A Double Crown Hexakis[(Di-µ-benzylthio) Nickel]Cluster: Synthesis, Structure and Properties①
2022-04-16LIUJIAAiQunZHANGQinFengJIANFngFng
LIU E JIA Ai-Qun ZHANG Qin-Feng② JIAN Fng-Fng
a (Institute of Molecular Engineering and Applied Chemistry,Anhui University of Technology, Ma’anshan, Anhui 243002, China)b (School of Chemical Engineering and Pharmaceutics,Henan University of Science and Technology, Luoyang 471000, China)
ABSTRACT The double crown hexakis[(di-µ-benzylthio) nickel] cluster, [Ni6(SCH2C6H5)12]·C2H5OH, was obtained by reacting C6H5CH2SNa with [(CH3)2CHOCS2]2Ni in EtOH. The results of electrochemical studies show that[Ni6(SCH2C6H5)12] is a quasi-reversible process. The crystal structure of [Ni6(SCH2C6H5)12]·C2H5OH is composed of discrete [Ni6(SCH2C6H5)12] and C2H5OH solvent molecules. Each Ni atom is surrounded by four S atoms of the µ2-SCH2C6H5 ligands in a distorted square-planar structure. The C6H5CH2S- side chains are arranged in the axial and equatorial positions, alternately, concerning the pseudo-hexagonal axis of the molecule. Strong π-π and C-H···π effects form the supramolecular nano-channel along the a-axis in the crystal, which wraps the solvent ethanol molecules in it. In addition, a wall is formed along the b- and c-axes, so that the ethanol molecules can freely enter and leave along the aaxis. These effects result in the ability of the title compound to adsorb and desorb ethanol molecules. Thermogravimetric analysis and powder X-ray diffraction at different temperature are provided to demonstrate this point.
Keywords: benzylthiol, nickel cluster, porous materials, cyclic voltammetry, thermogravimetry;
1 INTRODUCTION
Ordered porous materials, like metal-organic frameworks(MOFs) and covalent organic frameworks (COFs), have attracted increasing interest in recent time[1], and are a hot topic in materials chemistry[2,3]. However, supramolecular porous materials have many uncertainties due to their reliance on directed supramolecular forces. Unlike MOF and COF which use rigid structures or predesign, this type of material trades a significant portion of the strength of the bonds linking different building blocks in favor of soft-matter-type networks, which can spontaneously self-assemble in solution.
Porous supramolecular polymers are novel materials that are joined together by relatively weak coordination bonds. They form the molecular framework through hydrogen bonding orπ-πand C-H···πsupramolecular interactions, and often exhibit poor thermodynamic stability[4-6]. To the best of our knowledge, reports of thermodynamic properties of supramolecular polymers formed by these weak interactions are relatively rare[7-9]. In some cases, the molecular framework formed by these supramolecular interactions may create open interstices,holes, and channels that can occupy more than half of the volume of the entire crystal. These spaces can be occupied by solvent molecules[10,11]. A certain amount of porosity can be produced by eliminating these solvent molecules, and the supramolecular body frame is not damaged. Therefore,supramolecular organic frameworks (SOFs) pertain to the field of reticular chemistry broaden their definition so that they can encompass materials that are held together by interactions weaker than covalent or coordination bonds.
Metallacrowns form a class of multinuclear clusters that are analogous to crown ethers in both structure and function[12].Sulfur atoms are present widely in important materials and biomaterials, and some sulfur clusters formed with metals have important applications in materials[13]. However, the study of S-bridged variants remains limited[14].
In this paper, the crystal structure of a double crown hexakis-[(di-µ-benzylthio) nickel] cluster, [Ni6(SCH2C6H5)12]·C2H5OH(1), is reported, which is a kind of SOFs formed by supramolecular action. Also, it has adsorption and desorption properties for C2H5OH molecules and keeps the supramolecular structure intact. Powder X-ray diffraction and thermogravimetric analysis support this inference.
