High-elevation Adaptation of Motion Visual Display Modifications in the Toad-Headed Agamid Lizards (Phrynocephalus)
2022-03-26QiaohanHUYusongLINXiaQIUJinzhongFUandYinQI
Qiaohan HU ,Yusong LIN ,Xia QIU ,Jinzhong FU,3 and Yin QI*
1 Chengdu Institute of Biology,Chinese Academy of Sciences,Chengdu 610041,China
2 University of Chinese Academy of Sciences,Beijing 101400,China
3 Departments of Integrative Biology,University of Guelph,Guelph,Ontario N1G 2W1,Canada
Abstract Understanding the process of adaptation is a key mission in modern evolutionary biology.Animals living at high elevations face challenges in energy metabolism due to several environmental constraints (e.g.,oxygen supply,food availability,and movement time).Animal behavioral processes are intimately related to energy metabolism,and therefore,behavioral modifications are expected to be an important mechanism for high-elevation adaptation.We tested this behavioral adaptation hypothesis using variations of motion visual displays in toad-headed agamid lizards of the genus Phrynocephalus.We predicted that complexity of visual motion displays would decrease with the increase of elevation,because motion visual displays are energetically costly.Displays of 12 Phrynocephalus species were collected with elevations ranging from sea level to 4600 m.We quantified display complexity using the number of display components,display duration,pathways of display components,as well as display speed for each species.Association between display complexity and elevation was analyzed using the phylogenetic generalized least squares (PGLS) model.We found that both the number of display components and the average value of tail coil speed were negatively correlated with elevation,suggesting that toad-headed lizards living at high-elevation areas reduced their display complexity to cope with the environmental constraints.Our research provides direct evidence for high-elevation adaptation from a behavioral aspect and illustrates the potential impacts of environment heterogeneity on motion visual display diversification.
Keywords high-elevation adaptation,lizard,motion visual display,Phrynocephalus,signal complexity
1.Introduction
Understanding the process of adaptation is one key mission in modern evolutionary biology and high-elevation adaptation provides a fertile research ground (Cheviron and Brumfield,2012;Storz,2021;Sunet al.,2018;Yanget al.,2012).Environmental constraints at high elevations,including low oxygen partial pressure,low ambient temperature,strong ultraviolet radiation,and great daily and seasonal fluctuations,pose serious challenges to most animals,particularly for ectotherms who are more sensitive to environmental changes (Haoet al.,2019).Nevertheless,many species inhabit highelevation environments (Myerset al.,2000),and they often cope with these challenges by either suppressing total metabolism and oxygen demand,or increasing oxygen delivery efficiency and energy conversion (Liet al.,2018;Ramirezet al.,2007;Storzet al.,2010;Zhaoet al.,2020).Animal behavioral processes have an intimate relationship with energy metabolism (Biro and Stamps,2010;Kotiahoet al.,1998;Mowles,2014;Roset al.,2006;Suet al.,2020),and animals at high-elevation areas likely adjust their behavior to adapt the environment by either constraining the activity intensity or shortening the activity time (Wuet al.,2018;Zhuet al.,2020).For example,Anolislizards spent less time for activity and chose more open habitats with boulders for thermoregulation at high elevations (Muñoz and Losos,2017).Compared with physiological and genetic adaptation (Beall,2007;Quet al.,2021;Yanget al.,2014;Yanget al.,2016),behavioral adaptation at high-elevation environment has rarely been examined.
As a ritual behavior,social displays transmit specific information during animal communication (Laidre and Johnstone,2013).They play an important role in courtship,intra-sexual competition as well as territory defense,and their functions depend largely on complexity (Ordet al.,2001;Patricelli,2016;Petrieet al.,1991;Wuet al.,2018).A diversity of organisms rely heavily on communication with a variety of different modalities (e.g.,motion visual displays,olfactory,vocal;Endler,1992;Laidre and Johnstone,2013;Nelson and Jackson,2007).Physical movement-based motion visual displays are common in lizards’ communication (Bianet al.,2019;Fleishman and Pallus 2010;Rosenthal,2007),and their complexity varies greatly among closely-related species due to different selection pressures (Nadhurouet al.,2016;Ord and Martins 2006).These motion visual display signals are energetically costly and complex displays often stimulate anaerobic metabolism (Bennettet al.,1981;Biro and Stamps,2010;Clark,2012;Roset al.,2006;Wuet al.,2018;Zhu,2020;Zhuet al.,2021).For example,compared with resting and moving,male wolf spiders (Hygrolycosa rubrofasciata) increase their metabolic rate 22 times during drumming behavior (Kotiahoet al.,1998).However,long anaerobic metabolism produces high concentrations of acid,which can affect blood and muscle pH,disrupt enzyme function and oxygen transport (Bennett,1978).For highelevation dwellers,we would expect them to reduce their motion visual display complexity to cope with the challenging environment and to avoid adverse impact from anaerobic metabolism.
