lnsights into myelin dysfunction in schizophrenia and bipolar disorder
2022-04-01MarcelaValdTovarAlejandraMonserratRodrguezRamrezLeslyeRodrguezrdenasCarloSoteloRamrezBeatrizCamarenaMarcoAntonioSanabraisJimnezctorSolChagoyJesArguetaGermOctaviopezRiquelme
Marcela Valdés-Tovar,Alejandra Monserrat Rodríguez-Ramírez,Leslye Rodríguez-Cárdenas,Carlo E Sotelo-Ramírez,Beatriz Camarena,Marco Antonio Sanabrais-Jiménez,Héctor Solís-Chagoyán,Jesús Argueta,Germán Octavio López-Riquelme
Marcela Valdés-Tovar,Departamento de Farmacogenética,Subdirección de Investigaciones Clínicas,Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz,Mexico City 14370,Mexico
Alejandra Monserrat Rodríguez-Ramírez,Leslye Rodríguez-Cárdenas,Carlo E Sotelo-Ramírez,Beatriz Camarena,Marco Antonio Sanabrais-Jiménez,Departamento de Farmacogenética,Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz,Mexico City 14370,Mexico
Carlo E Sotelo-Ramírez,Jesús Argueta,Doctorado en Biología Experimental,Universidad Autónoma Metropolitana-Iztapalapa,Mexico City 09340,Mexico
Héctor Solís-Chagoyán,Jesús Argueta,Laboratorio de Neurofarmacología,Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz,Mexico City 14370,Mexico
Germán Octavio López-Riquelme,Laboratorio de Socioneurobiología,Centro de Investigación en Ciencias Cognitivas,Universidad del Estado de Morelos,Cuernavaca 62209,Morelos,Mexico
Abstract Schizophrenia and bipolar disorder are disabling psychiatric disorders with a worldwide prevalence of approximately 1%.Both disorders present chronic and deteriorating prognoses that impose a large burden,not only on patients but also on society and health systems.These mental illnesses share several clinical and neurobiological traits;of these traits,oligodendroglial dysfunction and alterations to white matter (WM) tracts could underlie the disconnection between brain regions related to their symptomatic domains.WM is mainly composed of heavily myelinated axons and glial cells.Myelin internodes are discrete axon-wrapping membrane sheaths formed by oligodendrocyte processes.Myelin ensheathment allows fast and efficient conduction of nerve impulses through the nodes of Ranvier,improving the overall function of neuronal circuits.Rapid and precisely synchronized nerve impulse conduction through fibers that connect distant brain structures is crucial for higher-level functions,such as cognition,memory,mood,and language.Several cellular and subcellular anomalies related to myelin and oligodendrocytes have been found in postmortem samples from patients with schizophrenia or bipolar disorder,and neuroimaging techniques have revealed consistent alterations at the macroscale connectomic level in both disorders.In this work,evidence regarding these multilevel alterations in oligodendrocytes and myelinated tracts is discussed,and the involvement of proteins in key functions of the oligodendroglial lineage,such as oligodendrogenesis and myelination,is highlighted.The molecular components of the axo-myelin unit could be important targets for novel therapeutic approaches to schizophrenia and bipolar disorder.
Key Words:Myelin sheath;Oligodendroglia;Schizophrenia;Bipolar disorder;White matter
lNTRODUCTlON
Currently,it is widely understood that optimal functioning of the central nervous system (CNS)depends on synaptic connections and multidirectional interactions between neuronal and glial cells.One of the closest glial-neuronal interactions in the CNS occurs between oligodendrocytes and neurons through myelination.Myelin ensheathment induces axonal compartmentalization to form nodes of Ranvier,i.e.,specialized domains that increase the conduction speed of action potentials.The saltatory propagation and speed of these electrical impulses depends on axon caliber,but primarily on myelin features such as the number and length of internodes as well as myelin width and compaction[1].Therefore,myelination allows neuronal circuits to be finely tuned and synchronized and,as such,plays a key role in maintaining the proper connectivity between brain structures to support higher integrating processes,such as perception,memory,or cognition.Furthermore,through myelination,oligodendrocytes also provide metabolic support to axons[2,3],contributing to their structural and functional integrity,which is a requirement for homeostasis of the human brain.
Myelination is a neurodevelopmental process that begins during the third trimester of pregnancy and increases steadily during childhood and early youth until it reaches a slow-increasing plateau in adult life[4,5].This process is adaptive,with neural activity being one of the main factors driving myelin plasticity[6].Moreover,because the myelin sheath is a specialized structure made up of multiple layers of plasma membrane,from which most cytoplasm is extruded,its composition is enriched with lipids(approximately 70% of its content) and proteins[7].These proteins have important functional roles,such as providing anchorage between myelin lamellae,attaching axons and myelin at paranodal regions,signaling and interacting with cytoskeletal elements within oligodendrocytes[7,8].As with any other cell type,the plasma membrane in oligodendrocytes is subject to homeostatic turnover;thus,to manage this large energy requirement,their metabolic rate is higher than that of other cell types[9].This characteristic renders myelinating oligodendrocytes more vulnerable to cellular stress and oxidative damage generated by reactive oxygen/nitrogen species[10].In the case of injury and myelin loss (demyelination),endogenous repair mechanisms are triggered and remyelination occurs.For either developmental/adaptive myelination or remyelination to occur,oligodendrocyte precursor cells (OPCs)distributed along the brain must differentiate and mature to acquire myelinating capacity[11,12].Oligodendrogenesis involves differential expression of proteins and other molecules and a dramatic increase in morphologic complexity,which implies crucial and extensive rearrangements of the oligodendroglial cytoskeleton[13,14].
Due to the intrinsic complexity of oligodendrocyte morphology and functioning and the importance of myelination/remyelination processes for CNS homeostasis,impairments in oligodendroglial lineage may be associated with brain disorders.Within the last two decades,great effort has been made to determine and describe neuronal and glial alterations that contribute to the etiology of mental illnesses[15-18].Of the studied mental disorders,we focused on schizophrenia and bipolar disorder because these neuropsychiatric illnesses present a chronic and deteriorating course that imposes a large burden,not only on patients but also on society and health systems.These disorders are long-lasting,severe mental health conditions that share genetic characteristics and alterations to brain structure and function,and neurobiological pathways[19-21].Among the main pathophysiological mechanisms shared by these conditions is glial dysfunction[22,23],specifically related to myelination,which is the focus of this text.
