Effects of T-2 Toxin Exposure on Bone Metabolism and Bone Development of Mice
2022-04-06CuiYilongCaoZhengZhangJianSongMiaoandLiYanfei
Cui Yi-long, Cao Zheng, Zhang Jian, Song Miao, and Li Yan-fei
College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China
Abstract: T-2 toxin is the most widespread mycotoxin in crops, feed and food, which poses a serious threat to body health. Bone is the main target tissue for T-2 toxin accumulation. Ingestion of food contaminated by T-2 toxin is the main cause of Kashin-Beck disease. However, the specific mechanism of bone damage caused by T-2 toxin is still unclear. In this study, a total of 40 male C57BL/6N mice were divided into four groups and orally treated with 0, 0.5, 1.0 and 2.0 mg • kg-1 body weight T-2 toxin for 28 days. The results showed that exposure to T-2 toxin led to weight loss, bone mineral density reduction and femoral structural damage of mice. In addition, osteoblast-mediated bone formation was inhibited, and osteoclast-mediated bone resorption was enhanced. Meanwhile, the levels of bone metabolism-related hormones including parathyroid hormone, calcitonin and 1, 25-dihydroxyvitamin D3 were reduced. More importantly, it was found that the level of neuropeptide Y (a neurohormone) was decreased. These results provided a new perspetive for understanding the osteotoxicity of T-2 toxin.
Key words: T-2 toxin, bone metabolism, parathyroid hormone, calcitonin, 1, 25-dihydroxyvitamin D3, neuropeptide Y
Introduction
T-2 toxin is widely present in field crops, stored grains, feed and food, can enter human and animal bodies through digestive tract, respiratory tract and skin, and has toxic effects on nervous, immune, digestive, reproductive and skeletal systems (Daiet al., 2019; Escriváet al., 2015). T-2 toxin is listed as the most dangerous source of natural food contamination by the World Health Organization, but T-2 toxin contamination events still occur frequently (Yanget al., 2016). In 2019, the detection rates of T-2 toxin contamination in feed, corns and grains in Europe were 36%, 40% and 49%, and the highest detection level reached 4 134 μg•kg-1(Gruber-Dorningeret al., 2019). T-2 toxin pollution poses a serious threat to livestock and poultry production performance, animalderived food safety and human health. Exploring its toxicity mechanism can provide a strong basis for the prevention and control of public health problems.
Bone is the main target tissue for the accumulation of T-2 toxin, which can cause bone dysplasia in humans and animals (Shiet al., 2021). Ingestion of food contaminated with T-2 toxin is the main cause of Kashin-Beck disease (Maet al., 2020). Exposure to T-2 toxin can cause animal bone deformities and even fractures, restrict exercise and diets, and cause economic losses to the breeding industry (Sunet al.,2021). However, the molecular mechanism of bone dysplasia caused by T-2 toxin has not yet been determined. Previous studies on bone injury caused by T-2 toxin mainly focus on cartilage. Studies have shown that T-2 toxin exposure leads to apoptosis of growth plate chondrocytes of mice and poultry, resulting in degradation of cartilage matrix, thinning of growth plate cartilage and susceptibility to fracture (Guanet al., 2013; Liuet al., 2014; Zhanget al., 2020b). Bone metabolism is the main physiological process to maintain the normal function of the skeletal system, but there is no research on the relationship between T-2 toxin and bone metabolism (Tobeihaet al., 2020). The balance between osteoblastmediated bone formation and osteoclast-mediated bone resorption is an important event that reflects the state of bone metabolism (Suzukiet al., 2020). Exploring the state of bone formation and bone resorption in bone injury caused by T-2 toxin can strengthen the understanding of its osteotoxicity.