Quantifying display complexity can be difficult,and three methods are often used.The number of distinct displays or components in displaying repertoire is the most classical measurement,and has been widely used in assessment of display complexity inAnolislizards (Decourcy and Jenssen,1994;Greenberg and MacLean,1978;Jenssen,1978;Ord and Martins,2006;).For example,Martinset al.(2004) used the number of head-bobbing displays as an indicator of signal complexity.With advancement of the information theory,a systembased method has recently been used,in which the sequence variation among display components in succession was used as an indicator of display complexity (Fischeret al.,2017;Ord and Martins,2006;Shannon and Weaver,1949).The higher the variation,the higher the signal complexity and the more information it may transmit (Peckreet al.,2019).Furthermore,variations within display components (e.g.,speed,duration) have also been used as indicators of signal complexity (Freeberget al.,2012;Hammerschmidt and Fischer,2008;Vehrencamp,2000).
The toad-headed agamid lizards (genusPhrynocephalus) provide an excellent model system to explore the display behavior adaptation to high-elevation environments.These lizards are widely distributed in a large elevational range from sea level to 4 600 m and display a variety of motion visual signals.For example,both males and females ofP.vlangalii,a high-elevation species,use tail coil and tail lash in territory defense and courtship,and the speed of tail display likely encodes levels of individual threat (Qiet al.,2011;Qiuet al.,2018).Several lowlandPhrynocephalusspecies show more complex displays besides tail coil and tail lash,such as limbs flapping and body turning (Lin,2020).In addition,the athletic ability ofP.vlangaliidepends largely on elevation,and individuals from high altitudes have relatively weak locomotor performance compared with low-elevation individuals (Wuet al.,2018a).Furthermore,our recent studies have shown that speed of tail display inP.vlangaliiis mainly regulated by anaerobic metabolism,and fast display speed is associated with high blood lactate concentration (Zhuet al.,2020;Zhuet al.,2021).
In this study,we explore the relationship between highelevation adaptation and complexity of motion visual display in the genusPhrynocephalus.We collected display data from 12 species and quantified the display complexity using the motion visual signal digitization method (Hedrick,2008;Peterset al.,2016).The association between display complexity and elevation was analyzed using the phylogenetic generalized least squares (PGLS) model.We predict that complexity of motion visual displays would decrease with elevation,because motion visual displays are energetically costly and total metabolism is constrained at high elevations.
2.Materials and methods
2.1.Species and study sitesWe collected motion visual display data from 12Phrynocephalusspecies in the Gobi Desert of northwestern China and the Qinghai-Tibetan Plateau (Figure 1).Lizards in different social context produce different signal,and all displays in this study were under a male-male social context,i.e.,male residents competing with male intruders.Habitats for each species are similar and mostly arid,varied from rock substrate to sand dunes,with sparse and low vegetation.Elevation of study sites varied from 604 m (P.alpherakii) to 4 550 m (P.erythrurus).For two widespread species,P.axillarisandP.versicolor,we examined two populations and their elevations were averaged between the populations.The study site and sampling information is presented in Table 1.
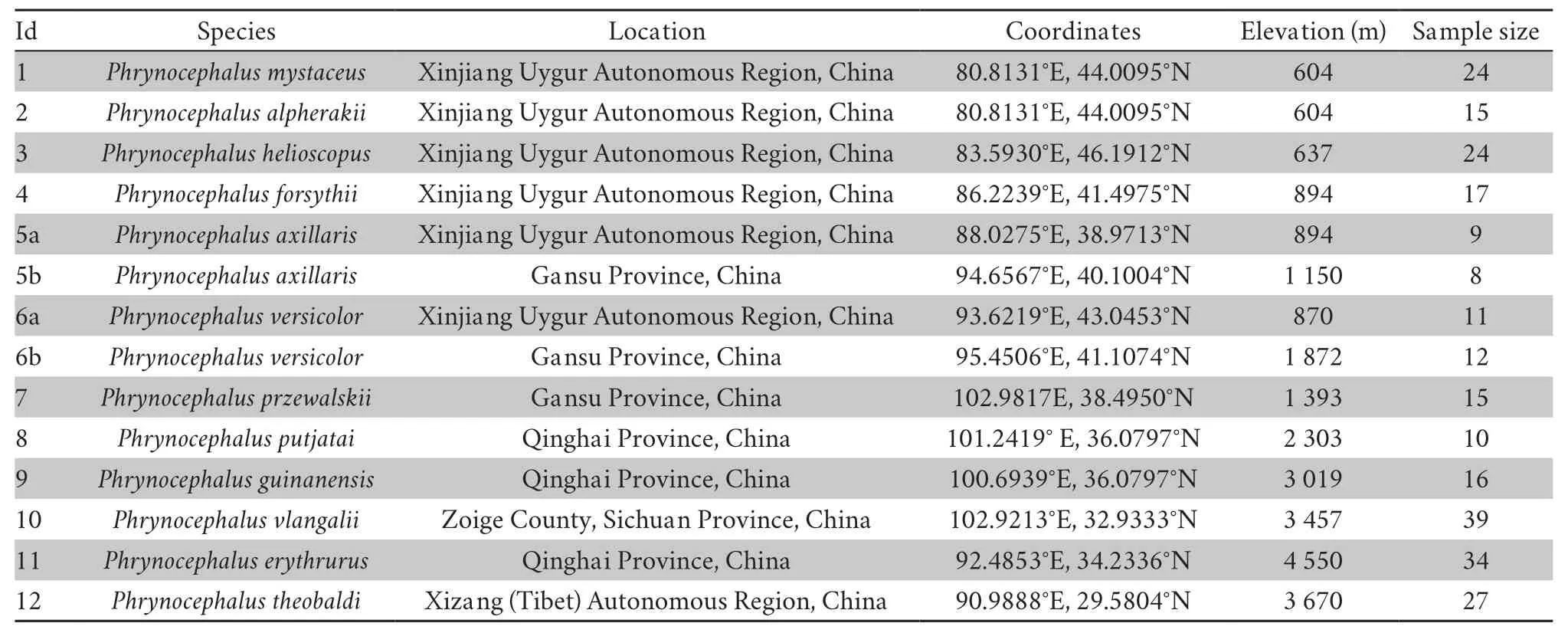
Table 1 Study site and sample size information of the 12 Phrynocephalus species examined in this study.
