Development Status and Enlightenment of Precision Medicine in China
2022-03-20ShiYuYangYuHuRongboXiangYouTianLijuan
Shi Yu,Yang Yu,Hu Rongbo,Xiang You,Tian Lijuan
(School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China)
Abstract Objective To analyze the development status of precision medicine in China and summarize the problems so as to put forward some countermeasures to promote its high-quality development.Methods Literature research and comparative research were used to study the development status of precision medicine in China from the perspectives of market environment,product research and development (R&D) and policy environment.Results and Conclusion There are some problems in the field of precision medicine in China,such as technical barriers,less investment in new drug R&D,and imperfect regulatory measures.Therefore,some solutions are put forward.First,enterprises should be encouraged to strengthen their cooperation with innovative R&D.Second,the compensation system of drug patent protection period must be established as soon as possible.Lastly,overseas experience should be learned with scientific supervision.
Keywords:precision medicine;development status;market scale;PD-1/L1;innovative R&D
Precision medicine is a new method of diagnosing and treating diseases,which transforms the“average”illness curing into the“precise”personalized treatment,bringing hope to the previously unconquered clinical diseases and some chronic diseases.Therefore,the application of precision medicine has attracted much attention.The significance of precision medicine lies in“precision”,which is derived from the achievements brought by high-tech innovation,the application of cross-genome sequencing,medical big data and other disciplines.And it is mainly supported by pharmacogenetics and biomarkers.Global precision medicine has just started in its early stage,and it becomes a key field for major multinational pharmaceutical enterprises.The highquality development of precision medicine will play an important role in China’s medical construction.This paper aims to analyze the development status of precision medicine in China from the perspectives of market environment,policy environment and listed products,so as to find out the problems and challenges in its application.Then,some suggestions and countermeasures are put forward to promote the development of precision medicine.
1 Overview of precision medicine
In 2011,the National Science Board in the United State put forward the concept of precision medicine for the first time in its report“Towards Precision Medicine:Building Biomedical Research Knowledge Network and New Disease Classification System”.In this concept,subtypes of diseases with different causes can be classified as the same disease.A new disease classification system integrating multiple genome data,clinical data and environmental data should be established.Then an individual centered,multilevel of human disease database can be set up by using systematic biological strategies.Based on this,a biomedical knowledge network can be formed for accurate classification of diseases[1].At the“2018 China Policy Summit on Precision Medicine”,some renowned scholars from home and abroad,on the basis of the development of precision medicine in recent years,broadly defined it as a new approach of treating disease that combined modern science and technology with the traditional medicine.It recognizes the essence of human body function and diseases,and obtains maximum individual and social health benefit in the most effective,safest and economical way[2].
As the life cycle of medical service,precision medicine is mainly composed of four aspects:precise diagnosis,precise prevention,precise treatment and precise drug,and the application types and relationships are shown in Fig.1.Precise diagnosis and prevention represented by gene sequencing,liquid biopsy and prenatal diagnosis,and precise treatment and drug represented by specific tumor markers such as cancer therapy,immune cell therapy and gene therapy have been popular in the global pharmaceutical market and clinical research,making a major breakthrough in the field of precision medicine.