Bone metabolism also depends on the regulation of some hormones including parathyroid hormone (PTH), calcitonin (CT) and 1, 25-dihydroxyvitamin D3 (1, 25-(OH)2-D3), and their levels in the body reflect the balance between bone formation and bone resorption (Liet al., 2011a). In addition, neuropeptide Y (NPY) is a hormone secreted by nerve cells in the hypothalamus that is widely present in the central and peripheral nervous systems (Shende and Desai, 2020). The NPY is involved in biological processes, such as inflammation, sleep and feeding behavior, and recent studies have confirmed that it is involved in bone metabolism (Linet al., 2021; Lvet al., 2021). Exploring the changes of these hormone levels in T-2 toxin-induced bone impairment might screen out its pathogenic and therapeutic targets.
Materials and Methods
Animals and treatment
All the procedures were approved by the Animal Ethics Committee of the Northeast Agricultural University (Harbin, China). Forty male C57BL/6N mice of fourweek-old (Liaoning Chang-sheng Biotech, Benxi, China) were randomly assigned to four groups (n=10 per group) and orally treated with 0 (control group, CG), 0.5 (low-dose group, LG), 1.0 (medium-dose group, MG), 2.0 (high-dose group, HG) mg•kg-1body weight T-2 toxin (Purity≥99.8%, Qingdao Pribolab, China) for 28 days. The dose of T-2 toxin was based on the LD50of mice (Liet al., 2011b). The mice were kept in a sterile room with a light/dark cycle of 12 h and free access to water and food at 22℃.
Sample collection
On the 29th day, the mice were euthanized by inhalation of carbon dioxide after being weighed. Blood was collected from the eyeball and serum was separated.
Bone mineral density determination
The femora were taken out and the bone mineral density (BMD) was measured with a Bone Densitometer DEXA machine (Diagnostic Medical Systems Corporation, France).
Histopathological analysis
The femoral metaphysis was fixed in 4% paraformaldehyde for three days, and then decalcified with 10% EDTA for 30 days. The specimen was embedded in paraffin and cut into 5 µm thick sections. Then the sections were respectively stained with hematoxylin and eosin (HE), bone alkaline phosphatase (BALP) (Nanjing Jiancheng Bioengineering Institute, China) and tartrate-resistant acid phosphatase (TRACP) (Nanjing Jiancheng Bioengineering Institute, China). Each staining was performed using an optical microscope (Pannoramic MIDI, Hungary) to randomly collect images in three fields of views.
Determination of bone formation markers in serum
The levels of bone gamma-carboxyglutamic acid protein (BGP), BALP and carboxy-terminal propeptide of type I procollagen (PICP) in serum were analyzed using ELISA kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturers' protocol. All the samples were analyzed in triplicates.
Determination of bone resorption markers in serum
The levels of C-terminal cross-linking telopeptide of type I collagen (CTX-I), amino terminal peptide of type I collagen (NTX-I) and TRACP in serum were analyzed using ELISA kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturers' protocol. All the samples were analyzed in triplicates.
Determination of bone metabolism-related hormone levels
The levels of PTH, CT, 1, 25-(OH)2-D3 (Nanjing Jiancheng Bioengineering Institute, China) and NPY(Shanghai Zhuo Cai Technology, China) in serum were analyzed using ELISA kit according to the manufacturers' protocol. All the samples were analyzed in triplicates.
qRT-PCR analysis
The femora were cut into pieces and placed in a mortar, liquid nitrogen was added, and the femora were ground with a pestle. Trizol (Beyotime, China) was added to extract the total RNA, and then transcribed into cDNA according to the kit instruction (TransGen Biotech, China). The relative expression levels of NPY andβ-actin were analyzed by ABI 7500 real-time PCR detection system. qRT-PCR reaction conditions were pre-denaturation at 50℃ for 2 min, 95℃ for 10 min; denaturation at 95℃ for 15 s; annealing and extension at 60℃ for 1 min, 40 cycles; 95℃ for 15 s, 60℃ for 1 min, 95℃ for 15 s. NPY primer sequence: F: 5'-CTCGTGTGTTTGGGCATTC-3', R: 5'-TAGTGTC GCAGAGCGGAGTA-3'.β-actin primer sequence: F: 5'-GTTGGAGCAAACATCCCCCA-3', R: 5'-ACGCG ACCATCCTCCTCTTA-3'.