Taking the above information into account,the aim of this work was to gather and discuss the evidence that myelin dysfunction at the cellular and subcellular levels may underlie the white matter(WM) macroscale connectome alterations evidenced by neuroimaging in schizophrenia and bipolar disorder,thereby supporting the disconnection hypothesis that explains the symptomatic domains of these clinical entities.For this purpose,we first provide a brief overview of the main structural features of myelin.Next,we present the evidence of myelin alterations at the microscale levels (cellular and subcellular) found in postmortem samples from schizophrenia and bipolar disorder patients.Then,we briefly compare the main findings at these levels.Finally,we review evidence at the macroscale level from neuroimaging techniques and find consistent support for dysconnectivity among key brain regions in these disorders.These neuroimaging techniques are the main methods that allow us to obtain information about brain structure and function from patients during the course of their illnesses.
BRlEF OVERVlEW OF MYELlN STRUCTURAL FEATURES
Each myelin internode is a specialized structure of multiple membrane lamellae.The first membrane layer-closest to the axon-is called the adaxonal membrane.Between the axon and the adaxonal membrane is the periaxonal space[7].Flanking each internode,paranodal loops make contact with the axon through the cell adhesion proteins neurofascin 155 (NF155;on the oligodendrocyte) and contactinassociated protein 1 (Caspr1)/contactin 1 (on the axon).In juxtaparanodal regions,myelin-axon interactions are mediated by contactin 2 and Caspr2,and the voltage-gated K+channels Kv1.1/1.2 are enriched at the axolemma.Contactin’s cytoplasmic domains provide anchors for scaffold molecules of the paranodal-nodal-paranodal cytoskeleton,specifically for the 4.1B protein,the αII/β2SP heterotetramers (both actin-interacting proteins) and ankyrin B (AnkB)[24].
In a mature myelin internode,the adaxonal layer is relatively loose compared with the tightly compacted myelin lamellae,and its cytoplasmic content is slightly higher,which allows the functional presence of signal transduction molecules and oligodendroglial cytoskeletal components such as septin filaments[25].These components are also present at paranodal loops.In contrast,the structure of compact myelin is almost withdrawn from the cytoplasm;thus,intracellular membranes are in tight apposition,with myelin basic protein (MBP) playing a key role in regulating the hydrophobic forces between them[7].
As previously mentioned,the molecular composition of myelin is highly enriched in lipids,which account for approximately 70% of its wet weight.Myelin membranes have a higher cholesterol content than other membranes (at approximately 1.6-fold) and are characteristically enriched with galactosphingolipids and plasmalogens,which are asymmetrically distributed among the bilayer leaflets.The extracellular leaflet is enriched in galactosylceramide and its sulfated form,sulfatide,as well as phosphatidylcholine and sphingomyelin,whereas the intracellular leaflet is rich in ethanolamine plasmalogen and other phospholipids.The lipid components of the extracellular leaflet form discrete domains known as lipid rafts,which often contain membrane proteins and are frequently involved in signaling and/or myelin component turnover.For further review of myelin lipids,see[26,27].
Myelin-specific structural proteins also are distributed according to their functions.Interestingly,at least five out of eleven CNS myelin-specific proteins are categorized as intrinsically disordered proteins.This set of physicochemical attributes accounts for their flexibility and multifunctionality,which are important for a plastic structure such as myelin[28].An exhaustive description of the structural and functional features of every myelin-specific protein is beyond the scope of this review and has been further addressed elsewhere[8,29].Figure 1 illustrates the main CNS myelin-specific proteins at their common locations in the myelin sheath,as well as the lipidic composition of myelin membranes.

Figure 1 Myelin in the central nervous system.
EVlDENCE OF MYELlN ALTERATlONS AND OLlGODENDROGLlAL DYSFUNCTlON lN POSTMORTEM SAMPLES OBTAlNED FROM SCHlZOPHRENlA PATlENTS
The analysis of postmortem samples provides valuable information about the structural and biochemical alterations present in the brains of patients with neuropsychiatric disorders.In the last 20 years,several reports by Uranovaet al[30] have described the main ultrastructural alterations in oligodendrocytes and myelinated fibers found in patients with schizophrenia.
With electron microscopy and morphometry or with a stereological approach and Nissl-stained sections,they extensively analyzed the prefrontal cortex (PFC),specifically the gray matter layers of Brodmann’s area 9 (BA9) and BA10[30-33] and their adjacent WM[34-36],as well as the caudate nucleus[21,33,37,38],hippocampus[33,38,39] and anterior putamen[40].
Their analysis of myelinated fibers found concentric lamellar bodies and interlamellar abnormal inclusions,swelling of periaxonal oligodendrocyte processes and ultrastructural signs of axonal atrophy[21,33,38,39].They characterized six types of abnormal myelinated fibers that were present in patients with schizophrenia and that could correlate with the predominant presence of positive or negative symptoms,age or illness duration[35].
Oligodendrocytes showed consistent signs of dystrophia,apoptosis and/or necrosis,and in most of the studies,their numerical density was significantly reduced in patient samples[31,33,37,40].Oligodendrocyte clusters,which are thought to be involved in activity-dependent myelination,were also consistently reduced[37,40].In the oligodendrocytes,mitochondria were the main altered organelle,with a significant reduction in numeric and volume density and even intramitochondrial accumulation of lipofuscin granules[30,34,36,38].These findings suggest that not only is the numerical density of oligodendrocytes affected in schizophrenia,but that their energy and redox metabolism is also compromised.
Interestingly,both perineuronal and pericapillar oligodendrocytes showed signs of dystrophy in patient samples[33,34] suggesting that oligodendrocytes may be involved both in the disrupted transmission of neuronal information and in metabolic dysregulation.In addition,these studies found dystrophic ameboid microglia adjacent to dystrophic oligodendrocytes[30,34] and myelin concentric lamellar bodies engulfed by astrocytes[21],implying the involvement of other glial cells in myelin pathology in schizophrenia patients.
Hofet al[41,42] found a significant decrease in both the total number of oligodendrocytes and the number of oligodendrocytes expressing the 2',3'-Cyclic-nucleotide 3'-phosphodiesterase (CNP) marker in cortical layer III of BA9 and the WM of the superior frontal gyrus with a stereological analysis on samples from patients with schizophrenia.Additionally,the number of oligodendrocyte clusters in the WM was significantly reduced[42].Other studies reported a decreased number of oligodendrocytes in the anterior principal thalamic nucleus[43,44],centromedian thalamic nucleus[44],thalamic internal capsule[45],hippocampus[46,47] and anterior cingulate WM[48] of schizophrenia patient samples.In the latter structure,oligodendrocytes expressing disintegrin and metalloproteinase domain-containing protein 12 (ADAM12) were examined[48].ADAM12 is predominantly expressed in oligodendrocytes and has been suggested to play a role in myelination and neurodevelopmental processes,as well as in higher cognitive functions[49].
