The serum and breath Raman fingerprinting methodfor early lung cancer and breast cancer screening
2018-04-23
(School of Physics, Dalian University of Technology, Dalian Liaoning 116024, China)
IntroductionMany countries have proposed the "early discovery, early diagnosis, and early treatment" strategy to overcome the cancers, however there is currently no satisfactory consensus on how to achieve this. The main reason is because there is no established "early screening" strategy occurring before the "early discovery, early diagnosis, and early treatment" strategy. A precondition for early treatment is early diagnosis, and a precondition for early diagnosis is early detection. Thus, the foundation of early detection needs to have methods for early cancer screening. In the past, an insufficient focus on and investment in research and development for early cancer screening has prevented much progress, relegating it to just a vain slogan. Many cancer patients, especially lung cancer patients, are already in the advanced stages of cancer at diagnosis, and they miss the window for successful treatment or prolonging survival time. The aim of this paper is to report the progress of our serum-Raman fingerprinting method to screen for lung cancer and breast cancer, to explore the feasibility of our breath-Raman fingerprinting method to screen for lung cancer, and to propose that a combined breath- and serum-Raman fingerprinting method to screen for lung cancer can increase the specificity of detection of early lung cancer.
1 Pre-research results of serum raman fingerprinting screening for early-breast disease
1.1 Professor Wang Yongcai, a professor of shedding cytopathology, provided the pathological diagnosis data and serum samples of early stage breast disease patients to the Dalian University of Technology. We analyzed the SERSp data in serum samples from patients with breast disease (58 cases) and healthy controls (35 cases) on our assembled inverted microscope + Raman spectrometer (Renisaw) using silver nanoparticles to enhance the Raman signal. Of the 58 cases of breast disease, 14 were cases of breast cancer, 18 were cases of benign tumors, 21 were cases of hyperplasia (including 5 cases of atypical hyperplasia), and 5 were cases of mastitis. The majority of the serum samples were rare samples of early stage breast disease. The average SERSp with the internal standard of 725 cm-1Raman-peak is shown in Figure 1.
The serum analysis results using hierarchical cluster analysis (HCA) for the 58 cases of breast disease patients and the 35 cases of healthy controls are shown in Figure 2.
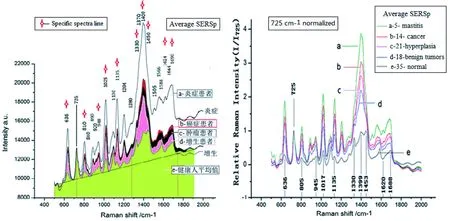
Figure 1 The average SERSp of serum of the 5 breast disease groups and the control group
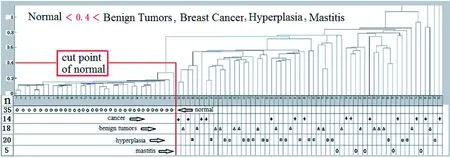
Figure 2 The HCA results of 58 patients with breast disease and 35 healthy persons[1]
The true positive rate of distinguishing between breast disease patients and healthy volunteers is 100%, with an error rate of 0. The accuracy rate as distinguished from inflammation is as follows: hyperplasia 81%, benign tumor 61%, and breast cancer 50%. It is difficult to distinguish between inflammation, cancer, benign tumors, and hyperplasia using HCA because the individual data are too dispersed. However, it can be observed from the average SERSp in Figure 1 that specificity between inflammation, cancer, benign tumors, and hyperplasia exists. Their average SERSp can be distinguished from each other and there are some regularities that can be further differentiated with further investigation. Of the 58 cases of breast disease, 5 cases are precancerous-atypical hyperplasia serum samples. We combined these SERSp data with 6 cases of preoperative ductal carcinoma patient data and 29 cases of healthy control data. The principal component analysis (PCA) results are shown in Figure 3.
