Study on intestinal flora of acupoint catgut embedding intervention in female patients with abdominal obesity:study protocol for a randomized controlled trial
2022-04-15QiFuLiJiaLeiFengXingHeZhangLiLiXuanXiangZengSiWenZhaoMengKongYunQiuZhengChongHuiXingTaiPinGuo
Background
Obesity is a type of chronic metabolic disease characterized by the excessive accumulation or abnormal distribution of fat in the body.With the development of the social economy and changes in people’s lifestyles,the morbidity of obesity is increasing annually and has become a serious global public health problem.The latest global epidemiological data indicate that more than 2.0 billion adults worldwide are overweight[1].The global overweight and obese populations are predicted to reach more than 2.16 billion and 1.12 billion,respectively,by 2030[2].Especially in China,which obese population has reached the world’s highest number,with 34.3%overweight and 16.4% for obesity in adults(≥ 18 years)[3].Obesity not only brings a heavy medical burden to society but also seriously affects people’s health.A meta-analysis about medical care costs of obesity found that in 2014,the US spent $1,901 on costs attributed to obese individuals and $149.4 billion at the national level[4].
The World Health Organization(WHO)defined obesity as a chronic metabolic disease characterized by abnormal or excessive accumulation of fat in the body[5].Excessive fat accumulation adversely increases the risk of developing multiple morbidities,such as cardiovascular disease,obstructive sleep apnea,type 2 diabetes mellitus(T2DM),psychiatric disorders,musculoskeletal disorders,liver disease,and even cancers[6-10].Obesity is also strongly associated with mortality[11].Surveys show that about 4 million deaths worldwide were caused by obesity or overweight in 2015[12].Abdominal obesity(AO)is the more dangerous type of obesity.When the body mass index(BMI)is only mildly elevated,but the waist circumference(WC)is larger,the prevalence of coronary heart disease and mortality will also increase[13].AO is more strongly correlated with most of the above-mentioned diseases,such as cardiovascular,cancer,T2DM,and obstructive sleep apnea,than general obesity[14-17].
Currently,the treatments for AO mainly include lifestyle changes(dietary interventions,exercise),medication,and bariatric surgery[18].Previous studies showed that lifestyle modification includes behavioral therapies,diet changes,and physical exercise,which requires at least 6 months or more to achieve bodyweight loss.Many patients lack high self-discipline and have difficulty adhering to it[19,20].Pharmaceutical drugs also have active effects but may rebound after stopping.Moreover,the side effects(gastrointestinal and menstrual disorders,possible liver damage)and instability limit its clinical application[21,22].Surgical intervention is also useful for obesity.A recent review reported that the most effective long-term treatments for severe obesity complicated by T2DM are bariatric procedures,but it is also associated with high costs,high surgical safety requirements,high risk of postoperative complications,and strict indications[23].Since obesity is a disease that requires long-term treatment,like hypertension,more and more people are seeking better alternative treatments.Acupuncture is favored because of its safety and effectiveness without toxic side effects.
Acupoint catgut embedding(ACE)as a subtype of acupuncture,which is a treatment method of infixing surgical chromic catgut sutures into corresponding acupoints to form a continuous acupoint stimulation,which has been proven to be used to treat many diseases,such as perimenopausal syndrome,chronic urticaria,depressive neurosis,refractory insomnia,obesity,etc.[24].It is generally considered to be more effective than ordinary acupuncture or electroacupuncture in the treatment of obesity[24,25].Recent research related that the beneficial effects of ACE in treating obesity,which is likely through improving leptin resistance in obese women,but the exact mechanism remains completely unclear[26].There are still clinical randomized controlled trials(RCTs)to further validate the efficacy and possible mechanisms of ACE for obesity[27].The relationship between intestinal flora and metabolic disorders has been a hot research topic in recent years.Studies have shown that obesity is related to changes in the intestinal flora and changes in serum metabolite levels corresponding to gut microbial patterns[28].Studies in acupuncture and herb have shown that the mechanism of action anti-obesity is related to the modulation of intestinal flora[29,30].Based on this,we conjecture that the mechanism of efficacy of ACE for obesity may be related to altered intestinal flora,but no study has yet confirmed this.The purpose of this study is to elucidate the intestinal flora mechanism of ACE for obesity.