Not all neuropathological studies of postmortem schizophrenia brain samples showed significant differences in oligodendrocyte densities,e.g.,in the cingulum bundle[50],BA9 adjacent WM[23,51],and BA10 adjacent WM[34],no changes in oligodendrocyte cell densities were found between schizophrenia and control samples.In contrast,an increased density of prohibitin(+)-oligodendrocytes was reported in the right dorsolateral prefrontal WM of schizophrenia patients[52].The authors suggested that prohibitin may be upregulated in oligodendrocytes as a result of mitochondrial stress and/or dysfunction in schizophrenia.
mRNA expression of neural/glial antigen 2 (NG2) was augmented in the putamen of schizophrenia patients[53],suggesting that there could be an increased density of OPCs.Additionally,a study by Kernset al[45] supported the hypothesis that in schizophrenia OPCs may fail to exit the cell cycle and differentiate into mature myelinating oligodendrocytes.In BA9 WM,there was no significant difference in NG2(+)-cells but a significant reduction in cells expressing oligodendrocyte transcription factor 2(OLIG2),suggesting an overall reduction in the oligodendroglial lineage[54].
MYELlN SUBCELLULAR/BlOCHEMlCAL ALTERATlONS lN POSTMORTEM SAMPLES OF PATlENTS WlTH SCHlZOPHRENlA
Proteomic approaches have been used to determine that the main myelin structural proteins are differentially expressed in schizophrenia postmortem brain samples;in most of the studies,these proteins were significantly downregulated.For most of the myelin structural proteins,altered transcriptomic levels have consistently been reported[53,55-57],and in some cases,single-nucleotide polymorphisms(SNPs) at their codifying genes have been associated with schizophrenia (Table 1).This is the case for MBP[58-61],CNP[57,60-63],proteolipid protein (PLP)[60,62,64],myelin-associated glycoprotein (MAG)[62,64,65],and transferrin[66-68].The latter is not a structural myelin protein,but is essential for oligodendrocyte homeostasis and survival[69,70].Downregulation of myelin oligodendrocyte glycoprotein (MOG)[58,60-62,71] and claudin-11[62,64] at the proteomic and transcriptomic levels has been reported,although no SNPs of the corresponding codifying genes have been associated with schizophrenia.Several brain regions have been analyzed by proteomic studies,such as the dorsolateral PFC BA46[59,62,71],PFC BA9 gray and WM[68],PFC BA10[60],anterior PFC[65],orbitofrontal cortex[64],anterior temporal lobe[58],corpus callosum[61],cerebellum,posterior cingulate cortex and caudate nucleus[63].

Table 1 Studies that reported single-nucleotide polymorphisms associated with schizophrenia in myelin/oligodendrocyte genes
Consistent with findings of overall downregulation of myelin-specific proteins,mRNA levels ofOLIG1[22],OLIG2[22,55,72-74] andSOX10[22,55,72],corresponding to oligodendroglial lineage transcription factors,were significantly reduced in postmortem schizophrenia brain samples.Additionally,the expression of theNG2,PGDFRAandGALCgenes (the former two coding for markers of OPCs and the latter for a marker of immature oligodendrocytes),was consistently downregulated in patient samples[22].Quaking (QKI),an RNA-binding protein with a key role in the posttranscriptional regulation of myelin-specific genes,mRNA levels were significantly reduced in postmortem samples of schizophrenia patients[72,75-77].Moreover,SNPs in bothOLIG2andQKIgenes have been associated with this mental disorder.
In addition to the previously mentioned gene association studies,a functional glial-specific gene set analysis based on genome-wide association data reported three main oligodendroglial gene sets,i.e.,lipid metabolism,gene transcription and oxidation-reduction,which were strongly associated with an increased risk for schizophrenia[78].Furthermore,gene expression profile analysis of CNP(+)-cells revealed nine differentially regulated signaling pathways associated with oligodendrocyte differentiation[54],strongly suggesting oligodendrogenesis impairment in schizophrenia.
Proteomic studies of schizophrenia-derived postmortem brain samples have also consistently revealed that many cytoskeletal components are differentially expressed in this disorder.Dynamic cytoskeletal rearrangements are crucial for oligodendrogenesis since this process implies a dramatic increase in oligodendroglial morphologic complexity.Additionally,actin-cytoskeleton dynamic assembly and disassembly are critical for axon ensheathment during the myelination process[79-81].Several actin-interacting proteins are involved in these rearrangements,including gelsolin and cofilin,actin filament-severing proteins that drive actin cytoskeleton disassembly,which is essential for proper myelin wrapping[79].Gelsolin is specifically expressed in myelin-forming cells[82] and is present in the different stages of oligodendroglial lineage differentiation[83].Transcriptomic and proteomic analyses of postmortem brain samples have shown that both gelsolin[61,68,72] and cofilin[59,63,64] are dysregulated in schizophrenia.Similarly,the oligodendrocyte-specific protein ermin,also known as juxtanodin,is downregulated in the anterior temporal lobe and upregulated in the dorsolateral PFC in patients with schizophrenia[58,59].Ermin is an F-actin binding protein that is expressed at late stages of oligodendrocyte maturation.It may play a key role in the formation of multiple oligodendroglial processes and the dramatic changes in morphology as these cells acquire the capacity for myelination[84,85].
Septin heteromeric filaments (SEPT2/SEPT4/SEPT7/SEPT8) form at the adaxonal myelin layer and at paranodal loops.These filaments act as molecular scaffolds,mediating axo-glial signaling and compartmentalization of mature myelin.Their loss or deficit has been associated with the formation of myelin outfoldings that impair the rapid propagation of nerve impulses[25,86,87].The four septins involved in these filaments are differentially expressed in proteomic analyses of postmortem schizophrenia brain samples[62,63].
α/β-Spectrin oligomers are important components of the membrane-bound cytoskeleton at the axolemma.At the paranodal and juxtaparanodal regions of the axon beneath a myelin internode,these oligomers interact with proteins such as 4.1B,adducin and AnkB to form a scaffold that mediates the interaction of the cytoplasmic tails of contactins and other axo-glial adhesion molecules with the actin filaments and the actin rings found along the axon.These proteins are also relevant because they are crucial for the paranodal-nodal-paranodal cytoskeleton,which is a specific arrangement of cytoskeletal protein oligomers and polymers underlying the proper assembly and plasticity of the nodes of Ranvier[24].All of these proteins are differentially regulated in schizophrenia postmortem brain samples[58,59,62-64].