Western blot analysis
The femora were cut into pieces and placed in a mortar, liquid nitrogen was added, and the femora were ground with a pestle. Proteins were extracted by adding RIPA lysis buffer (Beyotime, China) and electrotransferred to PVDF membranes (Millipore, Bedford) by SDS-PAGE. Then the membranes were incubated with primary antibodies recognizing the NPY (1 : 1 000, Santa Cruz, USA) orβ-actin (1 : 2 000, Sungene Biotech, China) overnight at 4℃. Then, these membranes were exposed to Mouse IgGκ BP-HRP (1 : 5 000, Santa Cruz, USA) for 2 h at 37℃. Protein bands were imaged with Amersham Imager 600 and analyzed with Image J software.
Statistical analysis
Data analyses were performed with SPSS 22.0 statistical software (SPSS Incorporated, USA). Data were expressed as mean±standard deviation (mean±SD) from at least three independent experiments performed in triplicates. *P<0.05 and **P<0.01 were considered statistically significant compared to the CG.
Results
Effects of T-2 toxin exposure on growth and bone development of mice
With the increase in the exposure dose of T-2 toxin, the body weight (Fig. 1A) and BMD (Fig. 1B) of mice gradually decreased, but compared with the CG, the LG was not statistically significant (P>0.05), and the MG and the HG were statistically significant (P<0.05,P<0.01). HE staining showed that as the exposure dose of T-2 toxin gradually increased, the trabecular structure was disordered and the number of bone cells gradually decreased in the femora (Fig. 1C). These results indicated that T-2 toxin exposure caused growth inhibition and bone impairment of mice.
Effects of T-2 toxin exposure on bone formation of mice
With the increase in the exposure dose of T-2 toxin, the levels of BGP (Fig. 2A), BALP (Fig. 2B) and PICP (Fig. 2C) in the serum of mice gradually decreased, but compared with the CG, the LG was not statistically significant (P>0.05), and the MG and the HG were statistically significant (P<0.01). The BALP staining results showed that the number of BALP-positive cells in the femora gradually decreased as the dose of T-2 toxin increased (Fig. 2D). These results indicated that T-2 toxin exposure inhibited bone formation of mice.

Fig. 1 Effects of T-2 toxin exposure on growth and bone development of miceA, Effects of T-2 toxin exposure on body weight of mice (n=10); B, Effects of T-2 toxin exposure on BMD of mice femora (n=10); C, Effects of T-2 toxin exposure on microstructure of mice femora. Yellow arrow indicates trabecular bone. Blue arrows indicate bone cells. Scale bar: 50 μm. *P<0.05, **P<0.01.
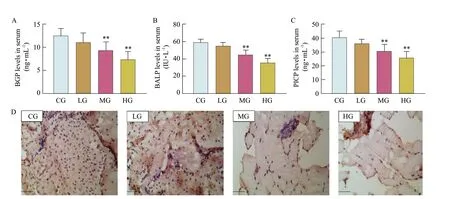
Fig. 2 Effects of T-2 toxin exposure on bone formation of miceA, Level of BGP in serum (n=6); B, Level of BALP in serum (n=6); C, Level of PICP in serum (n=6); D, Representative images of BALP staining. Blue-black particles are BALP staining positive cells. Scale bar: 50 μm. **P<0.01.
Effects of T-2 toxin exposure on bone resorption of mice
With the increase in the exposure dose of T-2 toxin, the levels of CTX-Ⅰ (Fig. 3A), NTX-Ⅰ (Fig. 3B) and TRACP (Fig. 3C) in the serum of mice gradually increased, but compared with the CG, the LG was not statistically significant (P>0.05), and the MG and the HG were statistically significant (P<0.01). The TRACP staining results showed that the number of the TRACP-positive cells in the femora gradually increased as the dose of T-2 toxin increased (Fig. 3D).These results indicated that T-2 toxin exposure enhanced bone resorption of mice.