2 EXPERIMENTAL
2. 1 Materials and physical measurements
All the chemicals were of analytical reagent grade and used directly without further purification. Elemental analyses were measured with a Perkin-Elmer 1400C analyzer. Infrared spectra were recorded on a Nicolet 170SX spectrometer using pressed KBr pellets in the 4000~400 cm-1range. TG and DTG curves were recorded on a NETZSCH-Geratebau GmbH thermoanalyser underflow of N2in the temperature range of 20~800ºC at a heating rate of 10 ºC/min. Powder X-ray diffraction data were collected by PXRD D/MAX-500. Electrochemical measurements were done by the Autolab PGSTAT-30 electrochemical workstation by using Au electrode as a working electrode, Pt slices (0.8cm × 0.8cm) as the auxiliary electrode and Ag/AgCl electrode as the reference electrode.
2. 2 Preparation of [Ni6(SCH2C6H5)12]·C2H5OH (1)
The mercaptobenzyl alcohol (B) was obtained by reacting benzyl chloride with thiourea (A) according to a known procedure[15]. Nickel xanthogenate (D) was obtained by reacting sodium thiolate (C) with aqueous metal sulfate. The compound(E) was obtained from the reaction of B and D, with the reaction steps shown in Scheme 1. The detailed process is as below: to a warm solution of benzylthiol (1.853 g, 14.92 mmol)in C2H5OH (30 mL) was added C2H5ONa (1.016 g, 14.93 mmol) under stirring, and the mixture was refluxed for 30 min with the color of the solution turning orange. Then D was added to the orange solution and reacted for 4 h and cooled to room temperature, followed by filtering the brown solution. The obtained red-brown precipitate was washed with C2H5OH three times. The yield was 69.3% based on mercaptobenzyl alcohol for [Ni6(SCH2C6H5)12] (E). Dark brown block-shaped single crystals suitable for X-ray analysis were obtained by slowly evaporating a mixture of CH3COOC2H5and C2H5OH for 1.Anal. Calcd. (%) for [Ni6(SCH2C6H5)12]·C2H5OH: C, 54.99; H,4.83. Found (%): C, 55.08; H, 4.87. IR (KBr pellet, cm-1):3082(w), 3059(s), 3026(vs), 2923(m), 1600(vs), 1583(w),1493(vs), 1452(vs), 1422(m), 764(vs), 696(vs), 666(m).

Scheme 1. Step of the synthesis of compound [Ni6(SCH2C6H5)12] (E)
2. 3 Structure determination for[Ni6(SCH2C6H5)12]·C2H5OH (1)
A suitable crystal of 1 (0.40mm × 0.25mm × 0.32mm) was selected for data collection performed on an Enraf-Nonius CAD-4 diffractometer with graphite-monchromated Mo-Kαradiation (λ= 0.71073 Å,T= 293 K). A total of 9518 independent reflections were collected in the range of 3.15≤2θ≤53.95°, of which 6135 were observed withI> 2σ(I) and used in the succeeding refinement. Empirical absorption correction was carried out using SADABS program[16]. Using Olex2[17],the structure was solved by the SHELXT[18]structure solution program using Intrinsic Phasing and refined by full-matrix least-squares techniques based onF2with the SHELXL[19]. All non-H atoms were anisotropically refined, while the hydrogen atoms were located by difference Fourier synthesis and refined isotropically. Atomic scattering factors and anomalous dispersion corrections were taken from International Tables for X-ray Crystallography[20]. A summary of the key crystallographic information for [Ni6(SCH2C6H5)12]·C2H5OH is given in Table S1-1. Selected bond lengths and bond angles are listed in Table 1.