Methods
Study design
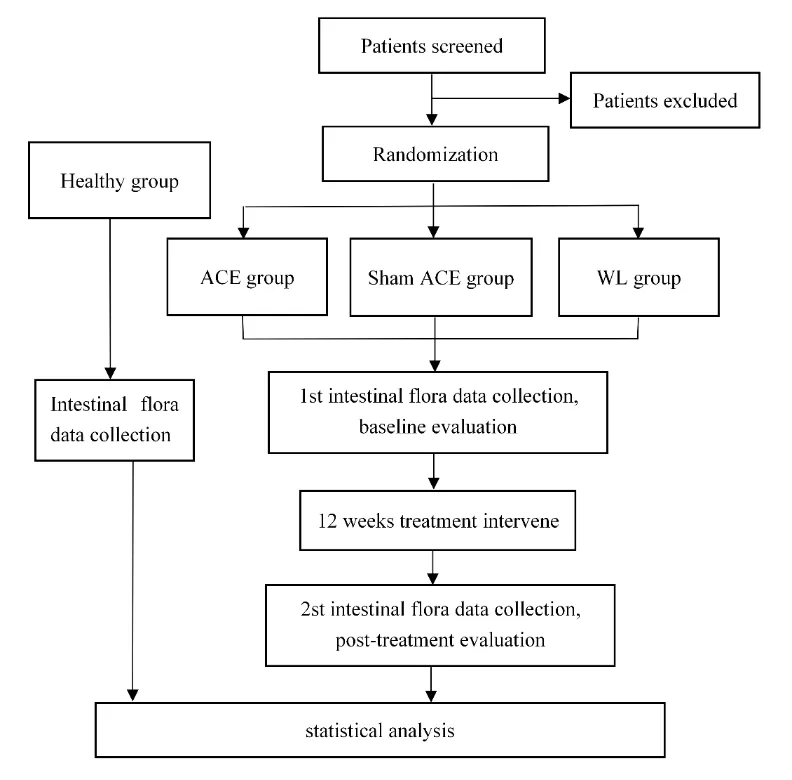
This randomized,single-blind,parallel-group study will be enrolled with 75 participants,of which 60 eligible female patients diagnosed with AO and 15 healthy female participants.60 female AO patients will be blinded to group assignment and randomized to either ACE group,sham ACE group,and waiting list(WL)group,with 20 patients in each group.Each patient in the ACE group and sham ACE group will receive one ACE treatment per week for 12 consecutive weeks,and patients in the WL group will not receive any treatment;only their data will be extracted.The primary outcome is the mean change in BMI over 12 weeks.Secondary outcomes include WC,body weight(BW),visual analog score(VAS)of appetite,and Gastrointestinal Symptom Rating Scale(GSRS).High-throughput 16S ribosomal ribonucleic acid(rRNA)gene sequencing will be used to detect intestinal flora in each group.All outcomes will be evaluated at baseline and the end of treatment.
Study setting
Collection time:the fecal samples should be collected within 1 week before treatment and within one week after 12 weeks of treatment,2 times in total.Fasting is required the night before the collection,and the collection should be done on an empty stomach in the early morning of the next day.Each collection tube will be labeled with the participants’ names,dates,and times of the sample will be collected.All samples will be returned to the laboratory as soon as possible and stored in a -80 °C refrigerator until analysis.It can also be stored in a household refrigerator at -4 °C,but that must be handed over to the researcher for subsequent analysis within 24 hours.
Patient recruitment
A public recruitment advertisement will be designed to recruit the patients online or offline.(e.g.WeChat public account,websites,outpatient clinics).Physicians will determine whether patients are eligible to participate in this study based strictly on inclusion and exclusion criteria.The included patients will sign an informed consent form before the trial.The schedule of patient enrolment,intervention,and assessment is illustrated in Table 1.
The North Wind woke her betimes next morning, and puffed19 himself up, and made himself so big and so strong that it was frightful20 to see him, and away they went, high up through the air, as if they would not stop until they had reached the very end of the world

Participant safety
The safety assessment of ACE will include two aspects.On the one hand,each patient’s blood pressure,heart rate,routine blood parameters,and hepatorenal function will be tested as a safety index before randomization and at the end of treatment.On the other hand,during the treatment,any adverse events such as pain,allergic reactions,local hematoma,infection,syncope,and other severe events will be managed immediately and documented in the case report forms(CRFs)carefully and reported to the study director and the ethics committee of The Sports Specialist Hospital of Yunnan Province.If the adverse event is severe and associated with the trial,the participant will be withdrawn from the study and given appropriate treatment.
Participants
AO patients
The WT group will not receive any intervention.The patients will be asked to receive relevant tests,fecal samples collecting,and scale evaluations at the same time as the other two groups and delayed ACE therapy for free after a waiting period of 12 weeks.
The exclusion criteria will be:(a)extremely obese patients(BMI ≥40 kg/m
);(b)Secondary obesity,such as obesity caused by endocrine disease(Cushing syndrome,thyroid disease,hypothalamic disease,pituitary disease,gonadal disease,etc.)and medication(glucocorticoid or antipsychotics);(c)pregnancy,lactation,or who have fertility requirements or are unable to provide adequate contraception within the next 6 months;(d)mental disorder,cardiovascular disease,liver and kidney impairment,immunodeficiency,diabetes,blood disease;(e)with a history of surgery for obesity.(f)with symptoms of the gastrointestinal system,such as active peptic ulcers/bleeding or previous recurrent ulcers/bleeding;(g)previously undergone major intestinal excision or major gastrointestinal surgery;(h)taken antibiotics for 3 days or more in the last 3 months;(i)with long-term dysmenorrhea;(j)tend to bleed,or who are allergic to alcohol or animal protein.
The dropout criteria will be:(a)do not meet the inclusion criteria but are mistakenly enrolled;(b)decide to withdraw from the study;(c)occurrence of severe adverse events(AEs)or complications that result in stopping the trial;(d)violate the protocol or refuses to give feedback on treatment information.
Healthy participants
The inclusion criteria will be:(a)BMI:18.5-23.9 kg/m
;(b)females aged between 18-40(including 18 and 40 years old);(c)have certain writing and reading ability to understand the entire process of research;(d)signed informed consent forms;(a)no other trials were taken within three months.
The exclusion criteria will be:(a)any one of the following criteria are excluded from this study;(b)mental disorder,cardiovascular disease,liver and kidney impairment,immunodeficiency,diabetes,blood disease;(c)with symptoms of the gastrointestinal system,such as active peptic ulcers/bleeding,or previous recurrent ulcers/bleeding;(d)previously undergone major intestinal excision or major gastrointestinal surgery;(e)take antibiotics for 3 days or more in the last 3 months;(d)with long-term dysmenorrhea.