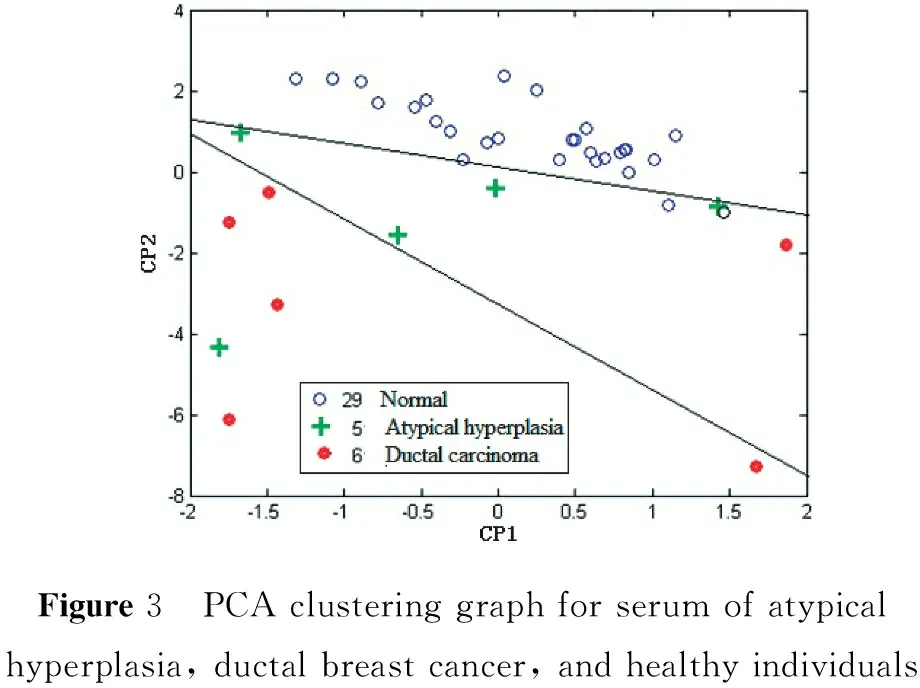
Figure3 PCAclusteringgraphforserumofatypicalhyperplasia,ductalbreastcancer,andhealthyindividuals
As seen in Figure 3, it is possible to distinguish between the PCA clusters of the three groups: atypical hyperplasia, ductal carcinoma, and healthy controls. There are 4 cases of atypical hyperplasia in the transition zone between the ductal carcinoma grouping and the healthy control grouping; the fifth case is severe atypical hyperplasia (clinically may be attributable to cancer). These cases, though few, confirm that the sensitivity and specificity of the serum-Raman fingerprint screening method can not only identify early ductal carcinoma cases but also detect and screen precancerous dysplasia cases in the breast. We found that the Raman fingerprint screening method has a very low detection capability limit, which went beyond our expectations.
1.2 A comparison of the results of the Raman breast disease screening method and the immune CA153-chemiluminescence screening method The results of the serum-Raman fingerprint screening of the 58 cases of patients with breast disease and the 35 healthy controls were compared with the results published, which reported results obtained using the conventional CA153 immune chemiluminescence method to screen serum of 59 cases of breast cancer and 35 healthy controls. Table 1 shows the comparison, and demonstrates that the sensitivity and specificity of the serum-Raman fingerprinting screening method for breast disease and breast cancer is much higher than the routine serum CA153 immune chemiluminescence screening method.
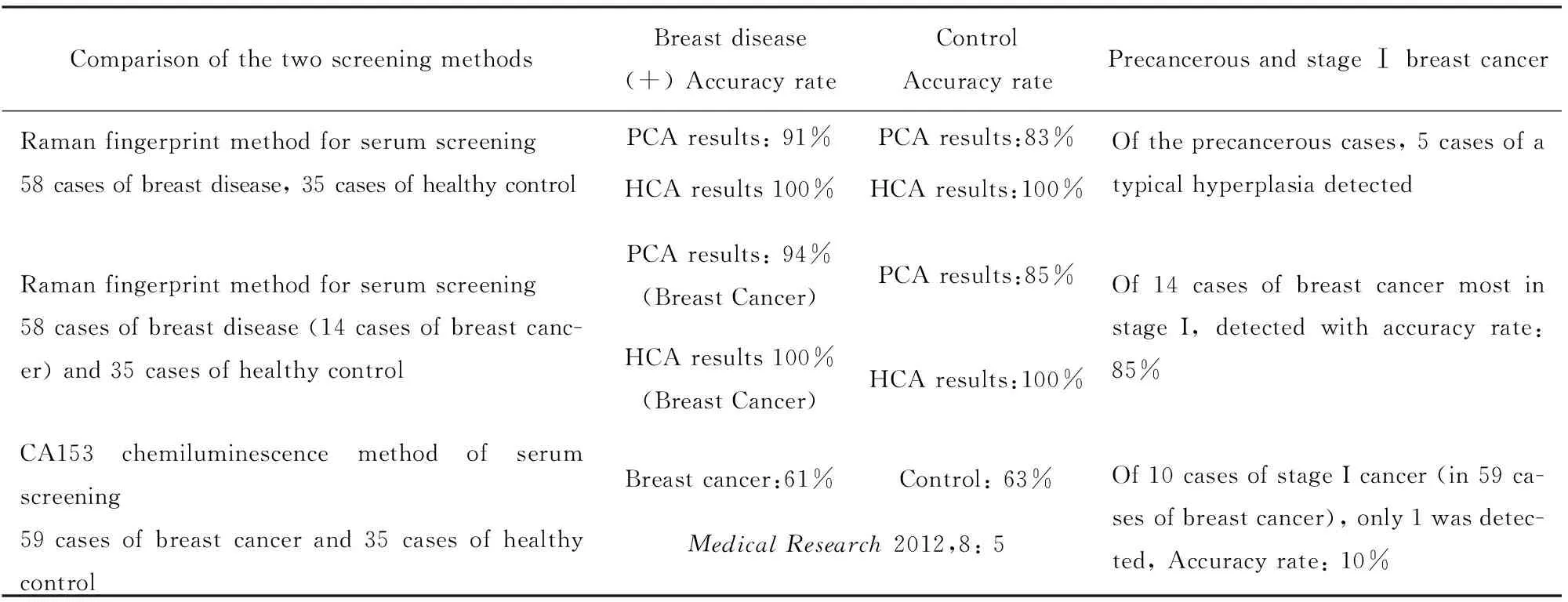
Table 1 Comparison of the Raman fingerprint and conventional CA153 screening methods
2 Results of the serum Raman fingerprinting method for lung cancer screening
2.1 The average SERSp and PCA analysis results for lung cancer screening The Dalian University of Technology cooperated with Jing Tong, a director of blood chemistry, to complete a serum Raman fingerprinting screening study[1]with 43 lung cancer patients and 41 healthy volunteers. Eight specific Raman lines are selected and shown in the mean SERSp graph (with an internal standard of 725 cm-1) in Figure 4. PCA is shown in Figure 5. The lung cancer screening accuracy rate is more than 95% with a false detection rate of 5% (two cases of false negatives), and the accuracy rate of screening healthy persons is 100% (41 total cases).