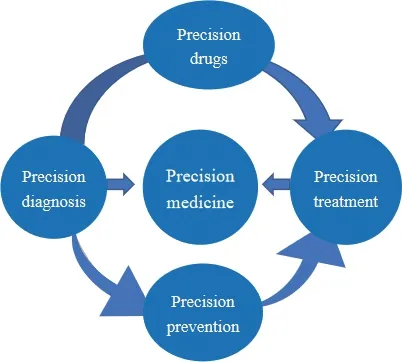
Fig.1 Application types and relationships of precision medicine
2 Current development status of precision medicine application in China
Currently,the research field of precision medicine mainly focuses on the treatment of tumors,chronic diseases andin vitrodiagnosis.As a country with high incidence of cancer and chronic diseases,it is necessary to start the development and research of precision medicine in China.(1) For data resources and platform construction,precision medicine research centers and platforms are constantly emerging.On July 5,2015,“Beijing-Tianjin-Hebei Precision Medicine Alliance for Cardiovascular Disease”was launched in Beijing Medical University,marking the initiative of precision medicine in cardiovascular field.In October 2016,Ningbo gene detection technology demonstration center became one of the first approved centers for the application of gene detection technology in China.Genome sample resources are the cornerstone of precision medicine research.The“100 000 genome projects”launched in December 2017 in China is the first major national project in human genome research.(2) For clinical research,such as immunotherapy,cell therapy,targeted therapy and other cutting-edge treatment technologies,China still faces many technical difficulties.In comparison,research institutions in Europe,the United States and Japan are in the leading position.(3) In terms of product application,there is a big gap between China and Europe,America and Japan as to target drugs,biomarkers represented by PD-1/L1,CAR-T cell therapy products and many other indicators such as the number of mature drugs on the market,market sales,domestic drug share.The following is an in-depth analysis of the development status of precision medicine in China from the perspectives of market environment,product R&D status and policy environment.
2.1 Market environment
According to industry research data[3],the market size of precision medicine in China exceeded 50 billion yuan in 2018,with a year-on-year growth rate of 16.2%.It is estimated that the market size of precision medicine would exceed 65 billion yuan in 2019,and the market size is expected to exceed 100 billion yuan by 2022.Fig.2 shows the market size of precision medicine in China from 2014 to 2019.
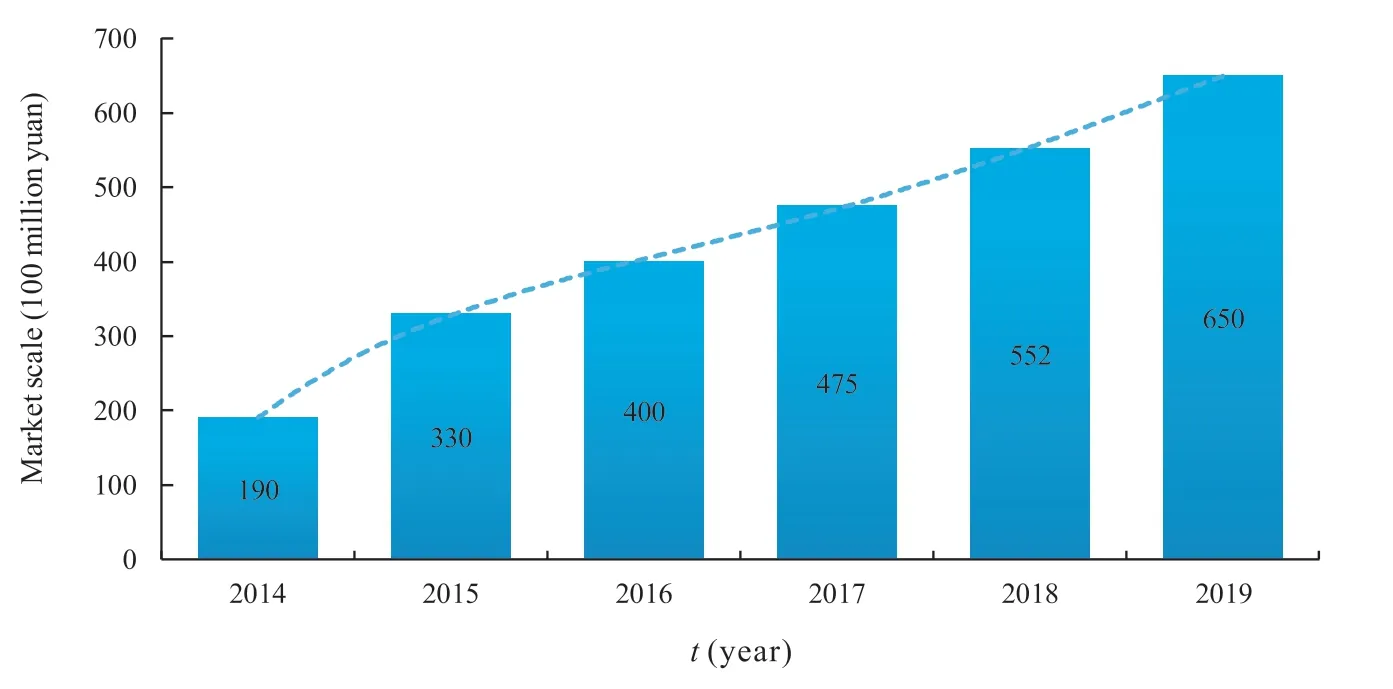
Fig.2 Market scale of precision medicine industry in China from 2014 to 2019
Due to China’s large aging population,lots of tumor patients and other factors,the market demand for precision medicine will increase greatly.In the early stage of the development of precision medicine,the market scale had been increasing by more than 10% year by year,showing a rapid growth rate and highly recognized market value.It also shows that the market demand has not been fully met.Therefore,China’s precision medicine has broad space and unlimited market potential.