I will tell you with pleasure, she answered, but where is the hurry? I want you to come back with me to the Green Castle, but I don t want to walk there, it is so far, and walking is so fatiguing99
Sample size
There is no consensus on the sample size for 16S rRNA gene sequencing.Based on similar studies and the research data analysis requirements of 16S rRNA gene sequencing,the sample size range of 15 to 20 cases shows enough statistical power for intestinal flora analysis[32,33].Finally,18 patients will be required for each group,allowing for a 10% withdrawal rate;60 participants will be recruited,with 20 participants in each intervention group.The healthy group will be considered for comparison only and will include the minimum criteria for the inclusion of 15 cases.
Randomization and allocation
All participants will be randomly assigned to three groups,including group A(ACE group),group B(sham ACE group),and group C(WL group)at a ratio of 1:1:1.Random numbers will be generated by computer and sealed in opaque envelopes by an independent research assistant.After participants accept the principle of random allocation,they will be randomly conducted to select an opaque envelope and obtain an allocation sequence number,which will be recorded in a CRF by the research assistant.The result of a participant’s allocation will be given to the operator in charge of ACE treatment.
Blinding
Considering the particularity of ACE manipulation,the operators cannot be blinded for the entire process.At the time of ACE operation,two groups of patients will be treated in separate rooms to avoid interaction.The acupuncturist and assistants will receive specialized training before participating in the study and will not disclose the allocation of the participants at any moment.Furthermore,to eliminate subjective bias,outcome assessors and statisticians are also blinded to group assignment.The allocation will only be revealed under some adverse events,such as severe allergy,serious infection,uncontrolled pain,etc.
Interventions
ACE will be operated by qualified TCM doctors with at least 3 years of clinical experience in acupuncture.
ACE group
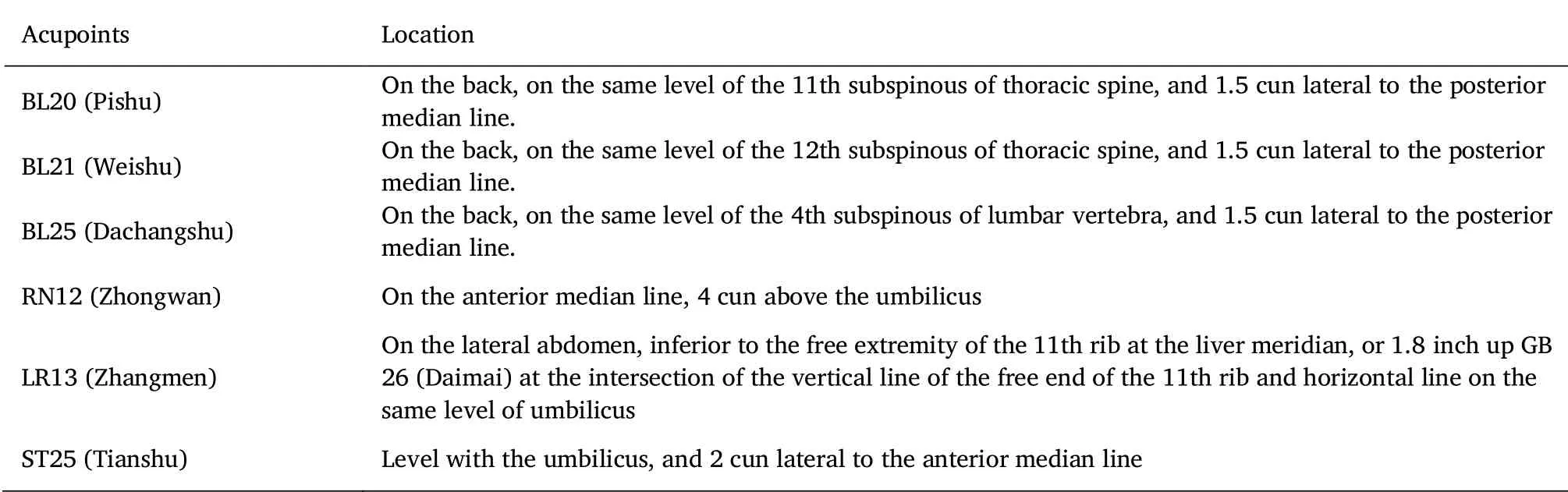
Acupoints selection:based on clinical experiences,previous research,and TCM theory of invigorating the spleen and resolving dampness,the back-shu points and front-mu points of the spleen,stomach,and large intestine will be selected as the prescription.It includes BL 20(Pi-shu),BL 21(Wei-shu),BL 25(Dachang-shu),RN 12(Zhong-wan),LR 13(Zhang-men),and ST 25(Tian-shu).All acupoints except Zhong-wan are selected bilaterally,for a total of 11 points.All acupoints will be positioned according to the national standard developed by the People's Republic of China in 2006(GB/T 12346-2006).The locations of the acupoints are shown in Figure 2 and Table 2.
One morning there was a young man bought some products from me. Firstly he bought 2 meters of 19mm diameter hosing and paid £3.50. After that he asked me some ideas about fish.