2.2 Comparison of the Raman screening method to the immune luminescence method using the same number of patients with lung cancer To date, there are no effective early screening methods for lung cancer except for the difficult, regular low-dose spiral chest CT monitoring procedure combined with biopsy[2-3]. In our serum screening study using Raman fingerprinting, 40 out of the 43 total lung cancer serum samples had undergone multiple routine immunization luminescence detections. Table 2 shows the results of the comparison of the Raman fingerprint screening method and the routine immune luminescence method in the same serum samples. PCA of Raman fingerprint method screening for lung cancer demonstrates a true positive rate of 95% and a false negative rate of 5%, while the conventional immune luminescence method for detection of lung cancer shows a true positive rate of 48~68% with a false negative rate of 32%~52%. These results indicate that the Raman fingerprint method for lung cancer serum screening has achieved the lung cancer screening requirements, while the conventional immune luminescence method for lung cancer screening has met the requirements with only one sample. Even though the immune luminescence method has been the routine clinical detection method, the CEA, CYFRA21, and NSE true positive accuracy rates and false negative rates for lung cancer screening do not meet the clinical requirements. In addition, we found that dynamic changes in both adenocarcinoma CEA levels and NSE levels in small cell carcinoma occur in an individual over time. This reflects treatment effects, lung cancer relapse monitoring, and judgment of prognosis, which indicates a more important reference value for clinical diagnosis.
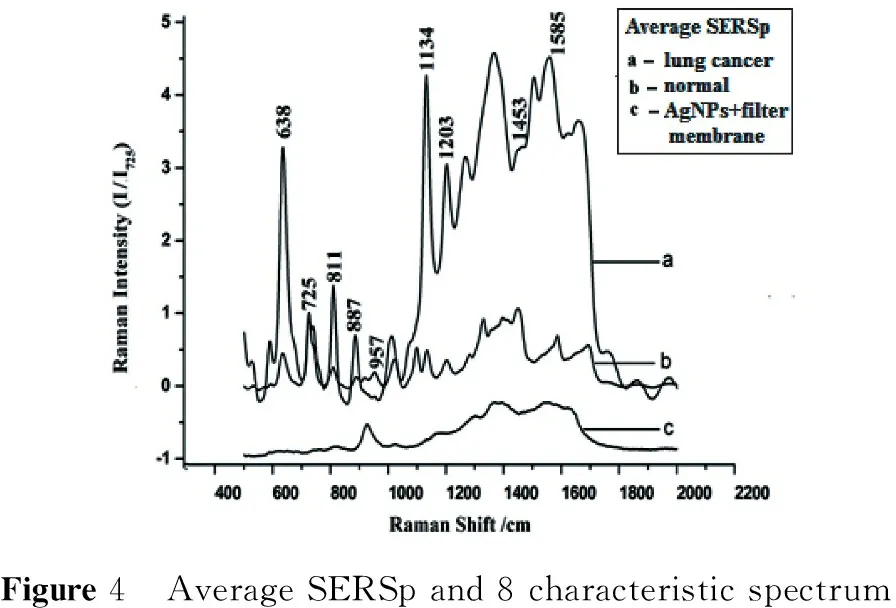
Figure4 AverageSERSpand8characteristicspectrum
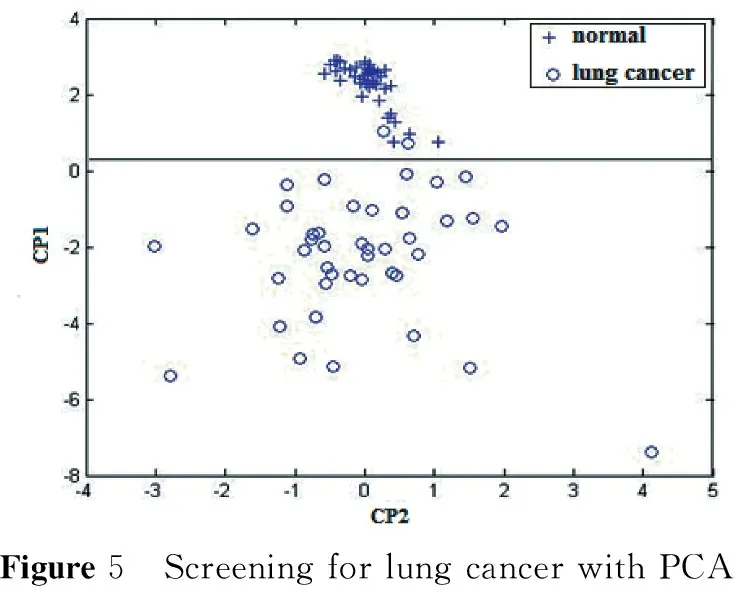
Figure5 ScreeningforlungcancerwithPCA
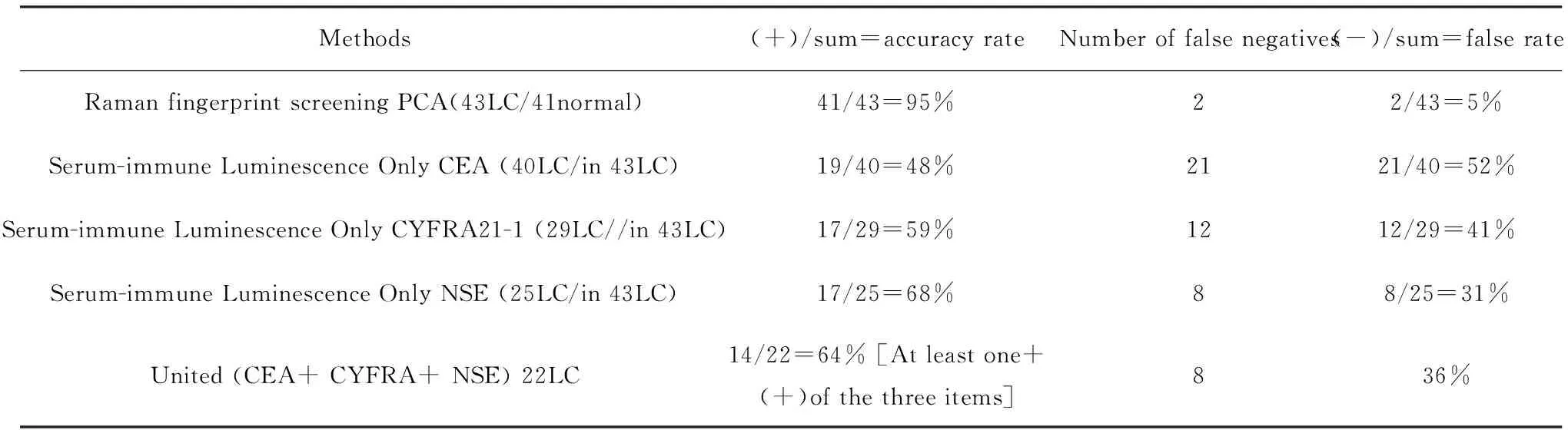
Table 2 Comparison of the Raman fingerprint method with the immune luminescence method using same serum samles screening
3 Using serum raman fingerprinting as a unified treatment of screening for lung cancer and early breast disease[4]
3.1 PCA of combined breast disease and lung cancer screening With the same 10 specific spectra lines (636,805,945,1 017,1 135,1 330,1 399,1 453,1 620,and 1 688 cm-1), and using the previous serum SERSp data from 58 cases of breast disease, 43 cases of lung cancer, and 35 healthy controls, we performed PCA (Figure 6) and determined the statistical likelihood ratio (equal to the true positive detection accuracy rate / the false negative rate) (Table 3).
