinate Carbon in 6σ + 2π Double Aromatic CBe42- Derivatives①