PD-1/L1 therapy is one of the most successful tumor treatments and one of the most representative applications of precision medicine.According to the data of the consulting company,the total sales of PD-1/L1 drugs in 2018 exceeded 15 billion dollars,and the domestic sales were about 6.4375 million dollars.On August 28,2018,China’s first PD-1 drug on the market,Opdivo developed by Bristol-Myers Squibb,exceeded 50 million yuan in its first-day sales.In 2019,the global sales volume of Keytruda reached 11.084 billion dollars,ranking the third in the global drug sales.Its domestic sales were about 100 million yuan.The domestic and global market space brings opportunities for Chinese enterprises to participate in the global competition.
2.2 Product research and development (R&D) status
The R&D and application of precision medicine embodies the most cutting-edge medical level and drug research technology in the world,which also reflects a country’s scientific research strength.
Taking representative PD-1/L1 drug as an example,we can see the development direction of precision medicine.A number of clinical data around the world have shown that PD-1/L1 drugs have a good inhibitory effect on multiple tumors.PD-1 drugs are a major breakthrough in the field of cancer treatment.The global marketing status of PD-1/L1 products is shown in Table 1.In July 2014,Opdivo developed by Bristol-Myers Squibb was first listed in Japan,which started the application for listing cancer immunotherapy.Currently,there are 10 PD-1/L1 antibody products on the market globally.China’s first PD-1 product,Opdivo,was approved to market in August 2018.By February 2020,eight immune checkpoint inhibitors had been approved to market in China,including 4 domestic PD-1,2 imported PD-1 and 2 imported PD-L1.Although China started late than the United States,Japan,since 2018,the R&D of tumor in domestic enterprises have been improved greatly.Besides,the reform of drug approval helps speed up the process of sales in China.These enterprises and multinational drug companies are competing in the domestic market now.In the future,they will be listed and sold in the United States and other countries.