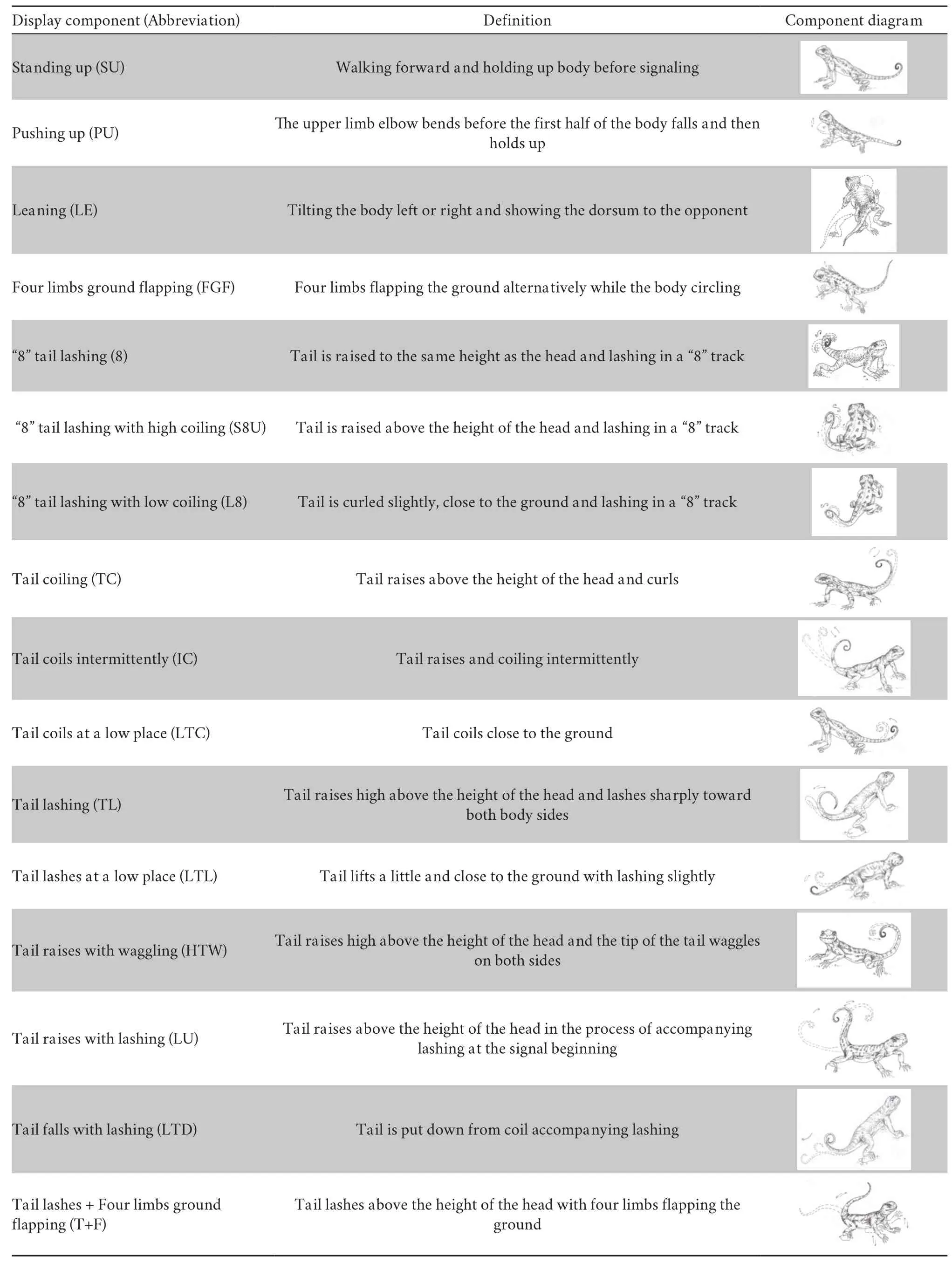
Table 2 Sixteen visual display components of the 12 Phrynocephalus species examined in this study.