Neurofilaments are important axonal cytoskeletal components.They belong to the intermediate filament IV category,and their composition is heteromeric,with light (NEFL),medium (NEFM) and heavy (NEFH) polypeptides as their main constituents.Internexin (INA) is also a component of these axonal structural filaments.Repelling forces among negatively charged phosphorylated residues on the neurofilaments contribute to the enlargement of axon caliber,e.g.,at internodes (Figure 2).Thus,phosphorylation/dephosphorylation of neurofilament polypeptides is a mechanism that regulates axon caliber,which influences molecular trafficking as well as the speed of nerve impulse conduction.Proteomic studies have found that the three neurofilament polypeptides NEFL,NEFM,and NEFH,as well as INA,are differentially regulated in postmortem brain samples from schizophrenia patients[57,61-64].

Figure 2 Main cytoskeletal components of the myelinated axon.
As expected,the actin and tubulin monomeric components of microfilaments and microtubules,respectively,as well as various microtubule-associated proteins,are altered in schizophrenia brain samples[58,61-63].The cytoskeleton mediates the essential functions of every cell in the organism.In the axo-myelin functional unit[88],the cytoskeleton is crucial for the following:Oligodendrogenesis;myelin formation,turnover and plasticity;assembly and remodeling of axonal specialized domains,such as the axon initial segment and nodes of Ranvier;myelin and axonal compartmentalization;anchorage for cell adhesion molecules involved in axo-glial junctions;and scaffolds for molecules involved in signal transduction.
As most of the myelin structural proteins are affected by schizophrenia and most of the cytoskeletal components are dysregulated,it is plausible to infer that overall dysfunction of the axo-myelin unit may underlie the compromised integrity of gray and WM and thus the functional disconnection observed in schizophrenia.
Metabolic dysfunction in schizophrenia has been suggested by positron emission tomography (PET)and magnetic resonance imaging (MRI),and mitochondrial alterations have been documented as mentioned above.At the proteomic level,dysregulation in the expression of enzymes involved in energy metabolism and the antioxidant system has been observed.For example,Martins-de-Souzaet al[59,89] found alterations in proteins involved in glycolysis (fructose-bisphosphate aldolase C and phosphoglycerate kinase 1),the Krebs cycle (citrate hydrolyase),the malate-aspartate shuttle (cytosolic malate dehydrogenase) and oxidative phosphorylation (mitochondrial ATP synthase F1 and F0 complexes) in postmortem dorsolateral PFC samples from schizophrenia patients[59,89].In addition,four subunits of mitochondrial respiratory complex I (NADH dehydrogenase [ubiquinone] (NDU)flavoprotein 2 (NDUFV2),iron-sulfur protein 3 (NDUFS3) and 6 (NDUFS6),and 1 beta subcomplex subunit 5 (NDUFB5)) are downregulated in the anterior temporal lobe of schizophrenia patients[58,89].
Increased amounts of oxidative reactive species are produced under high energy demand or mitochondrial dysfunction,as is suggested to occur in schizophrenia.Therefore,antioxidant enzymatic systems in schizophrenia are expected to be upregulated to counteract oxidative damage.However,the expression levels of three members of the glutathione transferase (GST) family (GSTM3,GSTTLp28,and GSTP1),carbonyl reductase 1 (CBR1),carbonyl reductase 3 (CBR3) and quinoid dihydropteridine reductase (QDPR),are reduced in the thalamus and PFC of schizophrenia patients (reviewed in[89]).As these results were obtained from brain homogenates,an interesting follow-up would be to assess whether these metabolic and redox alterations are present in oligodendrocytes.For this purpose,enriched cultures of patient-derived oligodendrocytes differentiated from induced pluripotential stem cells (iPSCs) and/or cocultures of these induced oligodendrocytes with neurons and other glial cells could be usefulin vitrotools for studying alterations in the oligodendroglial lineage in schizophrenia.
MYELlN ALTERATlONS AND OLlGODENDROGLlAL DYSFUNCTlON EVlDENCE lN POSTMORTEM SAMPLES OBTAlNED FROM BlPOLAR DlSORDER PATlENTS
Uranovaet al[51] also analyzed samples from patients with bipolar disorder,examining BA9 of the PFC,layers III and VI and the adjacent WM,BA10,the caudate nucleus and the anterior putamen.A stereological approach with Nissl-stained samples revealed a significant reduction in the numerical density of oligodendrocytes in the caudate nucleus and in the gray matter layers of BA9[32,37,51].In the adjacent WM,they found no difference between bipolar disorder samples and samples from control subjects[51].The number of oligodendrocyte clusters was also significantly reduced in the caudate nucleus[37] and in the anterior putamen,but the latter difference was observed only in male subjects[40].Electron microscopy analysis of the samples showed ultrastructural signs of apoptosis and necrosis of oligodendrocytes[21].
Oligodendrocyte numbers were significantly reduced in the thalamic anterior principal and centromedian nuclei,in postmortem samples from bipolar disorder patients with a clinical history of psychotic episodes[44].The age-related increase in oligodendrocyte number observed in control subjects was attenuated in this group of patients.The latter effect was also observed by Vostrikov and Uranova[90].Vostrikov and Uranova[90] also found significantly reduced oligodendrocyte densities in samples from BA9 Layer VI from bipolar disorder patients younger than 50 years old compared with those from corresponding age-matched controls[90].Hayashiet al[91] found a significant reduction in OLIG2(+)-cells using a flow cytometry approach in unfixed postmortem gray matter BA10 samples from bipolar disorder patients,which suggests an overall deficit in the oligodendroglial lineage.S100B(+)-oligodendrocyte density was decreased in the left alveus of the hippocampus from bipolar disorder patients[92].In contrast,Hercheret al[23] found increased oligodendrocyte density and CNP protein levels in BA9-adjacent WM in bipolar disorder patients compared with control samples.A further study also showed an increase in oligodendrocyte density along with deficits in axonal markers in prefrontal WM in bipolar disorder patients[93].In a systematic review of postmortem brain studies in bipolar disorder,Gigaseet al[94] found no difference in either neurons or glial cells and suggested that findings from existing studies should be validated.