3.2 Unified HCA of breast disease, lung cancer, and healthy control samples Using the same serum sample SERPs data and 10 specific spectral line data as in the unified PCA method above, a HCA was also preformed (Figure 7 and Table 4).
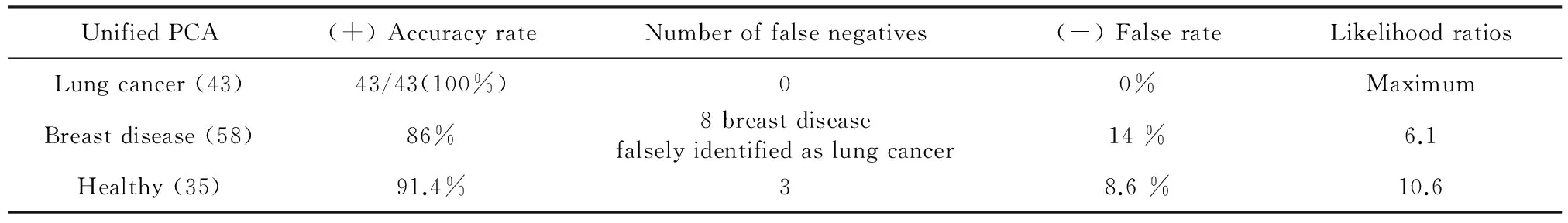
Table 3 Likelihood ratios of unified PCA for the lung cancer, breast disease, and healthy groups
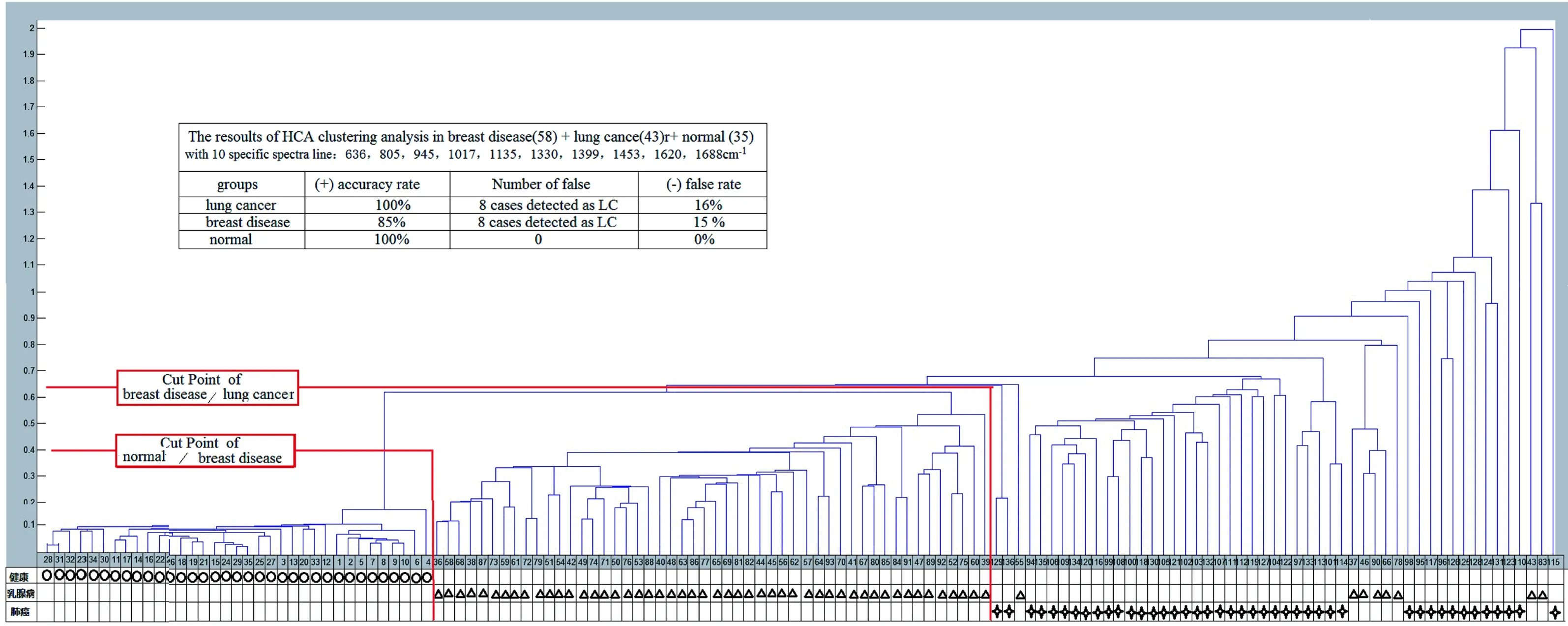
Figure 7 The unified HCA results for breast disease, lung cancer, and normal groups
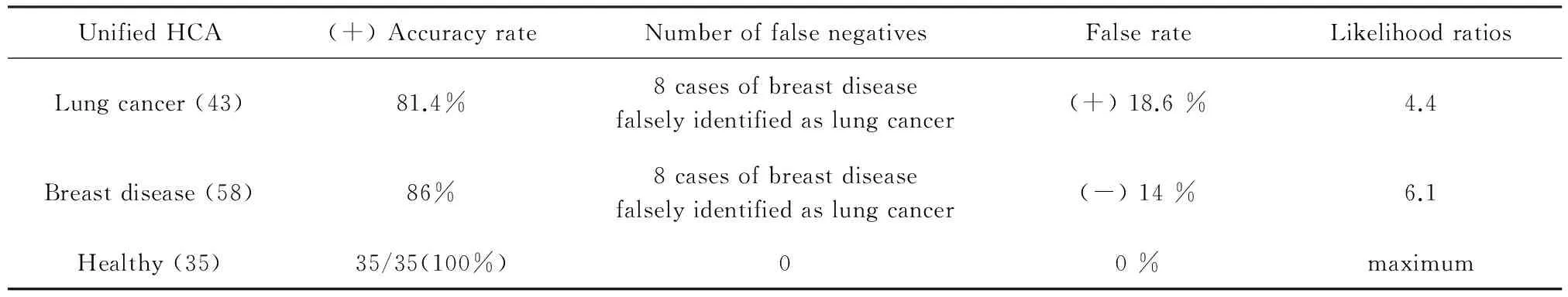
UnifiedHCA(+)AccuracyrateNumberoffalsenegativesFalserateLikelihoodratiosLungcancer(43)81.4%8casesofbreastdiseasefalselyidentifiedaslungcancer(+)18.6%4.4Breastdisease(58)86%8casesofbreastdiseasefalselyidentifiedaslungcancer(-)14%6.1Healthy(35)35/35(100%)00%maximum
3.3 Comparison of the likelihood ratios of the PCA and HCA and with the CA153 serum screening method for breast cancer The likelihood ratios of PCA and HCA for serum samples of breast disease, lung cancer and healthy controls are shown in Table 5. The difference in likelihood ratios between the two methods suggests that one may prefer PCA for lung cancer screening and HCA for healthy control screening, with the exclusion of lung cancer. The likelihood ratios for PCA and HCA are similar for breast disease.