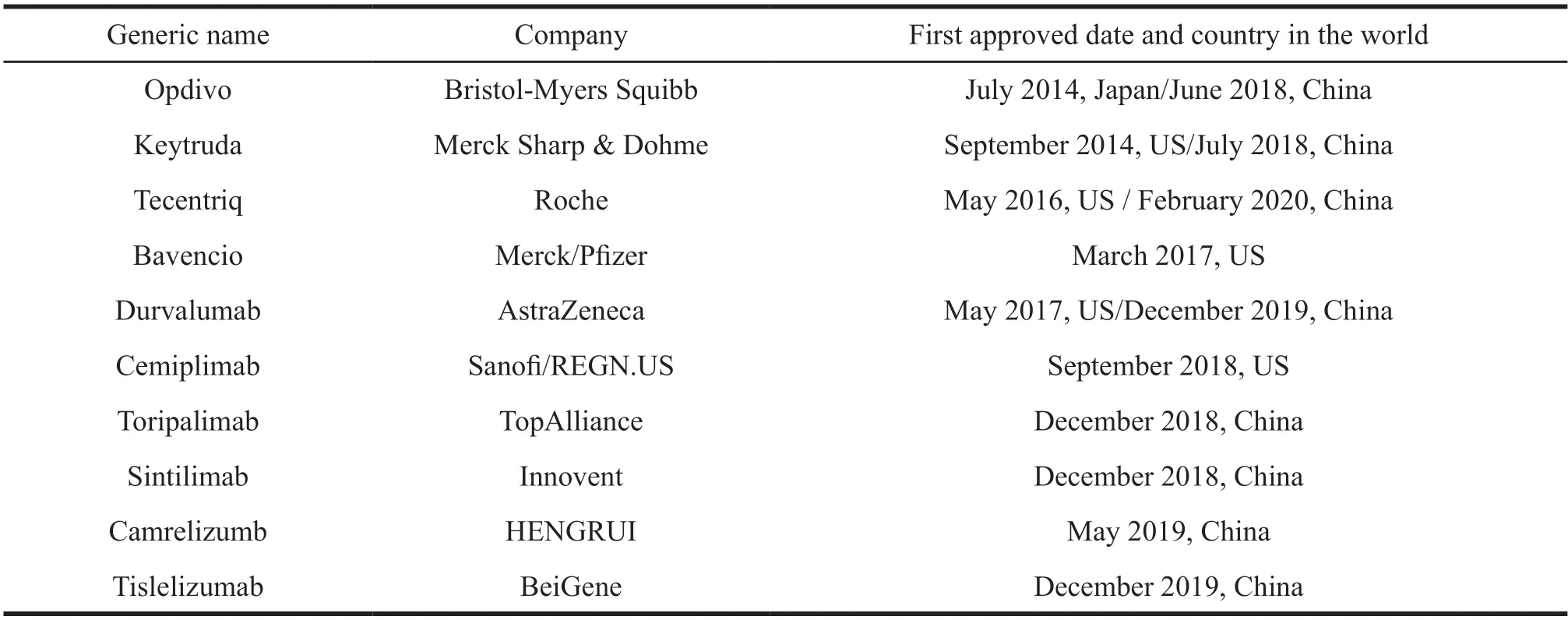
Table 1 Global PD-1/L1 product launch situation
In terms of precise diagnosis and prevention,the representative genetic detection is taken as an example.The gene testing market in China plays an active role in scientific research,clinical practice and commercial consumption.As an important link in the concomitant diagnosis,tumor gene detection has a guiding significance for the early intervention,treatment and prognosis of tumor therapy.According to research data[4],the market size of gene testing in China reached 60.329 billion yuan in 2018,and it is estimated that the market size will reach 101.549 billion yuan in 2020,with a compound growth rate of 37.87% (Fig.3).China’s gene detection market has great potential.As the industry penetration rate sinks from first-tier cities to second or third-tier cities,the market demand and market scale will continue to accelerate.

Fig.3 Market scale of gene detection industry in China from 2015 to 2020
Gene detection is still in the stage of standardized development in China,which is characterized by rapid market growth and low market penetration rate.Among the leading enterprises in the industry,China’s tumor detection business and second-generation sequencing technology NGS are monopolized by Roche Diagnosis,Illumina,Thermo Fisher and other multinational enterprises,but domestic enterprises,such as BGI,Berry Gene and others mainly focus on the prenatal screening market.
2.3 Policy environment
When precision medicine became a global hot research field,Chinese government also realized the important value of precision medicine to the improvement of national medical level.Therefore,a number of policies were introduced to promote the rapid development of precision medicine in China.The policies for the development of precision medicine can be mainly divided into three stages:the start-up stage,the promotion stage and the standard stage.
The startup phase is from 2015 to 2016.In March 2015,the Ministry of Science and Technology held the first strategic meeting on precision medicine and set up a high-level expert group.At the meeting,the Chinese version of the precision medicine plan was proposed.It was expected to invest 60 billion yuan in the development of precision medicine before 2030.The“Notice of the Ministry of Science and Technology on the Issuance of Guideline for Application of Key Projects”in 2016 such as precision medicine R&D plan was published in March 2016,starting the scientific research projects of precision medicine in China.