Significantly less intense myelin staining of the deep prefrontal WM was shown in bipolar disorder patients than in control subjects[95].Additionally,MBP immunostaining revealed decreased myelination of the hippocampal formation in female bipolar disorder patients than a corresponding sexmatched control group[96].In contrast,male patients showed increased MBP staining in the superior medullary lamina,which suggests sex differences in myelin alterations[96].To the best of our knowledge,no ultrastructural analysis of myelinated fibers has been conducted on bipolar disorder postmortem samples.
Perineuronal oligodendrocytes are located in the cerebral gray matter in close proximity to neuronal perikarya and less frequently near dendrites and neurites.Although their morphology is indistinguishable from that of other oligodendrocytes,it remains unknown whether perineuronal oligodendrocytes have a similar or different cell signature from that of typical myelinating oligodendroglial cells[97].Bipolar disorder patients showed cytochemical abnormalities of prefrontal perineuronal oligodendrocytes,correlating with cytochemical alterations of calbindin-containing GABAergic neurons and changes in gene expression levels[98].
MYELlN SUBCELLULAR/BlOCHEMlCAL ALTERATlONS lN POSTMORTEM SAMPLES OF PATlENTS WlTH BlPOLAR DlSORDER
Myelin structural proteins MBP,CNP,PLP and MOG were downregulated in postmortem brain samples from bipolar disorder patients[60].Consistent downregulation at the transcriptomic level was reported forMBPandCNP,and at this levelMAG,PLP,CLDN11,MOG,andMOBPwere also downregulated[22].Reduced mRNA levels were also reported forTF[22,53].The gene expression of the oligodendroglial lineage transcription factorsOLIG1,OLIG2andSOX10was downregulated.Additionally,transcript levels ofNG2andGALC,which correspond to markers of OPCs and immature oligodendrocytes,respectively,were significantly lower in bipolar disorder samples than in control samples[22].
Differential expression of cytoskeletal components of the axo-myelin unit has been reported in postmortem brain samples of bipolar disorder patients.In the WM adjacent to BA9,the β-tubulin protein level assessed by Western blot was significantly lower in patient samples than in controls[93].A proteomic approach found that the NEFL level was downregulated in bipolar disorder samples of BA10[60].Similarly,the neurofilament units NEFL and NEFM and INA,α-spectrin (SPTAN1),SEPT11 and tubulin polymerization-promoting protein (TPPP) were downregulated,whereas β-actin (ACBT) and the ARPC5 subunit of the actin-binding Arp2/3 complex were upregulated in hippocampal samples from bipolar disorder patients[99,100].The actin-bundling protein fascin (FASC) was also dysregulated in these samples[99].In samples of the dorsolateral PFC,NEFL,NEFM and INA were consistently downregulated,while α- and β-tubulins as well as SEPT5,SEPT6 and SEPT11 were upregulated[67].
As in schizophrenia,alterations in metabolic and redox pathways have been described for bipolar disorder.Studies using magnetic resonance spectroscopy have found a reduction in phosphocreatine and ATP in the frontal lobes and basal ganglia,while an increase in lactate levels was reported in postmortem gray matter samples from bipolar disorder patients.In addition,mitochondrial structure is altered,and mutations or polymorphisms in mitochondrial DNA associated with the respiratory chain have been reported[101].Furthermore,high levels of lipid peroxidation,nitric oxide concentration,and DNA and RNA oxidative damage were found in patient samples[102].There is evidence of dysfunctional attachment of the hexokinase 1 protein to the outer mitochondrial membrane in patient samples,which results in abnormal generation of mitochondrial reactive oxygen species and cellular oxidative stress[103].Additionally,impairment of redox modulation pathways in the frontal cortex is found in bipolar disorder patients[104].The antioxidant molecule glutathione has been reported at low concentrations in some brain regions and could contribute to oxidative stress[105,106];however,some patients present a significant increase in this molecule in the anterior cingulate cortex[107].These apparently contradictory results could reflect differential redox regulation or antioxidant capacity in diverse brain regions.
Due to their high metabolic rate and high lipid content in myelin-forming membranes,oligodendrocytes are especially vulnerable to oxidative stress.Therefore,a microenvironment prone to the generation of high amounts of oxidative molecules and an impaired antioxidant capacity,which seems to be characteristic of patients with schizophrenia or bipolar disorder,would certainly contribute to the dysfunction of the axo-myelin unit and subsequently impact the proper conduction of nerve impulses.
COMPARlSON OF ALTERED FEATURES OF OLlGODENDROCYTES AND MYELlN lN SCHlZOPHRENlA AND BlPOLAR DlSORDER
Several features are similarly altered by schizophrenia and bipolar disorder at the cellular level.Ultrastructural studies have revealed signs of oligodendrocyte apoptosis and necrosis[21],oligodendrocyte numerical density was significantly reduced in the caudate nucleus[37] and in BA9 gray matter layers of the PFC[32,51],and significantly fewer oligodendrocyte clusters were found in the caudate nucleus[37] and the anterior putamen[40].In BA9-adjacent WM,a stereological analysis found no differences in oligodendrocyte numerical density in either schizophrenia or bipolar disorder patients compared to that of the control samples[51].However,in schizophrenia,studies have reported decreased oligodendrocyte numerical density in the BA9- and BA10-adjacent WM[33,108],a significant reduction of OLIG2(+)-cells in the former[54],and a significant decrease in both total and CNP(+)-oligodendrocytes in the WM of the superior frontal gyrus[41,42].Additionally,significantly fewer ADAM12(+)-oligodendrocytes were found in the anterior cingulate WM[48].In contrast,two different studies reported increased oligodendrocyte density in the prefrontal WM in postmortem samples from bipolar disorder patients[23,93].One of these studies reported a concomitant decrease in axonal markers[93],which may imply axonal degeneration due to demyelination,which is consistent with an increase in oligodendrocytes at early stages of differentiation.In schizophrenia-derived samples,significantly more oligodendrocytes expressing prohibitin were found in the right dorsolateral prefrontal WM[52].Although prohibitin proteins can be found in other cell compartments,such as the nucleus or plasma membrane,their role in the inner mitochondrial membranes is key for modulating cell proliferation or apoptosis and for overall mitochondrial homeostasis[109-112].Therefore,altered oligodendroglial prohibitin expression is consistent with a previous work suggesting dysregulation of the cell cycle in oligodendrocytes in schizophrenia[113].Based on the findings of that work,Katselet al[113] suggested that postmitotic oligodendrocytes may abnormally re-enter the cell cycle,while a significantly increased level of NG2 in the putamen of schizophrenia patients suggested that OPCs failed to exit the cell cycle.Dysregulation of p57Kip2 gene expression in schizophrenia patient samples[113] could also be related to impaired oligodendrocyte maturation,since this protein has been characterized as an oligodendroglial differentiation competence marker[114-116].