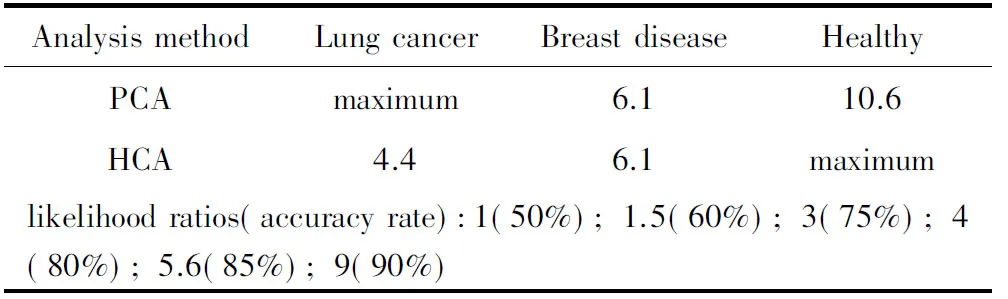
Table 5 Comparison of the likelihood ratios of PCA and HCA of Raman-screening for lung cancer and breast disease
4 Proposal for developing Raman fingerprinting screening methods using exhaled breath samples for early stage screening of lung cancer and other cancers as a complement to the serum-Raman fingerprint serum screening methods
4.1 In the metabolism of cancer tissues and cells, there are some Non-volatile small molecule biological markers released into circulation and some gas-phase small molecule biological markers of volatile organic compounds (VOCs) present in exhaled breath In the process of cancer cell metabolism, a number of small molecule cancer biomarkers are produced, with some that are easily dissolved and some that are difficult to dissolve in blood. The insoluble metabolic small molecular VOC biomarkers that come from lung or other cancer cells of the respiratory system or the digestive system do not dissolve in the circulating blood, but will instead immediately and directly interfuse into the exhaled breath out of patient's body. The insoluble metabolic small molecular VOC biomarkers that come from cancer cells in the other parts of patient's body will circulate through the blood to the lungs and interfuse with exhaled breath out of the patient's body. Therefore, to further develop and popularize early lung cancer Raman fingerprinting screening methods, we must not only use serum samples but also breath VOC samples, namely "united serum- and breath-Raman fingerprinting screening methods."
4.2 Progress of electronic nose research and SPME-GCMS have been shown to be very effective with breath-VOC sampling for early lung cancer screening Small molecular VOC cancer biomarkers in exhaled breath from a patient with lung cancer are collected using an airbag of standard design, and then detected using a combined system of solid phase micro extraction (SPME) and gas chromatography mass spectrometry (GCMS) and compared with the exhaled breath of a healthy control database. Dr. Hu Yanjie's doctoral dissertation[5]reported that 12 VOC biomarkers of lung cancer cells (including decane alkane, twelve alkanes, fourteen alkenes and fourteen alkanes, sixteen alkyl, 2-sixteen ketones, nineteen alkanes, and twenty alkyl VOCs) have been detected at significantly higher rates in lung cancer patients than in a healthy control population with SPME-GCMS. In 2003, Diana Poli reported[6]that the amount of decane-alkyl detected in the exhaled breath of patients with lung cancer decreased significantly after the patients underwent a lung cancer resection, which demonstrates that decane-alkyl is one of the most obvious lung cancer VOC biomarkers. In 2005, R. F. Machado et al. reported[7]the results of a lung cancer screening using an electronic nose with conductive polymer-carbon black composites-based sensor arrays. In a separate group of 76 individuals, 14 with and 62 without cancer, he obtained a (+) accuracy rate of 71.4% (10/14) and a (-) accuracy rate of 91.1% (57/62). In 2009, G. Peng et al[8-9]. Developed a sensor array with gold nanoparticles and a chemical resistance sensor combined with 9 kinds of organic receptors that could distinguish between lung cancer patients and the healthy control group. They further developed the technology, expanded the sensor array, increased the number of receptors to 14, and achieved a rapid discrimination of lung cancer, breast cancer, colorectal cancer, prostate cancer, and healthy control groups with breath samples[9]. In 2011, Orna Barash et al[10]reported in "classification of lung cancer histology by gold nanoparticle sensors" using a device that profiled VOCs in the headspace of (subtypes of) lung cancer cells using gold nanoparticle sensors that were suitable for detecting lung cancer-specific patterns of VOC profiles. This allowed for significant discrimination between (i) lung cancer and healthy cells; (ii) small cell lung cancer and non-small cell lung cancer; and (iii) two subtypes of non-small cell lung cancer: adenocarcinoma and squamous cell carcinoma. In 2013, Y.Y. Broza et al[11]reported that spherical AuNPs with an organic functionality sensor array used for identification of VOCs in exhaled breath enabled identification of malignant vs. benign cases (with stage Ia (n=7)+stage Ib (n=2)+stage IIa (n=3) lung cancer vs. Benign (n=5); sampling (1-2). Their results indicate that with a two sensor array (2-Nitro-4-trifluoro-methylbenzenethiol and Decanethiol) the CV1 of the multivariate discriminant factor analysis (DFA) of lung cancer is significantly higher than the Benign pre-surgery samples, and the CV1 of lung cancer is significantly reduced after surgery (Table 6). Results using another two sensor array (4-Methoxy-toluenethiol and Dibutyl disulfide) demonstrate that the CV1 of lung cancer pre-surgery is significantly reduced after surgery, while the CV1 of benign pre-surgery remains unchanged after surgery.