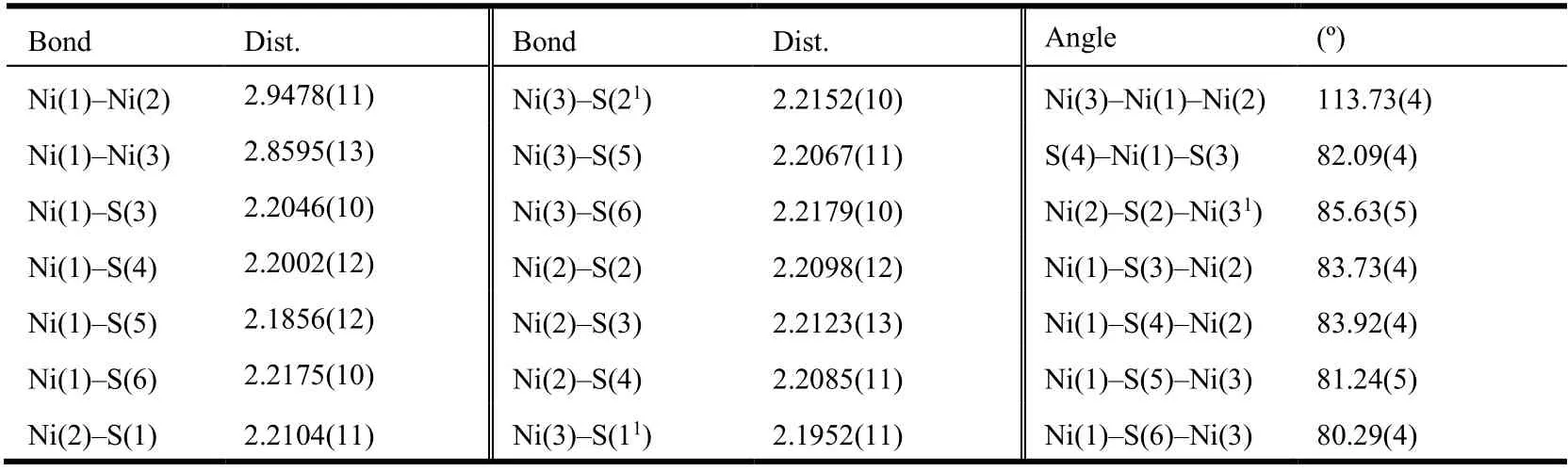
Table 1. Selected Bond Lengths (Å) and Bond Angles (º) for Compound (1)
3 RESULTS AND DISCUSSION
3. 1 Single-crystal X-ray diffraction studies
The crystal lattice of compound [Ni6(SCH2C6H5)12]·C2H5OH is made up of a centrosymmetric [Ni6(SCH2C6H5)12] molecule and disorder C2H5OH solvent molecules (Fig. 1). So far as we know, the toroidal nickel-sulfur architecture of cyclic-[Ni(SR)2]n(n = 4, 5, 6, 8, 9, 10, 11) has been reported[21,22]. All of them may be viewed as a regular convex n-polygon of coplanar nickel atoms with two thiolate sulfur atoms bridging each pair of adjacent nickel atoms. The upper and lower parallel polygons of coplanar nonbonding sulfur atoms, which form a prismatic polyhedron, are in a staggered conformation with then-polygonal Ni atoms; this parallel three-layer S/Ni/S sandwich was originally denoted as atiara[23]in Fig. 2.
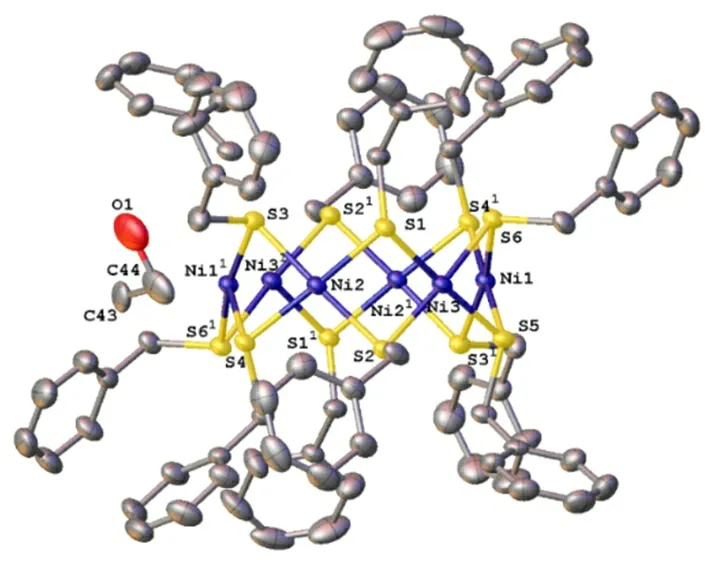
Fig. 1. An ORTEP drawing of compound (1).Symmetry operation: 1: -x, 1-y, -z.