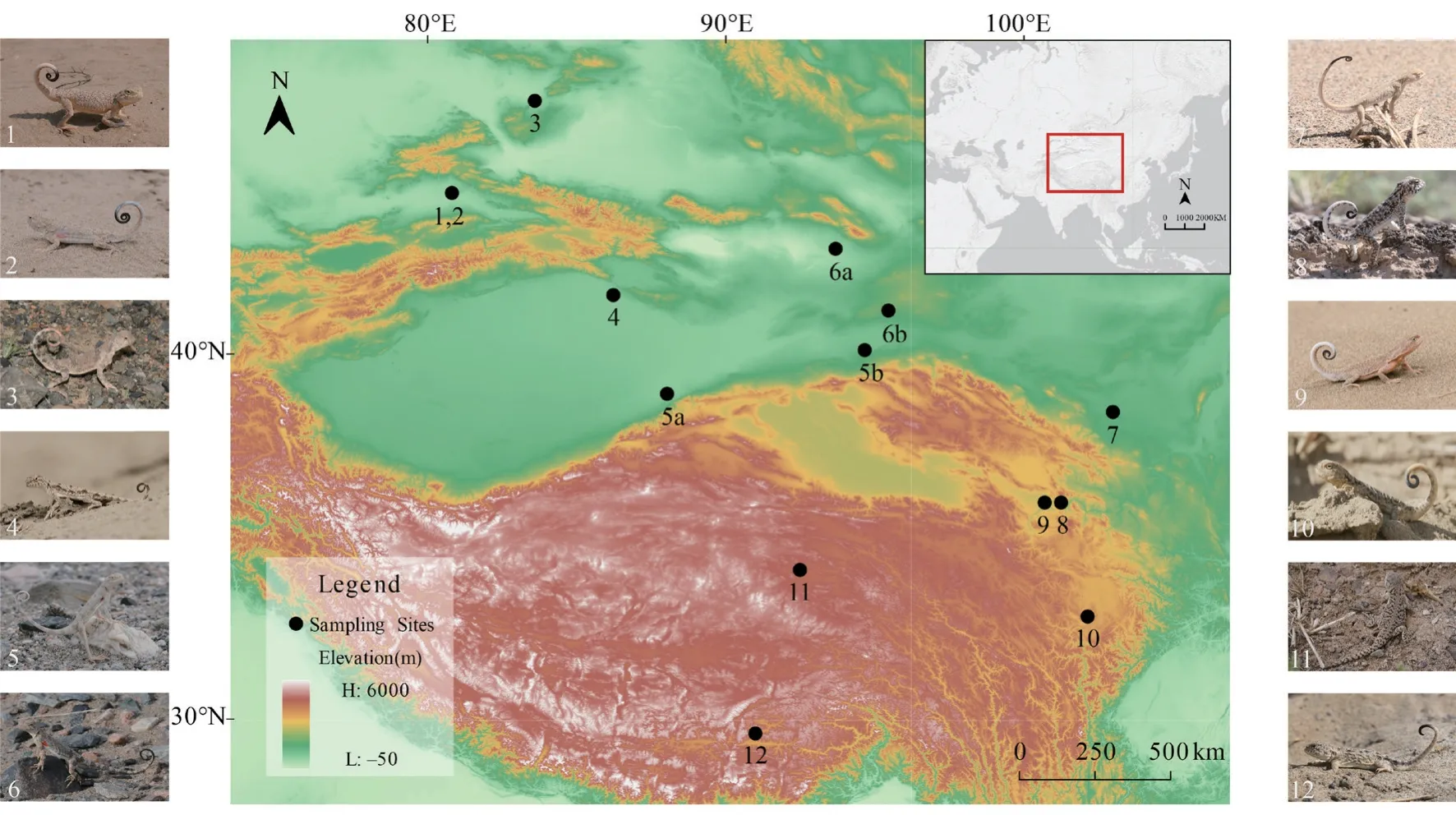
Figure 1 Map of study sites and the image of 12 Phrynocephalus species examined in this study.1.P.mystaceus,elevation 604 m.2.P.alpherakii,elevation 604 m.3.P.helioscopus,elevation 637 m.4.P.forsythii,elevation 894 m.5a.P.axillaris,elevation 894 m.5b.P.axillaris,elevation 1 150 m.6a.P.versicolor,elevation 870 m.6b.P.versicolor,elevation 1 872 m.7.P.przewalskii,elevation 1 393 m.8.P.putjatai,elevation 2 303 m.9.P.guinanensis,elevation 3 019 m.10.P.vlangalii,elevation 3 457 m.11.P.erythrurus,elevation 4 550 m.12.P.theobaldi,elevation 3 670 m.
2.2.Display signal data collectionWe first located and sexed target lizards using binoculars.Once a target was selected,one investigator (QH) set up a video camera (Sony HDR PJ670) at approximately three meters away from the target.After a five minute acclimation period,a second investigator (XQ) introduced an intruder lizard toward the target from four meters away.The intruder was tied with a 30-centimeter-long dental floss around the waist and was tethered with a 4-meter fishing rod,which allowed the lizard to move freely.At the same time,the first investigator started the camera and filmed the motion visual displays of the target lizard.We would end the trial if there was a potential conflict escalation or target did not show displays within two minutes.Immediately after filming,we captured the target lizards and measured their body temperature.To alleviate the effect of individual physiological condition on display complexity,we confined our display trials only during 11 am to 4 pm in sunny days when lizards are most active.To avoid impacts from potential previous social interactions and individual body size,a size-matched intruder collected from a different population was used for each target.After each trial,a ping-pong ball was placed at the exact location of the target and was filmed to serve as a scale in subsequent display digitization.