Effects of T-2 toxin exposure on bone metabolism hormones of mice
With the increase in the exposure dose of T-2 toxin, the levels of PTH (Fig. 4A), CT (Fig. 4B), 1, 25-(OH)2- D3 (Fig. 4C) and the NPY (Fig. 4D) in the serum of mice gradually decreased, but compared with the CG, the LG was not statistically significant (P>0.05), and the MG and the HG were statistically significant (P<0.01). The results of the qRT-PCR and western blot showed that the expression of the NPY in the femora gradually decreased as the dose of T-2 toxin increased (P<0.05,P<0.01) (Fig. 4E, F and G). These results indicated that T-2 toxin exposure disrupted hormone levels of mice.

Fig. 3 Effects of T-2 toxin exposure on bone resorption of miceA, Level of CTX-Ⅰ in serum (n=6); B, Level of NTX-Ⅰ in serum (n=6); C, Level of TRACP in serum (n=6); D, Representative image of TRACP staining. Red particles are TRACP positive cells. Scale bar: 50 μm. **P<0.01.

Fig. 4 Effects of T-2 toxin exposure on bone metabolism hormones of miceA, Level of PTH in serum (n=6), B, Level of CT in serum (n=6); C, Level of 1, 25-(OH)2-D3 in serum (n=6); D, Level of NPY in serum (n=6); E, NPY mRNA expression level; F, NPY protein expression level; G, Representative images of NPY protein expression. *P<0.05, **P<0.01.
Discussion
Exposure to T-2 toxin can cause bone dysplasia in human and animals, and exploring the mechanism of bone toxicity can provide a theoretical basis for clinical prevention and treatment. This research found that T-2 toxin caused growth inhibition and bone damage of mice, accompanied by an imbalance in bone formation and bone resorption. In addition, it was found that T-2 toxin disrupted the level of hormones of mice, including a neurohormone-NPY. These results provided a new perspective for understanding the osteotoxicity of T-2 toxin.
The BMD was an important indicator of bone strength (Cuiet al., 2021). Decreased BMD could cause osteoporosis and threaten bone health (Lupsa and Insogna, 2015). It was first found that T-2 toxin exposure reduced BMD in mice. In addition, the trabecular structures in the CG were arranged in an orderly manner and were rich in bone cells, but the T-2 toxin exposure damaged the microstructure of the femora. Similarly, Yanet al. (2011) found that T-2 toxin exposure caused histological changes in the tibia of rats. These results indicated that T-2 toxin caused severe impairment to the bone growth of mice.
The change of the BMD was mostly caused by the imbalance of bone formation and bone resorption (Bandeiraet al., 2017). The levels of BGP, BALP and PICP were widely used indicators in the bone formation (Cuiet al., 2014). BGP was secreted by osteoblasts to maintain the normal rate of bone mineralization, inhibited the rate of mineralization of growing cartilage and the formation of abnormal hydroxyapatite crystals (Oldknowet al., 2015). B-ALP could reflect the activity of osteoblasts and bone calcification, and was an essential active factor in the process of bone formation (Ravnet al., 2003). PICP in serum could reflect the synthesis of type I collagen and could assess bone formation (Seoet al., 2017). This study found that T-2 toxin exposure reduced the levels of BGP, BALP and PICP in the serum of mice, indicating that bone formation was inhibited. Further the BALP staining revealed that T-2 toxin exposure resulted in a decrease in the number of osteoblasts, which should be a key factor in inhibiting bone formation.