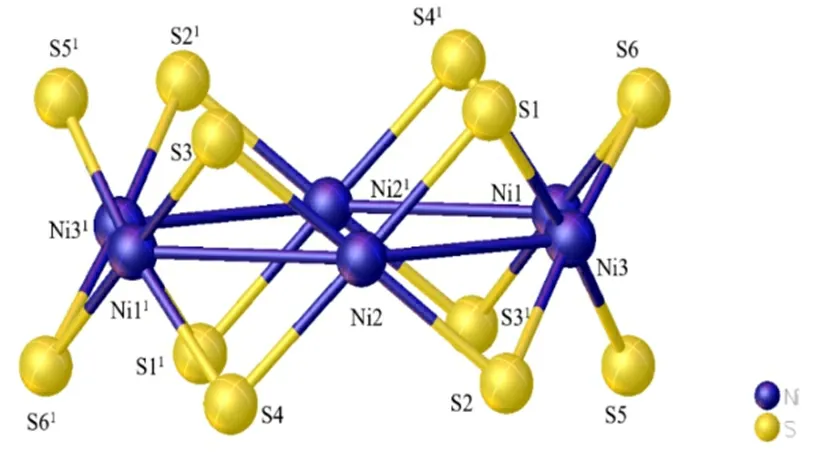
Fig. 2. Double crowns made of a Ni6 ring and twelve µ2-S atoms.Symmetry operation: 1: -x, 1-y, -z
The coordination geometry of the Ni atoms is approximately square planar. As a result, the edge-fused NiS4planes are connected into a ring. The six-nickel atoms approximately form a hexagon, with the Ni-Ni distances falling in the range of 2.859(17)~3.007(15) Å and the vertex angles of 113.70~126.16º. These values are very close to the Ni6 cluster reported in previous kinds of literature[24-26]. The diameter of the ring, defined as the average distance between two opposing nickel atoms, is 5.87 Å. Twoµ2-S bridges originating from two benzylthiol ligands connect the adjacent nickel atoms. As a consequence, the six nickel atoms nearly show virtual C6symmetry, and the twelveµ2-S atoms form double crowns, with one above and the other below the nickel ring, and the mean distance between the two S6planes to be 2.889 Å.
Owing to geometrical restrictions introduced by the bridging sulfur atoms, NiS4units are not strictly planar, with S-Ni-S bond angles ranging from 80.72º to 99.70º, and Ni-S-Ni bond angles between the adjacent subunits are in the range of 80.29~86.04º. The Ni-S bond lengths vary from 2.185 to 2.217 Å. Within the Ni6ring, the average Ni-Ni distance between adjacent faces is 2.938 Å, which shows feeble interaction in these nickel atoms but is similar to the Ni-Ni bond lengths found in other nickel clusters[23]. In addition, the triangle plane laminas (NiSNi) of the double crown are not perpendicular to the Ni6ring.
The disordered phenomenon of the solvent molecule ethanol in the crystal structure is obvious, and the disordered occupancy is 0.5. The disorder is a symptom of crystal imperfection,a signal of increased confusion degree. The disorder of the solvent molecules is easily lost from the point of view of the perfection of the crystal. This was confirmed by experiments,in which the title compound adsorbed and desorbed ethanol molecules.
There are some intermolecular interactions such as C-H···S hydrogen bonds,π-πstacking interaction and C-H···πsupramolecular interaction in the lattice structure, but only the latter twoplay an important role in the formation of micro-porous structures (Fig. 3). The interaction between twoπ-πforms a one-dimensional zigzag chain. And, the 1D chain was formed through intermolecular C-H···πstacking interactions. The carbon atom’s distances to the center of the benzene ring are 3.775 and 3.760 Å. Through these supramolecular interactions, the clusters formed a 2D planar structure.