Table6 The results of organic functionality of 3.5 nm spherical AuNPs sensor

Section sumary: The development of gold nanoparticle sensors in electronic noses and very high sensitivity SPME-GCMS for early lung cancer breath screening has provided favorable support for early lung and breast cancer breath-Raman screening[12], as well as for their cancer sub-types.
4.3 Using the results of GNPs array sensors and VOC biomarkers database of lung cancers with an electronic-nose to develop the breath-Raman screening method of lung cancer with VOC samples We have applied for an invent patent for a gas phase SERS Raman analysis chip to use for breath Raman detection for early lung cancer, or other cancer, screening. The patent application number is 2015101973328[13]. Although the detection principle is different in the GNPs sensor (modified receptors) for lung cancer screening technology with an electronic nose and the breath Raman gas phase SERS-GNPs lung cancer screening technology, there are many aspects that are exactly the same or similar. For example, the GNPs sensor array and SERS-GNPs or SERS-Ag-GNPs technology both modified the same receptors, the collection and pre-processing methods for the VOC gas phase samples are the same, and so on. (Table 7)
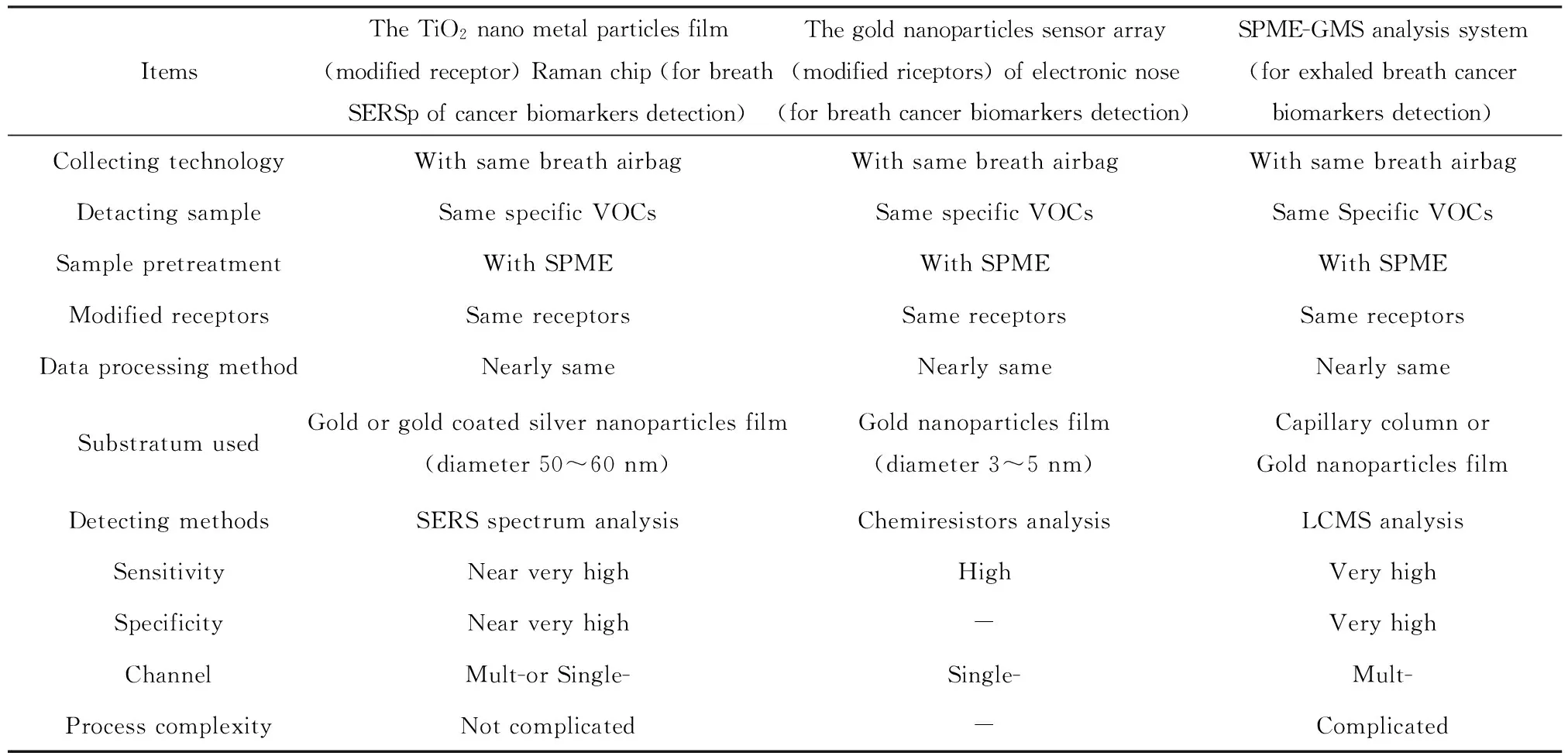
Table 7 The TiO2 nano-metal particles film Raman chip with receptor for breath Raman screening compared with the GNPs sensor array of electronic nose for cancer screening and SPME-GMS analysis system
5 Discussion and the focus of future research
5.1 Early lung cancer detection is the most difficult of all the cancer detections Our previous study results from screening for lung cancer and breast disease with serum samples have set a good preliminary foundation. However, in the tested 43 cases, only 2 were from patients with early (I stage) lung cancer. Thus, we need to expand the sample size of the screening study in the future including more patients with early stage cancer to create a database of serum-Raman fingerprint screening for lung cancer, and to perfect early stage lung cancer serum Raman screening.