Analysis processing:QIIME 2(QIIME 2,RRID:SCR_008249,URL:http://qiime.org/)and Greengenes database(Release 13.8,http://greengenes.secondgenome.com/)(DeSantis et al.,2006)will be applied.The classify-sklearn algorithm(Bokulich et al.,2018)(https://github.com/QIIME2/q2-feature-classifier)of QIIME2 will be used for analysis.According to each feature sequence of ASVs or representative sequences of each OTU,the pre-trained Naive Bayes classifier in the QIIME2 software with default parameters will be used for species annotation,to identify the corresponding taxonomic and its abundance information.Alpha diversity analysis,including species in richness,diversity,and evenness,will be used to assess the diversity of the intestinal flora;The number of high abundances and rare ASV/OTU in the intestinal flora can be visualized by using the Rank abundance curve(https://en.wikipedia.org/wiki/Rank_abundance curve).In addition,principal component analysis,principal coordinate analysis,Multidimensional Nonmetric scaling,and other methods will be used to characterize the bacteria in each experimental group.Then,the interrelationships between groups of intestinal flora will be performed through network analysis and the functional profiles of intestinal floras will be predicted by the software PICRUSt2(Phylogenetic Investigation of Communities by Reconstruction of Unobserved States)(Gavin M.Douglas,et al.,preprint).
Operation:The operation of ACE will refer to the relevant provisions of the Operation Standard for Acupuncture,Part 10:Catgut Implantation at Acupoints(GB/T 21709.10-2008)[34].Firstly,Patients take a supine position and routinely disinfect the skin around the points.Secondly,the assistant will place an absorbable catgut suture of an appropriate length at the front end of the trocar before the stylet is connected and handed to the acupuncturist.Thirdly,the skin where the acupoint is located will be tensed or lifted by the acupuncturist with the thumb and index finger of the left hand,and the needle will be inserted into the acupoint with the appropriate force by the right hand to the required depth.When a needling sensation(Deqi)occurs,the stylet will be pushed while the tube is withdrawn,which will embed the absorbable catgut suture into the muscular layer or subcutaneous tissue of the acupoint.Finally,a dry cotton ball can be used to press the acupoint for a few moments after the needle is withdrawn to avoid bleeding,and the patient will be told to keep the skin at the acupoint dry for 3 days.
Sham ACE group
The prescription and procedure of the operation for the sham ACE group will be the same as the ACE group.The only difference between the two groups is that the sham ACE group will not be placed in the absorbable catgut suture.The difference between the ACE group and the Sham ACE group is shown in Figure 3.


WL group
The diagnostic criteria will be:owing to this study will be conducted in China,participants must meet the diagnostic criteria in the guideline of "the Prevention and Control of Overweight and Obesity in Chinese Adults(Trial)",proposed by the Chinese Working Group on Obesity in 2003,published by the Chinese Center for Disease Control(CDC).At the same time,in order to obtain more reliable results,according to the characteristics of AO,the diagnostic criteria include the following two points,① BMI ≥ 28 kg/m
;② female waistline ≥80 cm.The inclusion criteria for the studies will be:(a)meet the diagnostic criteria for abdominal obesity;(b)females,aged between 18-40(including 18 and 40 years old);(c)have certain writing and reading ability to understand the entire process of research;(d)signed informed consent forms;(c)no other trials were taken within three months.
AnneLisbeth walked on, thinking of nothing at all, as people say, orrather her thoughts wandered, but not away from her, for thought isnever absent from us, it only slumbers
Healthy group
No intervention and only one fecal sample collection,data acquisition,and analysis.
Intestinal flora data acquisition and analysis
Collection of fecal samples:a total of 135 fecal samples will be obtained from 60 AO patients(before and after treatment)and 15 healthy participants.They will be given instructions by oral,video,or written teaching about collecting fecal samples,including usage of fecal kit,weight and volume of fecal sample,storage,and transport.The main tests are the species and the abundance of the intestinal flora.
This study will be conducted at The Second Affiliated Hospital of Yunnan University of Chinese Medicine and The Sports Trauma Specialist Hospital of Yunnan Province.This trial is reported following the Standard Protocol Items:Recommendations for Interventional Trials(SPIRIT)guidelines[31].A flowchart of the research procedure is shown in Figure 1.
Precautions for collection:(a)participants will be instructed to maintain their former diet,eating habits,and physical activity as usual,but to avoid foods rich in probiotics and prebiotics,such as yogurt.(b)participants should not take any food containing probiotics and prebiotics,such as yogurt,for 7 days before sample collection,and should also avoid spicy,raw,cold,and other stimulating foods.(c)if participants suddenly develop constipation or diarrhea before sampling,or the patient is in a menstrual period,it is advisable to delay sampling until the patient returns to normal bowel movements.(d)if participants have taken antibiotics for more than 3 days before sampling,it should be postponed for 1 month according to the time of taking the drug.(e)try to avoid urine contamination when collecting feces.
Then turning to Beauty, he said: Take your father into the next room, and help him to choose everything you think your brothers and sisters would like to have
Appliance selection:the instruments that will be used in this trial include disposable embedding needles(9#,Jiangsu Huahong Medical Instruments Co.,Ltd.,Jiangsu,China),absorbable catgut sutures(Shanghai Pudong Jinhuan Medical Products Co.,Ltd.,Shanghai,China),and disposable embedding aids(Yangzhou City Dragon Tiger Medical Instrument Factory,Jiangsu,China).