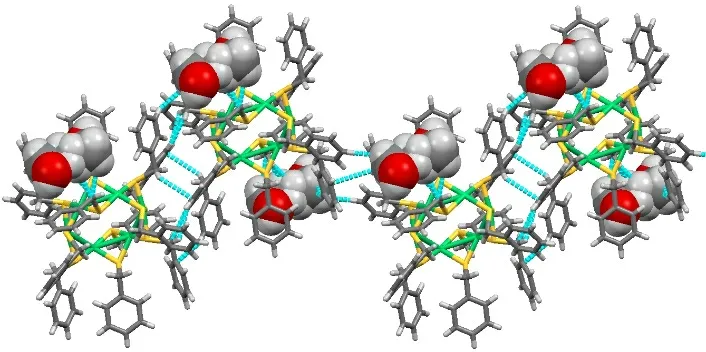
Fig. 3. 1D zigzag chain formed through intermolecular π-π stacking interactions and C-H···π stacking interactions

Fig. 4. (4a) Nanochannels along the a-axis with the disordered C2H5OH solvent molecules in it. (4b) Some phenyl rings hinder the solvent molecules from moving in and out along the b-axis. (4c) Some phenyl rings hinder the solvent molecules from moving in and out along the c-axis. C2H5OH solvent is represented by spacefill-style, and all H-protons are omitted for clarity. The benzene ring is in blue just to make it stand out
Along thea-axis, we can find anano-channel between these two supramolecular forces formed by the layer surface. And the disordered C2H5OH solvent molecule is right in thesenanochannels (Fig. 4a). No phenyl rings are influencing the C2H5OH solvent molecules to move in and out. But, alongb(Fig. 4b)andcaxes (Fig. 4c), the disordered C2H5OH solvent molecules are intercepted by the benzene ring, so they cannot move freely from these two directions. They can only move freely in and out of thea-axis. As we can see from Fig. 4, the benzene rings to block the movement of the ethanol molecules in theb- orcaxis direction. Therefore, we can infer that compound[Ni6(SCH2C6H5)12] can adsorb C2H5OH molecules and is a potential C2H5OH adsorption and desorption material. This performance was demonstrated in the TG analysis.
3. 2 Thermal analysis and adsorption/desorption C2H5OH experiment
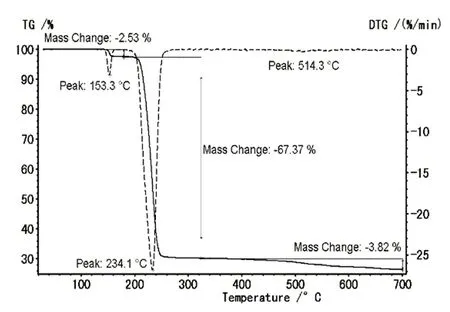
Fig. 5. Thermal analysis curve of compound 1
Thermal analysis curves of [Ni6(SCH2C6H5)12]·C2H5OH (1)are shown in Fig. 5. The decomposition of 1 is accompanied by three weight-loss steps corresponding to three peaks,respectively. During the first step, there is a small sharp peak at 153.3 °C with the weight loss of 2.53% assigned to the release of part C2H5OH solvent molecule (all loss calcd. to be-4.78%). Above 160 °C, in the second step the compound decomposes corresponding to another strong peak at 234.1 °C with the lost weight to be 67.37%. Based on the mass of 612.88,the residue may be Ni6S8(calcd. 67.56%). The results indicated that the C-S bond in the structure was broken and the benzyl group was volatilized into toluene, resulting in a strong endothermic peak change (C6H5CH3boiling point 110.4°C). Finally, a broad weight loss is observed in the temperature of 450~700 °C, with a small peak at 514.3 ºC and a weight loss of 3.82%. The residue could be Ni6S4based on the molecular formula (found 3.82%, calcd. 3.50%).
In order to check if compound [Ni6(SCH2C6H5)12] adsorbs C2H5OH or not, a simple experiment was carried out. We put 1.00 g of [Ni6(SCH2C6H5)12]·C2H5OH in a 160 °C furnace heating for 3 h. The dark brown crystal obtained dimmed and lost luster, also with a weight loss of 47 mg. Then it was put into a small beaker placed in a sealing big beaker containing 10 mL of ethanol. After keeping still for one day, it gained 23 mg weight increase, so compound [Ni6(SCH2C6H5)12] has adsorbed some ethanol molecules.