2.3.Display complexity measurementWe measured display complexity for each species using five parameters:the number of display components,display duration,number of pathways among different components,as well as tail coil and tail lash speed (Ord and Martins,2006;Shannon,1948).The component was defined as specific and repeatable display posture (Ramos and Peters,2017).Some components were shared by several species and others were species-specific,and therefore,the number of components represented an important aspect of display complexity (Freeberget al.,2012;Miller and Osmanski,2009;Vehrencamp,2000).The display duration was defined as the average time sustained by a species-typical display (Hammerschmidt and Fischer,2008;McComb and Semple,2005).For each species,the display sequence was mostly fixed,but individuals sometimes omitted some components under specific social context (Fischeret al.,2017).To establish the species-typical display sequence,we analyzed the motion visual display bout by bout and determined the starting and terminating component respectively for each species.We then calculated the transition probability (probability of one component followed by the other) between different components,and the display sequence was described as the sequence with the highest transition probability among components.The number of pathways between different components was determined using the number of different transition probability.The average speed of tail coil and tail lash were quantified following the methods outlined by Hedrick (2008) and Peterset al.(2016).Briefly,we extracted video footages of tail displays using the program iskysoft (iSkysoft Technology Corp.) and tracked the movement of tail tips in Matlab 2016b (MathWorks Inc.,Natick,MA,USA).The position of the tail tip was located in successive frames to generatex-ycoordinate data over time for each display.Thex-ycoordinates were then converted to millimeter using the ping-pang ball in the image as a scale (Peterset al.,2016;Wuet al.,2018).The Euclidean distance between successive digitized position points provided a vector of speed measurements for the whole sequence.We averaged these across the display sequence to determine the average speed.To reflect the potential difference between components,we calculated the speed for tail coil and tail lash respectively.To account for the effect of orientation of lizard relative to the camera,we categorized each display as either facing towards/away from the camera or at right angles to the camera,and calculated the display speed from different orientation respectively (Bianet al.,2016).
2.4.Data analysisTo examine the association between display complexity and elevation,we tested the relationships of the number of display components,display duration,number of pathways among different components,tail coil speed,and tail lash speed against elevation.To account for the phylogenetic non-independent effect,we used the phylogenetic generalized least-squares (PGLS) model in package“caper”(Ormeet al.,2013).Elevation was treated as the predictor variable,while number of components,display duration,number of pathways among different components,tail coil speed,and tail lash speed were treated as the response variable,respectively.A phylogenetic tree of the 12 study species (Figure 2) was constructed and modified from Solovyevaet al.(2018).We only used the display speed from facing towards/away from the camera to avoid the effect of orientation of lizards.
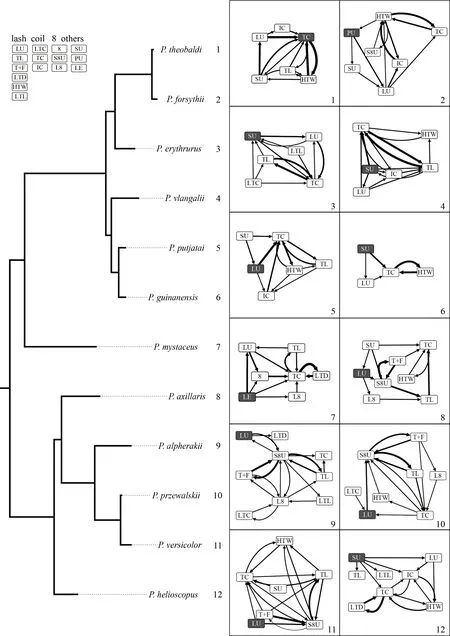
Figure 2 A phylogenetic tree of the 12 Phrynocephalus species examined in this study (modified from Solovyeva et al.,2018),along with schematic diagrams of the component network graph and display components for each species.
To test the phylogenetic effect on display complexity,we also estimated Pagel’sλfor the number of display components,display duration,number of component connections,tail coil speed,and tail lash speed.Pagel’sλwas estimated using the“phytools”package (Lynch,1991;Pagel,1999;Revell,2012),which ranges from 0 to 1,with“0”meaning weak phylogenetic effect and“1”meaning strong phylogenetic effect (Freckletonet al.,2015).Previous studies suggested that Pagel’sλis an effective measurement and typically performs better than other commonly used metrics for discriminating between more complex models of trait evolution (Münkemülleret al.,2012).All statistical analysis were conducted in R 3.6.2 (R Development Core Team,2019).
2.5.Ethical approvalAll applicable international,national,and institutional laws and guidelines for the care and use of animals were strictly followed.All activities were under permission from local conservation authorities and animal handling followed the approved protocols (protocol number 2017005,Chengdu Institute of Biology).