At the subcellular level,proteomic analyses have revealed that the four most abundant myelin structural proteins[117] (PLP,MBP,CNP,and MOG) are significantly reduced in schizophrenia and bipolar disorder.At the transcriptomic level,almost all myelin structural proteins,as well as the main oligodendroglial lineage markers and OPC markers,were significantly downregulated.This evidence strongly suggests that the oligodendroglial lineage is compromised at all differentiation stages in these disorders.Moreover,several axonal and oligodendroglial cytoskeletal components and cytoskeletalinteracting proteins are dysregulated in both schizophrenia and bipolar disorder.A deficit of myelin structural and cytoskeletal proteins in the axo-myelin functional unit may compromise myelin formation,compaction,remodeling and its overall integrity and functionality,which may imply a concomitant compromise in the assembly and functioning of the nodes of Ranvier and other axonal functional rearrangements.If nervous impulses are not properly conducted in terms of speed and precise timing,some connections would not be reinforced and could be lost,influencing the local connectome.At the macroscale connectomic level,which comprises long-range tracts,fine-tuning and synchronization of nervous impulse conduction is crucial,and even subtle alterations of myelin structural and functional features may have a detrimental impact on information processing and thus on cognitive functions and behavior.
These findings suggest that altered myelination,loss of oligodendrocytes and compromised energy and redox metabolism in oligodendrocytes of schizophrenia and bipolar disorder patients could correlate with the WM alterations observed by neuroimaging techniques.These mechanisms could explain,at least partially,the clinical manifestations observed in schizophrenia and bipolar disorder patients.The relationship between myelin and oligodendrocytes,WM and symptom domains can be systematically studied.In the following sections,we will address the evidence from imaging studies on dysfunctions in the nervous tracts and how the main symptoms correlate with these alterations,giving rise to the hypothesis of disconnection in mental disorders.
EVlDENCE OF WM ALTERATlONS lN SCHlZOPHRENlA AND BlPOLAR DlSORDER lN BRAlN lMAGlNG STUDlES
Structural and functional neuroimaging findings provide evidence of connectivity alterations that might be related to myelin dysfunction;the most extensive evidence comes from MRI studies[118].In the field of structural magnetic resonance imaging (sMRI),WM volume and density have been measured using techniques such as voxel-based morphometry (VBM)[119].VBM studies have found diminished WM volume and density in several brain regions of patients with schizophrenia,with main decreases in the frontal and temporal regions.A meta-analysis of VBM studies reported decreased WM in 150 foci.The affected tracts included the corpus callosum,internal capsule,fornix,anterior commissure,and an additional sixteen tracts[120].
Additionally,WM alterations have been detected by diffusion tensor imaging (DTI);this method evaluates subtle changes in WM,determining fractional anisotropy (FA).FA expresses the diffusion of water molecules along neural fibers.Water movement is inhibited when myelin sheaths are thick and well preserved (FA=1);in contrast,water moves easily along fibers in any direction when the myelin sheath is damaged (FA=0)[118].Although FA can indeed reflect changes in myelination,it could also reflect other tract properties,such as axonal ordering and axonal density[121].Therefore,Joneset al[121]urge caution when interpretating DTI-based measurements and not assume that they are direct indicators of WM integrity[121].Bearing this in mind,DTI studies have reported that patients with schizophrenia have a widespread decrease in FA[122].For instance,the ENIGMA-Schizophrenia DTI group analyzed 4321 individuals and found widespread FA reductions in 20 of the 25 analyzed regions in schizophrenia patients when compared with those of the controls[123].In addition,at least three meta-analyses have reported reduced FA in schizophrenia patients;they conclude that the tracts more frequently affected in these patients are the anterior corona radiata,the corpus callosum,the cingulate bundle,and the uncinate and arcuate fascicles[120,123,124] (Figure 3).Furthermore,functional MRI(fMRI) studies have also reported connectivity alterations in several circuits connecting frontal,limbic,temporal,and parietal regions in schizophrenia subjects,as well as alterations to the default network[125,126].

Figure 3 White matter alterations in schizophrenia.
As the evidence is extensive and complex,we will discuss the neuroimaging evidence of WM alterations in schizophrenia based on each of its main clinical domains.This will integrate the findings and highlight the importance of WM.The structural and functional WM alterations associated with the psychotic domain of schizophrenia include tracts and circuits that connect the frontal,temporal,and parietal cortexes[127].For instance,the arcuate fasciculus (AF) is frequently studied in regard to the psychotic domain;the AF connects temporal and parietal regions with the frontal lobe and is considered the main language processing tract of the brain because it connects Wernicke’s and Broca’s areas[128].DTI studies on schizophrenia patients have reported diminished FA on the AF when compared with that of controls[123].Additionally,some studies have related the decrease in AF integrity with thought disturbances,language alterations,and auditory hallucinations[129-131].
Furthermore,psychotic symptoms are related to functional connectivity alterations in the frontostriatal,frontotemporal,and frontoparietal circuits[132].The frontostriatal circuit comprises the connections between the PFC and basal ganglia[128].These regions have been extensively studied in relation to the dopaminergic hypothesis of schizophrenia[133].Some studies of resting-state fMRI analysis have indicated functional dysconnectivity between the dorsolateral PFC and basal ganglia in patients with schizophrenia,which is related not only to psychotic symptoms but also to cognitive alterations[134].In contrast,the frontotemporal circuit comprises connections from the PFC to temporal structures,including the auditory cortex and Wernicke’s area[128].Functional connectivity alterations in this circuit have been related to auditory hallucinations and the perceived reality of those hallucinations[134].
Alterations to the cingulum bundle,fornix,and inferior fronto-occipital fascicle are related to cognitive symptoms of schizophrenia[127,135].The cingulum bundle is a major connector between limbic,paralimbic,and neocortical structures,including the dorsolateral PFC,amygdala,paralimbic gyrus,and cingulate gyrus.This tract is implicated in self-monitoring,spatial orientation,and memory[128].Subjects with schizophrenia have lower FA on the cingulum bundle than controls,which has also been linked with executive dysfunction and impaired working memory in these same patients[136,137].The fornix is another WM structure implicated in cognitive function;this tract connects the hippocampus with other cortical structures and is implicated in memory and spatial learning[128].Patients with schizophrenia have compromised fornix integrity and disrupted functional connectivity between the PFC and the hippocampus[138,139].Further analysis of functional connectivity has provided evidence of alterations in the frontostriatal and frontoparietal circuits that are also related to cognitive dysfunctions in schizophrenia subjects[132].