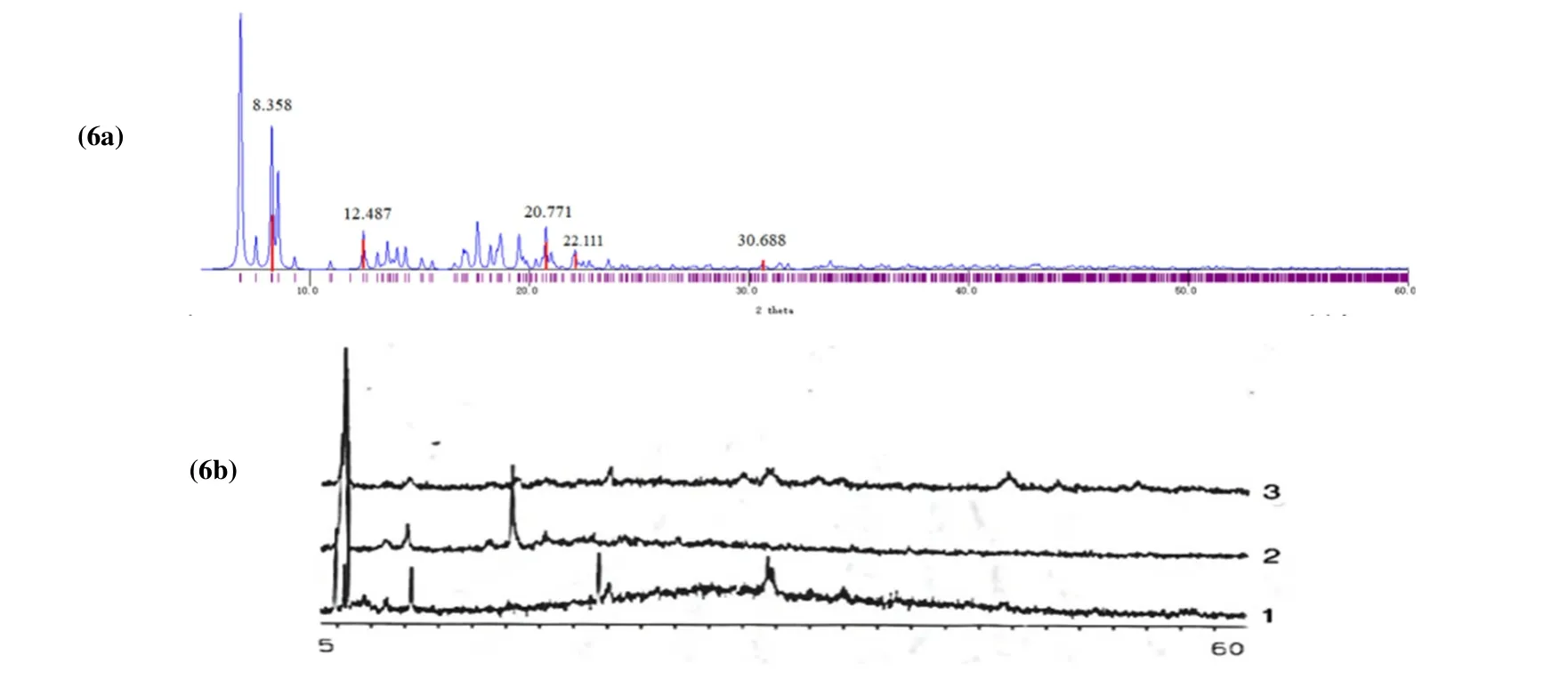
Fig. 6. (6a) Powder diffraction pattern of crystal structure conversion. (6b) PXRD patterns for measured as-synthesized sample 1, sample 2 after heating at 160 °C for 3 h, and sample 3 exposed to C2H5OH for more than 16 h
In order to verify whether the desolvation compound was consistent with the title compound (1), powder X-ray experiments were performed on the samples at different temperature stages. By analyzing the Powder X-ray Diffraction (PXRD)pattern of the material, the information of the composition of the material and the structure or morphology of the atoms or molecules in the material can be obtained. By comparing the diffraction pattern simulated by a single crystal with PXRD,we can roughly judge whether the host [Ni6(SCH2C6H5)12] is the same before and after desolvation. The calculated PXRD diffractogram from a single crystal structure (1) is provided, as shown in Fig. 6a. Also, three samples are obtained (Fig. 6b):1-original compound [Ni6(SCH2C6H5)12]·C2H5OH; 2-compound (1) at 160 °C to heat 3 h; 3-place 2 in a dryer with C2H5OH solvent for more than 16 h. Because the PXRD sample is to grind the powder into the tablet test, the accumulation of grains may appear. Some crystal face extinction makes the diffraction peak disappear. There is no such extinction in the simulated pattern of the single crystal. Therefore,the number of diffraction peaks of the simulated single-crystal pattern must be more than that of the PXRD one. From Fig. 6 we can see that, in the 2θrange of about 8.3°~12.5°, powder diffraction samples 1, 2, and 3 in Fig. 6b with Fig. 6a are similar, which all showed similar strong peaks. This indicates no major collapse has occurred. They are all the same to compound [Ni6(SCH2C6H5)12]. When some ethanol molecules were lost as sample 2, a new peak was found at about 17.7°and 18.6°, and the peaks at 20.7°, 22.1º and 30.6º disappeared.But, when sample 2 adsorbed the ethanol molecule and changed to 3, the new peaks at 17.7° and 18.6° cannot be found,and the missing peaks at 22.1° and 31.0º appear and recover again. This shows that the framework [Ni6(SCH2C6H5)12]remains unchanged when the C2H5OH solvent is removed. In addition, sample 2 can partially absorb ethanol molecules,except that the peak value is less than that of sample 1. As reported[27],π-πand C-H···πforces enable the staggered distribution of the benzyl to form a three-dimensional structure of the double-crown cluster, thus producing a porous supramolecular structure containing disordered ethanol solvent molecules[27].