3.Results
3.1.Signal repertoire and species-typical sequenceWe analyzed a total of 261 display bouts from 131 individuals of 12 species.Among all those displays,16 different components were defined,including tail lashing (TL),tail coiling (TC),“8”tail lashing (8),standing up (SU),push-up (PU),leaning (LE),tail lashing with four limbs flapping (T+F),and several others.Table 2 presents a complete list of all components and their definitions.Among them,the first three types (TL,TC,and 8) were basic components,and they might give rise to other components.For example,tail lashing (TL) might appear as tail lashing at a low place (LTL),tail raising with waggling (HTW),tail raising with lashing (LU),or tail falling with lashing (LTD).Tail coiling (TC) might appear as tail coiling intermittently (IC) or tail coiling at a low place (LTC),while“8”tail lashing (8) might appear as“8”tail lashing with high coiling (S8U) or“8”tail lashing with low coiling (L8) (Table S1).
Most species possess their own display repertoire and have a species-typical sequence.We provide a detailed description in Table S2.The index of display complexity,including number of components,display duration,number of pathways,average values for tail coil and tail lash speed are presented in Table 3.
3.2.Correlation between display complexity and elevationWe found significant and strong phylogenetic signal in display duration (λ=0.9918,P=0.0090) and number of display components (λ=0.6000,P=0.0266).The average speed of tail lash had a largeλbut a marginally significantP(λ=0.9711,P=0.0906),which we interpreted as having less strong phylogenetic signal.The average speed of tail coil had a largeλbut insignificantPvalue (λ=0.8356,P=0.9393),which could be due to the small number of species.The number of pathways among display components had a smallλand an insignificantPvalue (λ=0.1872,P=0.4771),suggesting minimum phylogenetic signal for this trait (Figure S1).
For the association between display complexity and elevation,the number of display components (β=-0.0007,P=0.0063,λ=0) and average tail coil speed (β=-0.0026,P=0.0459,λ=1) were negatively correlated with altitudes (Figure 3,Table 4).We found no association of the display duration (β=-0.0016,P=0.1985,λ=1),the number of pathways (β=-0.0008,P=0.2596,λ=0),the tail lash speed (β=-0.0071,P=0.1849,λ=1) with altitudes (Table 4).
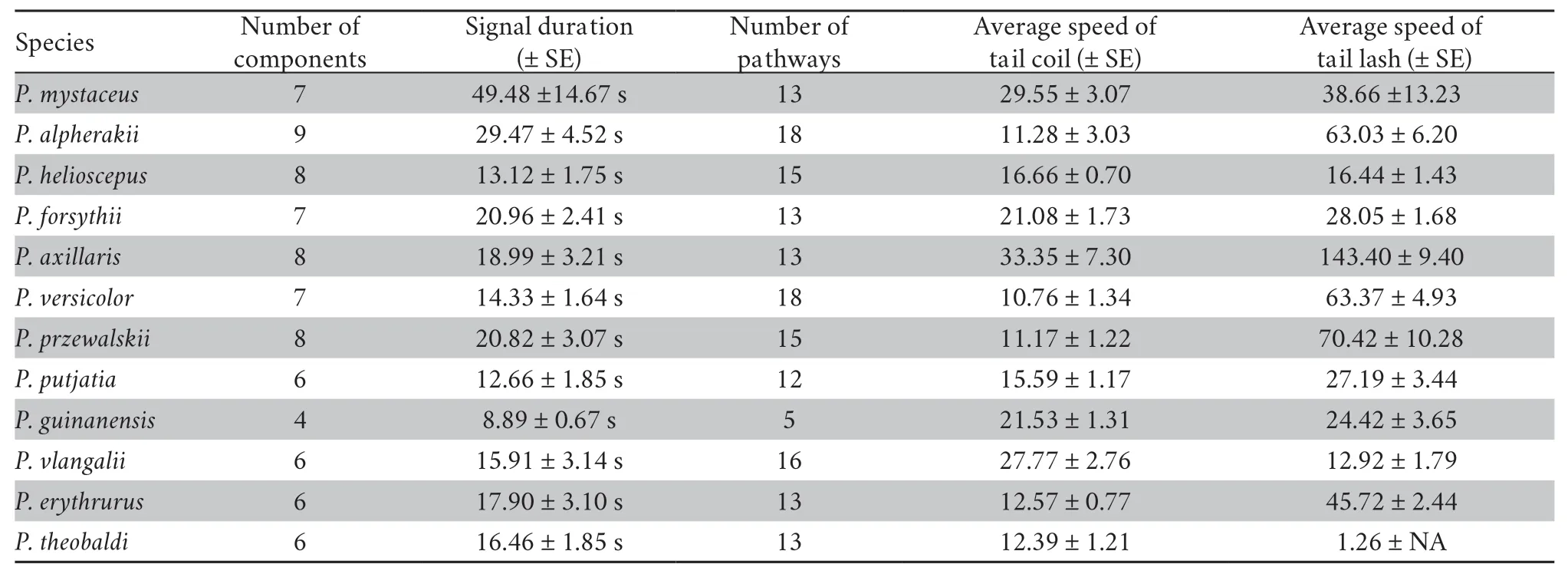
Table 3 Number of display components,average display duration (s),number of pathways among different components,and average values (cm/s) for tail coil speed and tail lash speed for each Phrynocephalus species.