Connectivity alterations have been associated with altered tract integrity of the uncinate fascicle;this tract connects the orbitofrontal and anterior dorsolateral cortex with the temporal lobe and is related to negative symptoms[128].At least two studies have demonstrated an association between low FA of this tract and flattened affect and lack of social engagement[140,141].As WM decline can be a consequence of demyelination,all of these neuroimaging results (that report WM reduction in important tracts underlying highly integrative brain functions) support the hypothesis that demyelination may be a key factor in explaining,at least in part,the symptoms of schizophrenia.
There are also extensive data on gray and WM changes that are associated with clinical characteristics,genetics,functional impairment,and treatment response for bipolar disorder[142,143].Currently,one of the main hypotheses about the neurobiology of this disease centers on the disconnection of prefrontal-subcortical networks and limbic structures associated with mood regulation[144].Diverse prefrontal-striatal-thalamic circuits that regulate the expression of sensorial,cognitive,and emotional data from cortical regions are altered in bipolar disorder patients.It is believed that the dysfunction of these networks explains the cognitive,behavioral,and affective manifestations of this disorder[145].The current fronto-limbic circuit disconnection model highlights the importance of WM in bipolar disorder.Evidence of WM alterations can be provided through structural or functional findings from neuroimaging techniques,with the most extensive evidence coming from MRI studies.
White matter hyperintensities (WMHs) are evident bright areas on T2 MRI sequences.These alterations are one of the most replicated findings in bipolar disorder[144].WMHs are lesions that are associated with vascular anomalies and neurodegenerative processes,such as demyelination,axon loss,or necrosis[146].These lesions are frequently found around the lateral ventricles (periventricular),deep WM,and subcortical gray matter (basal ganglia,thalamus)[147,148].At least three meta-analyses have linked the presence of WMHs with bipolar disorder,and it is estimated that approximately 39% of bipolar disorder patients have these lesions,compared with 18% of controls[149-151].The presence of WMHs in patients has been associated with the worst outcomes of the disease,such as hospitalizations,psychotic symptoms,suicide attempts,cognitive impairment,and treatment resistance[151-155].
In addition to WMH,there is also extensive evidence about WM volume alterations from different methodologies[156].Two meta-analyses that used a region-of-interest (ROI) approach reported a volume reduction in the corpus callosum of bipolar disorder patients,which is a structure of crucial importance for interhemispheric connectivity and is implicated in higher cognitive functions such as attention,memory,and language[124,156,157].However,no clear association was found between altered corpus callosum volumes and psychotic symptoms or suicidal ideation in patients[157-159].In contrast,Lavagninoet al[160] reported an association between volume reduction of the posterior corpus callosum and a higher number of affective episodes,hospitalizations,and incomplete remission of symptoms in female patients[160].Other studies and meta-analyses used VBM to evaluate the whole brain and reported a reduction in WM volume of the corpus callosum,corona radiata,posterior cingulum,and inferior longitudinal fasciculus in bipolar disorder[142,161] (Figure 4).
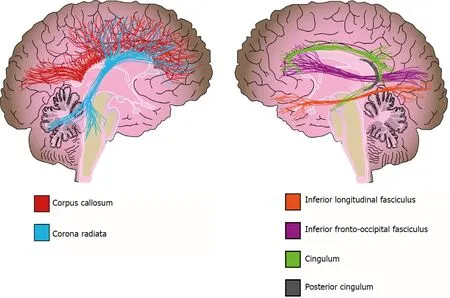
Figure 4 White matter alterations in bipolar disorder.
DTI studies of bipolar disorder have reported diffuse WM microstructural alterations[124,162,163],which are evident when tract integrity and WM volume are measured.Recent meta-analyses have found FA reductions in all major classes of WM tracts (commissural,association and projection fibers)with frequent reports of alterations in temporoparietal WM,the inferior fronto-occipital fasciculus,inferior longitudinal fasciculus,and left cingulum[124,164].A mega- and meta-analysis of the ENIGMA group revealed decreased FA in 29 ROIs,with the greatest effect sizes in the corpus callosum and cingulum of patients compared with those of controls[162].Voxel-based analysis of DTI (VBA-DTI) data has also found clusters of decreased FA and WM volume in prefrontal,temporal and parietal regions[164-166].Emsellet al[165] conducted a study on euthymic bipolar disorder patients and found a cluster extending from the prefrontal WM to the splenium of the corpus callosum and posterior cingulum bundle[165],whereas a VBA-DTI meta-analysis reported another two clusters in areas involved in emotional processing[164].Nortje’s meta-analysis identified a large cluster of decreased FA and mean diffusivity in the right temporoparietal WM,a region that is crossed by the inferior longitudinal fasciculus and inferior fronto-occipital fasciculus[164].The evidence suggests that the posterior WM contributes to cognitive deficits,while the alterations of anterior fibers are associated with affective symptoms of bipolar disorder[164,167].In conclusion,the previously discussed evidence suggests not only alterations to fronto-limbic connectivity but also dysfunction in parietal,fronto-occipital and interhemispheric connections,which may explain the cognitive and emotional manifestations of this disorder[167].
THE DlSCONNECTlON PARADlGM AND WM DYSFUNCTlON lN SCHlZOPHRENlA AND BlPOLAR DlSORDER
Taken together,the evidence discussed above highlights the importance of oligodendroglial cells for brain function;through myelin formation,they are involved in the precise synchronization of electrical impulses that propagate along nerve fibers connecting brain structures[168-170].Most long-distance connecting tracts in the CNS are heavily myelinated and comprise the WM.Although structural and functional WM alterations have been described in other mental illnesses,such as major depression,obsessive-compulsive disorder,attention-deficit/hyperactivity disorder,autism spectrum disorders,Alzheimer’s disease,and drug addiction[16,171],in this review,we focused on schizophrenia and bipolar disorder because these two neuropsychiatric illnesses share several clinical and pathophysiological features.