3. 3 Electrochemical properties
DMF was used as the solvent, 0.1 mol·L-1TBAP (tetrabutyl ammonium perchlorate) as the supporting electrolyte, and the complex concentration was 1.0 × 10-4mol·L-1. Scanning was performed in the range of 0.5~1.6 V, and cyclic voltammetry
4 CONCLUSION
In summary, we have reported one new member in the series of M6S12cyclic hexanuclear complexes. In this paper, a novel benzyl mercaptan metal crown cluster was synthesized, and the single-crystal structure of [Ni6(SCH2C6H5)12]·C2H5OH was obtained. By analyzing its crystal structure, these clusters are found to be micropore structures, and the solvent molecule can(CV) was used to study under room temperature anaerobic conditions.
Cyclic voltammetry is the most widely used method in the study of electrode dynamics. The peak current density (Ip) in cyclic voltammetry (CV) is a function of many kinetic parameters and an indicator of the reaction mechanism. The Ip analysis of reversible and irreversible reactions plays a key role in the analysis of CV experimental results. Fig. 7 shows the cyclic voltammogram of compound [Ni6(SCH2C6H5)12] at the scanning rate of 0.05 V/s. The half-peak method was used to measure the peak. The peak potential of oxidation is Ep2 =723 mV, and the peak current is Ip2 = 3.85 × 10-6A. The reduction peak potential is Ep1 = 457 mV, and the peak current is Ip1 = 7.23 × 10-6A. Oxidation peak reduction peak potential difference is 266 mV, and the ratio of peak current is Ip1/Ip2= 1.78. It is shown that the electrochemical behavior of 1 on the gold electrode is a quasi-reversible process[28-30].be included in these micropore channels. Therefore, they are potential adsorption materials. Compounds containing the sulfhydryl group have the potential to generate novel cyclic polynuclear complexes with promising structural features and properties. We believe that these results can strongly justify further work on the cyclic polynuclear systems with sulfhydryl and transition metal.

Fig. 7. Cyclic voltammograms of the complex [Ni6(SCH2C6H5)12] in DMF solution containing 0.1 M TBAP at a scan rate of 0.05 mV/s
杂志排行
结构化学的其它文章
- Structural and Electronic Properties of Lutetium Doped Germanium Clusters LuGen(+/0/-) (n = 6~19):A Density Functional Theory Investigation①
- Discovery of Benzimidazole Derivatives as Novel Aldosterone Synthase Inhibitors: QSAR, Docking Studies, and Molecular Dynamics Simulation①
- QSAR Models for Predicting Additive and Synergistic Toxicities of Binary Pesticide Mixtures on Scenedesmus Obliquus①
- Preparation, Crystal Structure and Fungicidal Activity of N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide Compounds①
- Antibiotic Silver Particles Coated Graphene Oxide/polyurethane Nanocomposites Foams and Its Mechanical Properties①
- Planar Tetracoordinate Carbon in 6σ + 2π Double Aromatic CBe42- Derivatives①