The active promotion phase is from 2016 to 2018.In May 2016,the State Council issued the“Outline of the National Innovation-Driven Development Strategy”,proposing the development of precision medicine and encouraging the R&D of genetic and chronic disease gene screening technologies.In July 2016,the State Council released the“13th Five-Year Plan for Scientific and Technological Innovation”,which strengthened the R&D of precision medical technology.In November 2017,the Ministry of Science and Technology issued the“Guidelines on the Construction of National Technology Innovation Center”,which listed precision medicine as a key construction field.The Ministry of Science and Technology launched three special projects of“precision medical research”from 2016 to 2018.In 2018,the national intellectual property office listed a number of precision medicine therapies as key support industries.
The normative development stage is from 2018 to present.In September 2018,the Biotechnology Coordination Committee issued the“Quality Control Specification for Preparation of Chimeric Antigen Receptor Modified T Cells (CAR-T Cells)”to promote the industry standards.In March 2019,the State Council promulgated the“Regulations of the People’s Republic of China on the Management of Human Genetic Resources”to strengthen protection,promote rational utilization and regulate the orderly development of the industry.In December 2019,medical services development committee released the guiding principles for the clinical application of new anti-tumor drugs (2019 edition),and the guidance of“targeted drugs”was added to it.
From 2015 to 2019,the State has introduced many policies to encourage and support the development of precision medicine.Meanwhile,with the rapid development of science and technology,especially medical technology,more measures of industry standards and scientific supervision will be crucial to the development of precision medicine in China.
3 Enlightenment and reflection
3.1 The problems of precision medicine in China
The breakthrough of precision medicine is based on the continuous updating and iteration of technology.But it also has the problem of technical barrier because there are difficulties in many links from laboratory R&D to clinical decision-making.56% of the market share of in vitro diagnostic reagent in China are taken by Illumina,Thermo Fisher,Roche,Abbott and other multinational companies.Mindray,Maccura,KHB,Meikang Biology and Autobio,which are among the top 5 domestic enterprises,only account for about 20% of the market share[5].Other problems include the small number of domestic innovative R&D enterprises,low investment,small product R&D coverage and no patent layout in overseas market.At the same time,as a new medical method emerging in recent years,government departments are also faced with many challenges in the management of precision medicine,such as regulatory concepts,industry access standards and standard formulation.
3.2 Strategies to promote high-quality development of precision medicine in China
Innovation is the cornerstone of the development and growth of an enterprise.For precision medicine,its market returns are huge as an innovative product.China’s government should encourage enterprises to invest more in innovative medicine by setting up industrial development support projects such as special R&D fund subsidies for precision medicine.A compensation mechanism for drug patent protection should be established as soon as possible to guarantee the innovative R&D of enterprises.Enterprises are encouraged to establish R&D alliance for precision medicine and share their experience with each other.Academic closure is not conducive to the development and innovation of precision medicine application.From the perspective of scientific supervision,the regulatory authorities should keep pace with the times,learn from the experience and measures of overseas supervision,and establish a set of national standard system.
It is believed that China will become a powerful competitor of precision medicine in the world with the technical breakthrough and policy promotion of precision medicine.
杂志排行
亚洲社会药学杂志的其它文章
- Research and Suggestions on the Development of Smart Hospital -Taking Hospital A in Liaoning for Example
- The Development Opportunities and Dilemmas of Telemedicine-Base on the Perspective of Medical Resource Distribution
- A Review of the Development and Effect of Contraceptive Counseling After Abortion
- Design of Pharmaceutical Care Process for Retail Pharmacies Based on Pareto Analysis
- Study on the Application of Quality Risk Management on Drug Collinear Production
- Analysis and Enlightenment of Pediatric Drug Registration Data in China