Intestinal flora sequencing:intestinal flora will be quantified by Knorigene Technologies Co.,Ltd.The main steps from extraction to sequencing include the following:(a)the CATB method will be applied to extract intestinal floras’ DNA,and the DNA will be quantified using Nanodrop.(b)polymerase chain reaction(PCR)amplification of target fragments.The sequence of the variable region V3 to V4 of the 16S rRNA gene,which can reflect the composition and diversity of the intestinal flora,will be targeted,and the corresponding primers will be designed according to the conserved regions in the series.(c)the PCR products are purified and recovered by magnetic beads,and then they will be quantified by fluorescence(fluorescence reagent is Quant-iT PicoGreen dsDNA Assay Kit,quantification instrument is Microplate reader(BioTek,FLx800).(d)sequencing library preparation.Based on the characteristics of the amplified 16S,a small fragment library will be constructed,and the library will be sequenced by double-end sequencing(Paired_End)based on Illumina's TruSeq Nano DNA LT Library Prep kit sequencing platform,and the corresponding reagent is MiSeq Reagent kit V3(600 cycles).(e)the Illumina MiSeq platform(Illumina,San Diego,USA)will be used for high-throughput sequencing to obtain the base sequence information of the corresponding microbiomes.
Outcome measurement
Clinical data
The primary outcome is the mean change in BMI.The BMI will be used to assess patients’ obesity levels and the effectiveness of ACE to AO.The BMI is calculated as BMI = weight(kg)/(height(m))
.Weight will be measured after the overnight fast using an electronic balance scale.Before being weighed,the participants will be asked to wear lightweight clothing and take off their shoes.Height will be measured using a wall-mounted stadiometer.
Secondary outcomes include WC,VAS of appetite,and GSRS.The WC will be measured twice by a standard measuring tape at the midpoint between the lowest rib and the iliac crest.The average of the two measurements will be taken.The VAS of appetite of Canadian E Doucet scholars will be used,consisting of 4 components,including the intensity of desire to eat VAS,the intensity of hunger VAS,the intensity of satiety VAS,and the intensity of willingness to eat quantity VAS,all on a 10-point scale,with 0 no appetite,minimal intake,1-3 light appetite,a small amount of intake,4-6 moderate appetite,moderate intake,and 7-10 strong appetite,huge intake[35].Participants will be asked to mark their level of appetite on the VAS.Participants will not be allowed to access their previous VAS records on subsequent sessions.The scale of the VAS of appetite is shown in Table 3.The GSRS is a widely used questionnaire for patients'gastrointestinal symptoms in the past 1 week,which is a 15-item specific scale covering 5 GI symptoms:reflux,abdominal pain,dyspepsia,diarrhea,and constipation.The GSRS is rated on a 7-point Likert scale from "asymptomatic" to "very severe";The higher the score,the more severe the symptoms[36,37].

Other safety indicators,including basal metabolic rate,blood pressure,heart rate,total cholesterol,triglyceride,low-density lipoprotein,and high-density lipoprotein,will be tested before and after treatment.
And then they arrived --- the minister s family and all my relatives in a clamor of doorbells and rumpled8 Christmas packages. Robert grunted9 hello, and I pretended he was not worthy10 of existence.
Intestinal flora data
The intestinal flora will be detected by high-throughput 16S rRNA gene sequencing,which is considered an intuitive and reliable assay to study the composition and dynamics of the gut microbial community at present[38].Through the relevant results of intestinal flora,the number and species of intestinal flora in patients with AO before and after the intervention will be obtained,and the results will be analyzed and compared.
All participants will be tested in the School of Acupuncture and Massage-Rehabilitation/The Second Affiliated Hospital of Yunnan University of Chinese Medicine.
Statistical analysis
Before data analysis,the research group will draw up a statistical plan,including the required data and method of data processing.
Intestinal flora data analysis
The differences in flora between the healthy group and the other three groups before the intervention will be compared and analyzed in terms of both flora type and diversity.
In times past, offering an apple was a symbol of love and affection (Philip 1997). The apple was sacred to Aphrodite and represented knowledge, especially sexual knowledge, fertility and love.Return to place in story.
Bettelheim also observes: Since all the treasure and jewels given the princess by her mother are of no help to her, this suggests that what a parent can give his child by way of earthly goods is of little aid if the child does not know how to use it well (Bettelheim 1975, 139).Return to place in story.
The differences in flora between the ACE group,sham ACE group,and WL group before and after the respective interventions and between groups will be compared in terms of both flora species and diversity.
“It does not matter,” answered the crow; “I will explain as well as I can, although it will be very badly done;” and he told her what he had heard. “In this kingdom where we now are,” said he, “there lives a princess, who is so wonderfully clever that she has read all the newspapers in the world, and forgotten them too, although she is so clever. A short time ago, as she was sitting on her throne, which people say is not such an agreeable seat as is often supposed, she began to sing a song which commences in these words:
Differences in intestinal flora between the three intervention groups will be correlated with clinical outcomes.
Clinical data analysis
The demographics,baseline characteristics,and efficacy of the participants will be analyzed with different methods by SPSS 22.0 statistical software(SPSS Inc.,Chicago,IL,USA).Qualitative data will be described as percentages or proportions and will be compared using chi-square(χ
)tests.Quantitative data will be expressed as mean ± standard deviation.For continuous variables,if the data are normally distributed,a one-way analysis of variance(ANOVA)will berepeated measures data,repeated measures ANOVA will be used to determine differences in the same group at 2-time points(baseline and the end of 12 weeks of intervention).Two-sided tests will be used during the analysis,and
-value < 0.05 will be considered as the threshold of statistical significance.