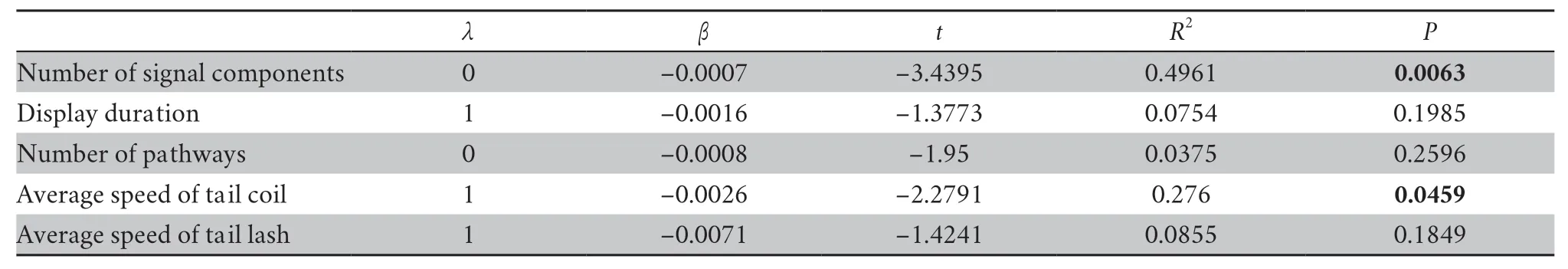
Table 4 The association between display complexity and elevation across 12 Phrynocephalus species.The display complexity was measured using number of display components,display duration,and number of pathways.Significant predictors are marked in bold.
4.Discussion
Our results clearly demonstrated thatPhrynocephalusspecies at high-elevation areas have modified their motion visual display signals.First,species at high elevations appear to have fewer display components in general (Figures 2-3).The negative correlation between elevation and the number of display components is clear and significant.Of all the highelevation species,P.guinanensishas the fewest components,but it is not the highest species.This species has an obvious colorful belly and trunk side for both male and female individuals,which most other species do not have.We postulate that the presence of colorful belly may compensate for the reduction of display components.P.forsythiipresents another interesting case.It is a part of the high-elevation clade but currently has a primary lowland distribution.It has a large number of display components similar to other lowland species.Its phylogenetic position suggests that it originated from a high-elevation ancestor and turned to lowland secondarily (Figure 2).Its large number of display components further supports the association between reduction in display component and high-elevation environment.Second,species at high elevations appear to have slower tail coil speed.Two other parameters,the display duration and tail lash speed,are also lower at high elevations,although the differences are not statistically significant.We suggest these modifications represent adaptations to highelevation environments of these lizards.Other behavioral modifications were also reported in lizards.For example,the horned lizards (Phrynosoma hernandesi) adjust their basking duration to cope with low temperature challenges when transplanted to high-elevation areas (Refsnideret al.,2018),andAnolislizards chose more open habitats with boulders forthermoregulation at high elevations (Muñoz and Losos,2017).
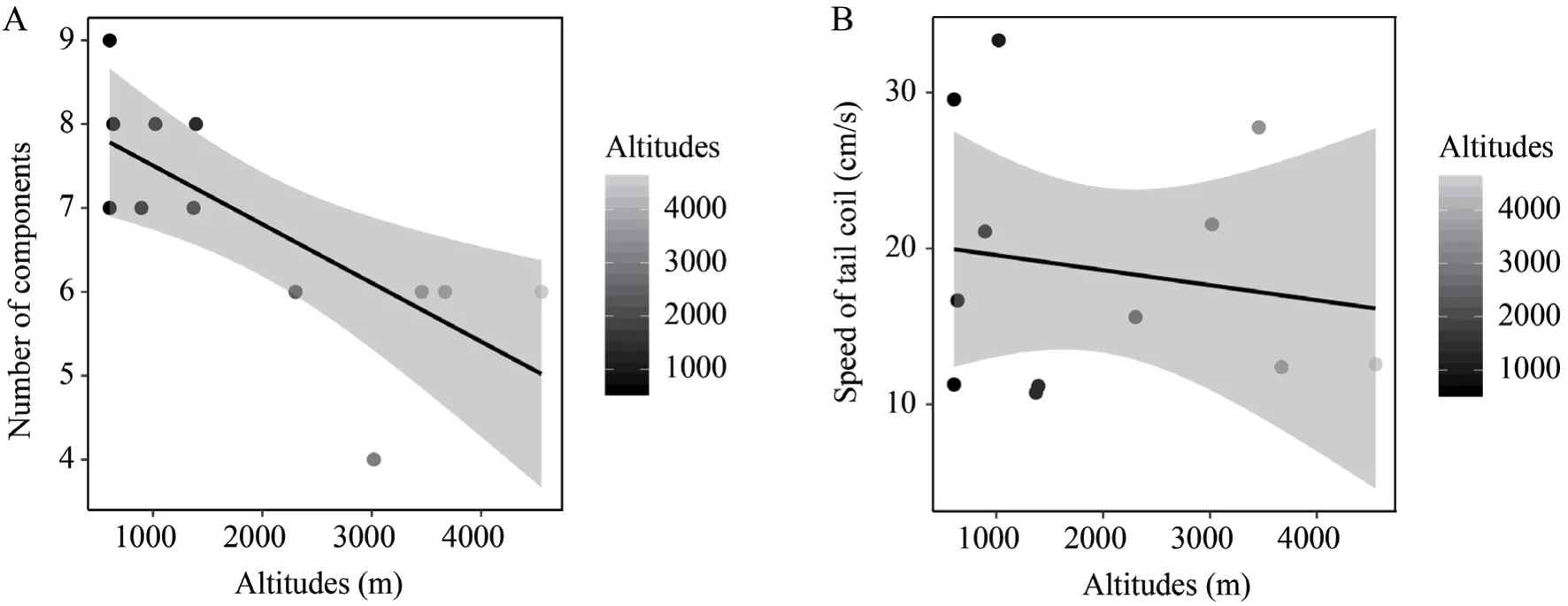
Figure 3 A:Correlation between number of display components and elevation (R2=0.4961,P=0.0063).B:Correlation between average tail coil speed and elevation (R2=0.2760,P=0.0459).