5.2 Because the concentration of biomarkers present at early cancer stages is at extremely low, trace levels, it is necessary to collect more information about cancer characteristics in early lung cancer screening Cancer specific information is obtained as completely as possible from gas phase samples of VOCs and serum liquid phase samples. Therefore, an early stage lung cancer screening method using the two samples united in Raman analysis methods is right and feasible. The combined breath-and serum-Raman fingerprint screening method would be an important improvement over only using the serum-Raman fingerprint screening method. With this combined method, all of the metabolized cancer biomarkers in the liquid phase and gas phase will be entirely collected, and the sensitivity and specificity of early lung cancer Raman screening is likely to reach the best possible level.
5.3 Of the three important strategies for overcoming cancer, "early discovery, early diagnosis, and early treatment," we recommend that research for early cancer screening methods be the highest priority of the cancer "early discovery, early diagnosis, and early treatment," strategies. It is the most important link and the key to overcoming cancer.
To overcome cancer is the twenty-first century’s great national systems engineering initiative, involving the popularization of early cancer screening, especially in high-risk populations. We firmly believe that the combined breath-and serum-Raman fingerprinting cancer screening method will be the most effective early lung cancer and early gastric cancer screening method.
Acknowledgements
This study was supported by a fund from the National Natural Science Foundation of China (NO.11074029); Yongcai Wang (Dalian Medical University, the Second Affiliated Hospital, Dalian 1160272), Yue Deng, Yi Zhang, and Jianhua Ding (Dalian University of Technology, School of Physics Dalian 116024), and Jing Tong (Dalian Fifth People's Hospital, Chest Tumor Hospital, Dalian 116000) were the collaborators of the reference[4], from which some figures were cited in this paper. Thanks to coporators: Ding Jian Hua, Zhang Yi, Li Dawei, Deng yue, Zhou Rongge, Liu Kun, Li Rui, Pan lujun, etc; Thanks to Dr Hung Ruo-pan for to review and some correct this manus cript.
【参考文献】
[1]Zhou Rongge,Wu Shifa,Tong Jing,et al.Using serum surface enhanced Raman spectroscopy study on the new method of lung cancer screening,topic exchange[J].Proceedings of development of national light scattering tech-nology and applications symposium,2014-04 (in Chinese).
[2]Zhu Jinfang, Su Yuewen, Feng Yuan. Theearly diagnosis and research development of blood tumor markers for lung cancer[J].Medical Review,2010,16(7):1015-1018 .
[3]Aberle DR,Berg CD,Black WC,et al.The national lung screening trial:overview and study design[J].Radiology,2011,258(1):243-253.
[4]Wu Shifa.The development of Raman fingerprint unified screening research for the lung cancer and early breast disease with serums[J].Proceeding of Tianjin 2015 Nobel prize winner medical summit and international symposium on cancer research,2015,05,08-10.
[5]Hu Yanjie.Study on screening and diagnostic value of lung cancer breath characteristic of VOCs[J].Doctoral Dissertation of Zhejiang University,2010-05-29 (in Chinese).
[6]Phillips M,Cataneo RN,Cummin AR,et al.Detection of lung cancer with volatile markers in the breath[J].Chest,2003,23(6):2115-2123.
[7]McCulloch Mng,Jezierski T,Broffman M,et al.Diagnostic accuracy of canine scent detection in early and late stage lung and breast cancers[J].Integr Cancer Ther,2006,5(1):30-39.
[8]Peng G,Tisch U,Adams O,et al.Diagnosing lung cancer in exhaled breath using gold anoparticles[J].Nat Nanotechnol,2009,4(10):669-673.
[9]Peng G,Hakim M,Broza YY,et al.Detection of lung,breast,coloreetal,and prostate cancers from exhaled breath using a single array of nanosensors[J].Br J Cancer,2010,103(4):542-551.
[10]Barash O,Peled N,Tisch U,et al.Classification of lung cancer histology by gold nanoparticle sensors[J].Nanomedicine,2012,8(5):580-589.
[11]Broza YY,Kremer R,Tisch U,et al.A nanomaterial-based breath test for short-term follow-up after lung tumor resection[J].Nanomedicine,2013,9(1):15-21.
[12]Barash O,Zhang W.Differentiation between genetic mutations of breast cancer by breath volatolomics[J].Oncotarget,2015,6(42):44864-44876.
[13]Wu Shifa.A with and without receptor modified titanium dioxide nano metal film Raman chip and manufacture method, patent application number:2015101973328,(2015-04).