Quality control
To ensure the objectivity of the study results,the study will be conducted in strict accordance with the basic requirements of a clinical randomized controlled trial,randomized grouping and enrollment,strict control of participant recruitment criteria,and evaluate the trial results by evaluators who are unaware of the characteristics of the groups,and perform a blind statistical analysis by a third party.All researchers,data collectors,and statisticians are involved in the study should strictly abide by the rules of the study.
To ensure a smooth study,specialized clinical training will be provided to all the clinical researchers who will receive specialized training before the initiation of the trial to familiarize each clinical researcher with the study’s process and implementation.
Among them,the operator of ACE should have at least 3 years of clinical work experience and be able to cope with any possible AEs during the treatment;The data collectors are responsible for keeping and managing various data and performing rigorous proofreading of the data.Moreover,keeping detailed records of participants’withdrawals and AEs during the study period and those who have already passed 1/2 course of treatment should enter the efficacy statistics;The statisticians will be fully responsible for data management and statistical analysis.Regular team meetings will also be held and fully documented during the conduct of the study.
What, said he, should be done to a certain person who has deceived everyone? and he proceeded to relate the whole story, ending up with, Now what sentence should be passed?
Data management and confidentiality
The researchers will record participants’ information on the CRFs and verify that data are collected timely,accurately,and fully.Personal information such as the patient's name,phone number,ID number,and medical records will be kept anonymously to prevent information leakage.All participants’ paper data will be kept by the researchers in a special cabinet and preserved for at least 5 years after publication.
In addition,the ethics committee of the Sports Specialist Hospital of Yunnan Province will periodically review the progress of the trial and monitor the collection,allocation,and concealment of data.The modification or termination of the trial can be implemented by the committee.The data monitoring committee is independent of the sponsor and has no conflict of interest.
Discussion
Obesity,as an epidemic public health issue in the world today,not only brings a heavy medical burden to society but also seriously affects people’s quality of life.In recent years,with the development of complementary medicine,acupuncture has been widely applied as an alternative therapy in the clinic,among which,ACE is more popular among obese patients because of its economical,time-saving,less frequent needling,and long-lasting efficacy.In the context of new evidence for the efficacy of ACE interventions in obesity,it is necessary to elucidate the mechanism of ACE in the treatment of obesity.The results of this trial are expected to provide further theoretical support for ACE in the clinical treatment of obesity.
At first the King and all the kingdom were terrified. All except the Princess, that is. She trusted her littlest knight and upon hearing the whole story set about immediately to make a healing salve for the dragon s eyes.
There is growing evidence that changes in the intestinal flora are associated with metabolism-related diseases such as obesity,and the intestinal flora consists of many bacteria that contribute to nutrient and energy regulation and they are closely related to the energy metabolism of the host[39,40].It was found that there are significant changes in the intestinal micro-ecosystem in obese people compared to normal people.Compared with the intestinal flora of lean people,the level of thick-walled bacteria in the intestinal tract of obese people is increased and accompanied by a decrease in the number of mimics[41].The study has shown that obesity can be effectively suppressed by intervening in the intestinal flora related to obesity[42].It was also found that acupuncture in the treatment of simple obesity can reduce BMI by increasing the number of intestinal Lactobacillus and Bifidobacterium and decreasing the number of Bacteroides and Clostridium perfringens[29].However,the current research on the mechanism of ACE to regulate intestinal flora to treat obesity is still limited.
Tsarevitch Ivan seated himself on the back of the Wolf joyfully26 enough. Take me, Gray Wolf, he said, to the Fire Bird that stole my father s golden apples, and instantly the Wolf sped away, twenty times swifter than the swiftest horse. In the middle of the night he stopped at a stone wall.
According to the theory of TCM,obesity is closely related to the digestive function of the spleen,stomach,and intestines.In the theory of acupuncture,the back points and the collection points are the gathering points of the qi of the internal organs on the back,chest,and abdomen,respectively.The back-shu points and front-mu points are the points where the qi of the internal organs converges in the back and chest and abdomen respectively.The two are often used together to bring out their synergistic effect in the clinic,which is also one of the classic combinations of acupoints in Chinese medicine,known as Shu-Mu points.An experimental study showed that electrical stimulation of the back-shu points "Dachangshu"(BL 25)and front-mu points "Tianshu"(ST 25)of the large intestine could improve diarrhea in IBS model rats by regulating the expressions of colon c-kit and TRPV1[43].Therefore,the back-shu points and front-mu points of the spleen,stomach,and large intestine are chosen as the prescription for ACE.
There are clinical trials with non-meridian non-acupuncture points as a control group in the clinic[44].Due to the special nature of ACE,the acupoints prescription for the control group in this trial will be the same as the observation group,with the difference that the absorbable catgut suture will not leave in the body after the needle is withdrawn.At the same time,considering that the sham ACE may produce clinical effectiveness,including some stimulation of acupoints and unavoidable psychological factors,we added the WL group to exclude this confounding factor.The healthy group was considered in the trial design aims to observe the differences in intestinal flora between AO patients and the healthy population,and thus to identify the key intestinal flora causing AO in patients.15 cases will be included because of financial considerations.