As can be inferred from the previously mentioned findings,the focus of investigations on the pathophysiology of schizophrenia and bipolar disorder has changed from alterations in specific regions to dysfunction in the connectivity of brain structures.This shift occurred first for schizophrenia,when the disconnection hypothesis was postulated more than twenty years ago,in response to the fact that several manifestations of schizophrenia,such as negative symptoms,cannot be fully explained by structural alterations to a specific cortical area[172-174].Researchers subsequently hypothesized that the clinical domains of schizophrenia might be due to widespread network dysfunction instead of only specific morphological alterations of specialized cortical regions[125].This paradigm shift in schizophrenia research quickly translated to other psychiatric conditions,and many studies have since tested the disconnection hypothesis in bipolar disorder[153].Functional MRI and DTI studies have reported an association between compromised WM integrity and clinical manifestations of these disorders[126,167].
In the following years,many neuroimaging studies have associated WM alterations found in psychiatric patients with executive function,functional impairment,affective symptoms,treatment response or resistance,suicidal thoughts and attempts,and the severity of symptoms,to name only a few traits[123,145,161,175].This overwhelming evidence has helped researchers to frame schizophrenia and bipolar disorder as multidimensional conditions with strong brain correlates at the macroscale connectomic level[125].Undoubtedly,further research from a neuroglial integrative perspective is necessary to unravel the anomalies at the cellular/subcellular level,i.e.,the microscale connectomic level that may underlie the complex clinical manifestations of these patients.
PERSPECTlVES:MYELlNATlON lS NOT AN EXCLUSlVE OLlGODENDROGLlALNEURONAL RELATlONSHlP
The axo-myelin interaction is so close that,by itself,it constitutes a functional unit with a complex and deeply intermingled physiology.However,both astrocytes and microglia interact with axo-myelin units and influence their function[176-179].Metabolic homeostasis andde novoformation or plasticity of myelin internodes and nodes of Ranvier are modulated by astrocytes and microglia.The main glialmediated modulatory mechanisms of myelin homeostasis include physical intercellular interactions through gap junctions,secretion of soluble factors and clearance of myelin debris.Dysregulation of these modulatory mechanisms may also underlie the pathophysiology of mental illnesses such as schizophrenia and bipolar disorder;however,scientific research on this topic is still limited.
During the last two decades,great advances have been made in our understanding of human CNS physiology and pathophysiology,and glial cells have been recognized as key players in neuropsychiatric disorders[15,180-182].Nevertheless,scientific psychiatry and patients with mental disorders would definitely benefit from a more integrative point of view at all research levels.
CONCLUSlON
Schizophrenia and bipolar disorder are multifactorial neuropsychiatric illnesses that share clinical manifestations and alterations to brain structure and function,genetic characteristics,and neurobiological pathways.Both are chronic and severe conditions that cause disability,reduce lifespan and impose a high burden on patients and society.The disconnection hypothesis of the pathophysiology of these two disorders is supported by alterations in WM tracts revealed by neuroimaging techniques.Alterations at the macroscale connectome level strongly correlated with the multidimensional clinical manifestations of these disorders;however,to better understand the correlates at the cellular and subcellular levels,it is necessary to obtain deeper insight into the main components of WM,i.e.,myelinated axons.Therefore,the pathophysiology of both the neuronal and oligodendroglial components of neural circuits and networks needs to be investigated.Twenty years since the first hypothesis implying oligodendrocyte/myelin failure as a hallmark of schizophrenia[183],a large amount of evidence at the connectomic,microscopic,proteomic,transcriptomic and genomic levels has accumulated for overall dysfunction of the axo-myelin functional unit in these patients.Although oligodendrocyte/myelin dysfunction has also been consistently reported in bipolar disorder,the same amount of scientific knowledge about axo-myelin pathophysiology in this psychiatric disorder is lacking,at least at the cellular and subcellular levels.Further research on schizophrenia and bipolar disorder is needed to better understand the axo-myelin molecular pathways that are dysregulated and to identify potential targets for the development of novel therapeutic alternatives.Several recent studies have focused on the effects of commonly prescribed antipsychotic drugs on oligodendrocytes/myelin[184].However,testing the effects of novel compounds intended to induce oligodendrogenesis and(re)myelination[116] in preclinical models of schizophrenia and bipolar disorder could also hold great promise for future research.
FOOTNOTES
Author contributions:Valdés-Tovar M contributed to the overall conception and design of the study;all authors carried out comprehensive literature search and wrote the first draft;Rodríguez-Ramírez AM contributed to the clinical perspective and figure design;Sotelo-Ramírez CE contributed to figure creation;Solís-Chagoyán H critically revised the manuscript;Valdés-Tovar M and Camarena B obtained funding;all authors assisted in a thorough revision of the manuscript and approved its final version.
Supported byFondo Sectorial de Investigación para la Educación (FSIE SEP/CONACyT) to MV-T,No.287115;and Fondo Sectorial de Investigación en Salud y Seguridad Social (FOSISS SS/IMSS/ISSSTE-CONACyT) to BC,No.261459.
Conflict-of-interest statement:Dr.Valdés-Tovar has received research funding from Fondo Sectorial de Investigación para la Educación (FSIE),SEP-CONACyT and Dr.Camarena has received research funding from Fondo Sectorial de Investigación en Salud y Seguridad Social (FOSISS),SS/IMSS/ISSSTE-CONACyT,during the conduct of the study.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See:https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Mexico
ORClD number:Marcela Valdés-Tovar 0000-0002-5540-7321;Alejandra Monserrat Rodríguez-Ramírez 0000-0002-9248-0472;Leslye Rodríguez-Cárdenas 0000-0003-2456-3353;Carlo E Sotelo-Ramírez 0000-0001-9924-1434;Beatriz Camarena 0000-0001-7737-501X;Marco Antonio Sanabrais-Jiménez 0000-0002-7722-5940;Héctor Solís-Chagoyán 0000-0003-0692-6931;Jesús Argueta 0000-0003-3668-1066;Germán Octavio López-Riquelme 0000-0002-8031-4522.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
杂志排行
World Journal of Psychiatry的其它文章
- Screening dementia and predicting high dementia risk groups using machine learning
- Prenatal nicotine alters development of the laterodorsal tegmentum:Possible role for attention-deficit/hyperactivity disorder and drug dependence
- Drug-induced stuttering:A comprehensive literature review
- Common outcome,different pathways:Social informationprocessing deficits in autism spectrum disorder and attentiondeficit/hyperactivity disorder
- Associated mortality risk of atypical antipsychotic medication in individuals with dementia
- Reduced paraoxonase 1 activities may explain the comorbidities between temporal lobe epilepsy and depression,anxiety and psychosis