While osteoblasts maintained bone remodeling, osteoclasts acted on the bone matrix to cause bone resorption (Raggatt and Partridge, 2010). CTX-I and NTX-I were only derived from mature bone type I collagen fibers, and their content changes could reflect the loss of bone matrix and were representative indicators for evaluating bone resorption (Herrmann and Seibel, 2008). The TRACP participated in the meta-bolism of mineralization in the bone matrix, and its level could reflect the activity of osteoclasts and the state of bone resorption (Nakasatoet al., 1999). In the study of cadmium and aluminum-induced bone injury of rats, an increase in the content of CTX-I, NTX-I and the TRACP was found, which was consistent with this research results (Chenet al., 2017; Sunet al., 2016).The TRACP staining also demonstrated the enhancement of osteoclast activity in the femora after T-2 toxin exposure. These results suggested that enhanced bone resorption was one of the factors that T-2 toxin caused bone damage in mice.
Bone metabolism included osteoclast-mediated bone resorption and osteoblast-mediated bone formation (Siddiqui and Partridge, 2016). Under normal circumstances, bone resorption and bone formation were maintained in a balanced state. When the balance was affected by unfavorable factors, it was broken and led to abnormal bone development and bone disease (Feng and McDonald, 2011). In this study, mice exposed to T-2 toxin had enhanced bone resorption, but weakened bone formation, which inevitably resulted in bone damage.
In addition, this study also analyzed the effect of T-2 toxin exposure on the levels of hormones related to bone development of mice. PTH could promote the proliferation and differentiation of osteoblasts, stimulate osteoblasts to produce growth factors and transformation factors, and then play an osteogenic effect (Halladayet al., 2001). CT could prevent the apoptosis of osteoblasts, and could also inhibit the bone resorption of osteoclasts (Devogelaeret al.,2006). 1, 25-(OH)2-D3 could affect the proliferation and differentiation of osteoblasts and the mineralization of extracellular matrix (Mahieu and Calvo, 1998). In this study, exposure to T-2 toxin reduced the levels of PTH, CT and 1, 25-(OH)2-D3 in the serum of mice, disrupted normal bone metabolism, and ultimately resulted in bone damage.
NPY was secreted by nerve cells in the hypothalamus and was widely distributed in the central nervous system and peripheral nervous system (Decressac and Barker, 2012). Previous studies on NPY mainly focused on neuroendocrine, cognitive function and eating behavior (Tatemotoet al., 1982). However, recent studies had shown that the expression of NPY in the hypothalamus was related to bone formation, and NPY was also widely expressed in bone cells and participates in bone metabolism (Chenet al., 2021; Lvet al., 2021; Zhanget al., 2021). When NPY gene silenced mice encountered a cold stimulus, their bone loss rate was three times that of normal mice (Parket al., 2017). Zhanget al. (2020a) found that overexpression of NPY in osteoblasts had a positive regulatory effect on osteogenic function. In this study, after exposure to T-2 toxin, the level of NPY in the serum of mice was reduced, and the expression of NPY in femora was also reduced. These results suggested that NPY might become one of the important targets for the prevention and treatment of bone diseases caused by T-2 toxin.
Conclusions
In summary, T-2 toxin exposure caused bone damage of mice, accompanied by an imbalance of bone formation and bone resorption, as well as a disorder of hormone levels related to bone metabolism. This study enriched people's understanding of the mechanism of bone damage caused by T-2 toxin, and provided a basis for discovering targets for the prevention and treatment of T-2 toxin-induced bone diseases.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Journal of Northeast Agricultural University (English Edition)
- Characterization and Optimization of Amylase Production in Strains LZ-10 and LZ-11 Belonging to Bacillus subtilis
- Comparative Analysis of Hydrolytic Amino Acids in Human and Cow Milk of Different Lactation Periods
- Cloning of GsTPS9 Gene from Glycine soja and Study on Its Responses to Stresses
- Effects of Sowing Periods on Growth and Development, Yield and Quality of Maize in Cold Area
- Nutrient Accumulation and Distribution in Cotton Promoted by Removal of Mulch Film