In addition,since the prevalence of obesity among women is higher than that of men,women are recruited as the participants of this experiment,and abdominal obesity is relatively more significant in women[2,45,46].What’s more,in clinical practice,the majority of patients on diet pills are women,and diet pills can have serious effects on female fertility in addition to the conventional side effects such as nausea,headache,and constipation[47].
“Oh, how I have been detained!” said the little maiden, “I wanted to seek for little Kay. Do you know where he is?” she asked the roses; “do you think he is dead?”
In this trial,in addition to BMI as the classical clinical evaluation index,GSRS,which is currently more widely used,will be used to assess changes in the gastrointestinal tract,as well as several aspects such as WC,body weight,appetite changes,and blood lipids to assess the clinical efficacy.More importantly,the main purpose of the study is to observe the changes in the intestinal flora,and 16S rRNA gene sequencing,which is currently more widely used,will be used to detect changes in the intestinal flora composition before and after treatment.We do not restrict the diet of the participants during the treatment period,they can maintain their diet,eating habits,and physical activity as usual during the study except for some that could interfere with the analysis of intestinal flora,such as taking probiotic foods or using antibiotics,which ensure the participant’s diet components,and levels of physical activity did not differ compared to before the treatment and also exclude the effects of dietary interventions to facilitate a more objective evaluation of the trial results.
One limitation of this study is the relatively small sample size,partly due to the relatively high cost of intestinal flora testing,but even so,our analysis of the gut flora results will not be affected.Also,our study will only observe changes before and after treatment and no follow-up will be done,also in consideration of the study funding.Secondly,the treatment period of this trial is relatively long,and the sham buried group may have some risk in blinding control because the absorbable catgut suture is not left in the patients,which requires good communication between the operator and the participants.
Conclusions
This study intends to focus on the idea of "ACE treats obesity by regulating intestinal flora",the more harmful and more prevalent female AO will be selected as the study object,the Shu-Mu ACE will be used as an intervention and high-throughput 16S rRNA gene sequencing analysis technology will be applied to identify the key intestinal flora causing abdominal obesity and the target intestinal flora of AO regulated by ACE in terms of intestinal flora species,diversity and functional gene prediction.We anticipate that the results will further support the clinical application of ACE for AO.
1.Endalifer ML,Diress G.Epidemiology,predisposing factors,biomarkers,and prevention mechanism of obesity:a systematic review.
.2020:6134362.
2.Kelly T,Yang W,Chen CS,Reynolds K,He J.Global burden of obesity in 2005 and projections to 2030.
.2008;32(9):1431-1437.
3.Pan XF,Wang LM,Pan A.Epidemiology and determinants of obesity in China.
.2021;9(6):373-392.
4.Kim DD,Basu A.Estimating the medical care costs of obesity in the United States:systematic review,meta-analysis,and empirical analysis.
.2016;19(5):602-613.
5.World Health Organization.Obesity and overweight.Fact sheet 2021.www.who.int/mediacentre/factsheets/fs311/en/.Accessed 9 June 2021.
6.Bogers RP,Bemelmans WJ,Hoogenveen RT,et al.Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels:a meta-analysis of 21 cohort studies including more than 300 000 persons.
.2007;167(16):1720-1728.
7.Jehan S,Myers AK,Zizi F,Pandi-Perumal SR,Jean-Louis G,McFarlane SI.Obesity,obstructive sleep apnea and type 2 diabetes mellitus:epidemiology and pathophysiologic insights.
.2018;2(3):52-58.
8.Rajan TM,Menon V.Psychiatric disorders and obesity:a review of association studies.
.2017;63(3):182-190.
9.Tallis J,James RS,Seebacher F.The effects of obesity on skeletal muscle contractile function.
.
2018;221(Pt 13):jeb163840.
10.Lauby-Secretan B,Scoccianti C,Loomis D,et al.Body fatness and cancer--viewpoint of the IARC working group.
.2016;375(8):794-798.
11.Flegal KM,Carroll MD,Kit BK,Ogden CL.Prevalence of obesity and trends in the distribution of body mass index among US adults,1999-2010.
.2012;307(5):491-497.
12.Afshin A,Forouzanfar MH,Reitsma MB,et al.Health effects of overweight and obesity in 195 countries over 25 years.
.2017;377(1):13-27.
13.Flegal KM,Graubard BI,Williamson DF,Gail MH.Cause-specific excess deaths associated with underweight,overweight,and obesity.
.2007;298(17):2028-2037.
14.Ritchie SA,Connell JM.The link between abdominal obesity,metabolic syndrome and cardiovascular disease.
.2007;17(4):319-326.
15.Zhang C,Rexrode KM,van Dam RM,Li TY,Hu FB.Abdominal obesity and the risk of all-cause,cardiovascular,and cancer mortality:sixteen years of follow-up in US women.
.2008;117(13):1658-1667.
16.Freemantle N,Holmes J,Hockey A,Kumar S.How strong is the association between abdominal obesity and the incidence of type 2 diabetes?
.
2008;62(9):1391-1396.
17.Zhao XL,Xu HJ,Qian YJ,et al.Abdominal obesity is more strongly correlated with obstructive sleep apnea than general obesity in China:results from two separated observational and longitudinal studies.
.2019;29(8):2535-2547.
18.Jensen MD,Ryan DH,Apovian CM,et al.2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults:a report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society.
.2014;63(25 PtB):2985-3023.