The reduced visual display complexity at high elevations inPhr ynocephaluslizards is likely caused by metabolic capacity constraints.Many ectothermic species living in harsh high-elevation areas have reduced metabolic capacity (e.g.,reducing oxygen consumption and metabolic rate) due to environmental constraints (Tanet al.,2021).For example,the lactate dehydrogenase (LDH) activity,an indicator of anaerobic metabolism,is lower inP.erythrurus(elevation 5 300 m) than inP.przewalskii(elevation from 1 000 to 1 500 m) (Tanget al.,2013).Furthermore,recent research showed that the intensity of tail displays inP.vlangalii(altitude from 2 000 to 4 500 m) was primarily regulated by anaerobic metabolism (Zhuet al.,2021).Therefore,reduction in their number display components and speed of display by the high-elevationPhrynocephalusspecies is most likely response to their low anaerobic metabolic capacity.
Alternatively,energy constraints on the nervous system at high-elevation environments may also contribute to the simplification of visual displays.The central nervous system regulates the movement of skeletal muscles in most vertebrates (Akinrodoye and Lui,2021;Kingsburyet al.,2019),and as accurate and complex communication signals,the tail displays ofPhrynocephalusmust be closely controlled by the brain.Nevertheless,the function of brain depends largely on continuous supply of blood sugar and oxygen (Erecińska and Silver,2001;Olesen,1986).High-altitude hypoxia undoubtedly imposes various constraints on brain functions and oxygen conditions during development can affect lizards’ cognition as well (Sunet al.,2014),which may lead to reduced display complexity.This has been partially evidenced by recent works on the Asiatic Toad (Bufo gargarizans) that individuals at highelevation areas have reduced brain sizes and limited movement capacity (Maiet al.,2017;Yaoet al.,2020).Future research on relationships between brain size and display complexity,as well as detection of the critical functional brain regions associated with display manipulation is needed.
One confounding issue with the observed motion visual display simplification in high-elevationPhrynocephalusspecies is social complexity.According to the social complexity hypothesis (SCH),animals living in complex groups likely encounter more social interactions,and may evolve complex communication signals to cope with social conflict and maintain social coalitions (Freeberg,2006;Freeberget al.,2012).Our field observations show thatPhrynocephalusspeciesat high-elevation areas are more territorial (e.g.,stay near burrow and defend territory intensively) compared with species in lowland;based on SCH,we would expect that high-elevation species possess more complex signals.This is the opposite of our results in this study.We assumed that this is likely a compensation way for the effect of display simplification,species from high-elevation area likely increase their territoriality and avoid unnecessary social conflict.Potential associations among social complexity,territorial defense,and display complexity remain to be explored and tested.
5.Conclusion
Lizards of the genusPhrynocephalususe complex and speciesspecific motion visual displays during social communication.Species living in high-elevation areas reduce their display complexity,particularly the number of display components and tail coil speed.This is likely associated with limited metabolic capacity and brain function.The genusPhrynocephalusprovides an excellent system for studying high-elevation adaptation,and more research should be conducted on this group.
AcknowledgmentsThis work was supported by grants from the National Natural Science Foundation of China (grant numbers:31872233,31572273) to Y.QI.
Statement of authorshipY.QI and J.Z.FU conceived and finalized the manuscript,Q.H.HU finished data analysis and prepared the draft,Y.S.LIN and X.QIU contributed to display collection and digitization.All authors read and approved the final version of the manuscript.
杂志排行
Asian Herpetological Research的其它文章
- Appendix 1
- An Annotated List of Lizards (Sauria:Squamata) Recorded from the People’s Republic of China
- Circadian Rhythm and Intersexual Differences in Auditory Frequency Sensitivity in Emei Music Frogs
- Sex But Not Altitude,Modulates Phenotypic Covariations Between Growth and Physiological Traits in Adult Asiatic Toads
- Offspring Sex Is Not Determined by Gestation Temperature in a Viviparous Lizard (Eremias multiocellata) from the Desert Steppe of Inner Mongolia
- Comparative Osteology of Two Far Eastern Species of Ratsnakes (Serpentes:Colubridae),Elaphe dione (Pallas,1773) and E.schrenckii (Strauch,1873),for the Purpose of Palaeontological Studies