19.Dombrowski SU,Avenell A,Sniehott FF.Behavioural interventions for obese adults with additional risk factors for morbidity:systematic review of effects on behaviour,weight and disease risk factors.
.2010;3(6):377-396.
20.Kesztyus D,Erhardt J,Schonsteiner D,Kesztyüs T.Therapeutic treatment for abdominal obesity in adults.
.2018;115(29-30):487-493.
21.Yanovski SZ,Yanovski JA.Long-term drug treatment for obesity:a systematic and clinical review.
.2014;311(1):74-86.
22.Halpern B,Halpern A.Safety assessment of FDA-approved(orlistat and lorcaserin)anti-obesity medications.
.
2015;14(2):305-315.
23.Arterburn DE,Telem DA,Kushner RF,Courcoulas AP.Benefits and risks of bariatric surgery in adults:a review.
.2020;324(9):879-887.
24.Huang CY,Choong MY,Li TS.Treatment of obesity by catgut embedding:an evidence-based systematic analysis.
.2012;30(3):233-234.
25.Guo TP,Ren YL,Kou J,Shi J,Sun TX,Liang FR.Acupoint catgut embedding for obesity:systematic review and meta-analysis.
.2015;2015:401914.
26.Chen IJ,Yeh YH,Hsu CH.Therapeutic effect of acupoint catgut embedding in abdominally obese women:a randomized,double-blind,placebo-controlled study.
.2018;27(6):782-790.
27.Zhou YM,Yan B,Yuan WQ,Yu HB,Yang ZX.Efficacy of verum and sham acupoint catgut embedding for treatment of obesity:study protocol for a randomized controlled trial.
.2019;20(1):644.
28.Thingholm LB,Rühlemann MC,Koch M,et al.Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition.
.2019;26(2):252-264.e10.
29.Xu ZT,Li RF,Zhu CL,Li MY.Effect of acupuncture treatment for weight loss on gut flora in patients with simple obesity.
.2013;31(1):116-117.
30.Kim BS,Song MY,Kim HJ.The anti-obesity effect of Ephedra sinica through modulation of gut microbiota in obese Korean women.
.2014;152(3):532-539.
31.Chan AW,Tetzlaff JM,Gøtzsche PC,et al.SPIRIT 2013 explanation and elaboration:guidance for protocols of clinical trials.
.2013;346:e7586.
32.Wang WT,Li YB,Wu QJ,Pan X,He XH,Ma XX.High-throughput sequencing study of the effect of transabdominal hysterectomy on intestinal flora in patients with uterine fibroids.
2020;20(1):98.
33.Liu J,Liu X,Xiong XQ,et al.Effect of vitamin A supplementation on gut microbiota in children with autism spectrum disorders-a pilot study.
.2017;17(1):204.
34.PtoATOSPAC E.National standard of the people's republic of China(GB/t 21709.10-2008)acupuncture technical operation specification part 10:acupoint catgut embedding.
2009;29(5):405-406.
35.Doucet E,Imbeault P,St-Pierre S,et al.Appetite after weight loss by energy restriction and a low-fat diet-exercise follow-up.
.2000;24(7):906-914.
36.Svedlund J,Sjödin I,Dotevall G.GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease.
.1988;33(2):129-134.
37.Revicki DA,Wood M,Wiklund I,Crawley J.Reliability and validity of the gastrointestinal symptom rating scale in patients with gastroesophageal reflux disease.
.1998;7(1):75-83.
38.Knight R,Vrbanac A,Taylor BC,et al.Best practices for analysing microbiomes.
.2018;16(7):410-422.
39.He XY,Zheng NN,He JJ,et al.Gut microbiota modulation attenuated the hypolipidemic effect of simvastatin in high-fat/cholesterol-diet fed mice.
.2017;16(5):1900-1910.
40.Turnbaugh PJ,Ley RE,Mahowald MA,Magrini V,Mardis ER,Gordon JI.An obesity-associated gut microbiome with increased capacity for energy harvest.
.2006;444(7122):1027-1031.
41.Ley RE,Turnbaugh PJ,Klein S,Gordon JI.Microbial ecology:human gut microbes associated with obesity.
.2006;444(7122):1022-1023.
42.Liang C,Guo M,Liu T,et al.Profiles of gut microbiota in children with obesity from Harbin,China and screening of strains with anti-obesity ability in vitro and in vivo.
.2020;129(3):728-737.
43.Li KG,Guo MW,Tan LH,et al.Comparison of effects of electroacupuncture at "Dachangshu"(BL 25)or "Tianshu"(ST 25)on visceral sensitivity,c-kit and TRPV1 of irritable bowel syndrome rats.
.2018;38(6):625-629.
44.Du YZ,Zhang LL,Liu W,et al.Effect of acupuncture treatment on post-stroke cognitive impairment:a randomized controlled trial.
.2020;99(51):e23803.
45.Ford ES,Giles WH,Dietz WH.Prevalence of the metabolic syndrome among US adults:findings from the third National Health and Nutrition Examination Survey.
.2002;287(3):356-359.
46.Mozumdar A,Liguori G.Persistent increase of prevalence of metabolic syndrome among U.S.adults:NHANES III to NHANES 1999-2006.
.2011;34(1):216-219.
47.Shyh G,Cheng-Lai A.New antiobesity agents:lorcaserin(Belviq)and phentermine/topiramate ER(Qsymia).
.2014;22(1):43-50.
