Analysis and Enlightenment of Pediatric Drug Registration Data in China
2022-03-20SuNaTianLijuan
Su Na,Tian Lijuan
(School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China)
Abstract Objective To analyze the current status of pediatric drug development in China from the perspective of pediatric drug registration and clinical trials so as to provide a reference for its improvement.Methods The registration and clinical trials explicitly labeled as pediatric drugs were analyzed by using the Insight Database,National Medical Products Administration (NMPA),and other publicly available information.Results and Conclusion The number of applications for pediatric drug registration was 196,and 104 were for domestic drugs,accounting for 53.06%.The number of pediatric drugs included in the priority review was 116,and 70.18% have completed the review.However,the number of new pediatric drug marketing applications is still not optimistic.Only 5 applications for pediatric drug registration are accepted by Center for Drug Evaluation (CDE),accounting for 4.63%.There are difficulties in recruiting pediatric subjects in clinical trials,with 42.08% of completed recruitment projects.Besides,there are few institutions for drug clinical trials,the distribution of institutions and professional certification are uneven.Therefore,enterprises should be encouraged to carry out research and development of pediatric drugs from two aspects:improving the policy for pediatric drugs and strengthening the clinical management of pediatric drugs to guarantee their safe use.
Keywords:pediatric drug;drug registration;data analysis
With the opening of China’s second-child policy,the total population of children in China has grown steadily.By the end of 2018,the population aged 0 to 15 years (including less than 16 years old)is about 248.6 million,accounting for 17.8% of the total population[1].Public information shows that in China’s existing more than 3 500 pharmaceutical preparations,only 1.7% are for pediatric use.There are more than 4 000 pharmaceutical enterprises in China,but only 5% of them produce pediatric drugs[2].With the increasing demand for pediatric drugs,the issue of drug safety for children due to the shortage of pediatric drugs has emerged.To further encourage innovative research and development of pediatric drugs and speed up the marketing of them,China introduced a drug priority review system in February 2016,which included pediatric drugs in the priority review process..This article analyzes the registration status and clinical trials of pediatric drugs in China after the implementation of the priority review system so that some references can be provided for the development of pediatric drugs.
1 Analysis of the information on pediatric drug registration
This article uses Center for Drug Evaluation(CDE) of National Medical Products Administration(NMPA),the Insight Database,and the Drug Intelligence Database as information sources to retrieve information on drug registrations labeled as“infant”and“children”.Then statistical analysis is made according to the year of acceptance (2015-2019)and registration category.The results showed that the number of drug registrations from 2015 to 2019 was 13 071,of which 196 (1.50%) were for pediatric drugs.Among them,104 (53.06%) were for domestic pediatric drugs and 92 (46.94%) were for imported drugs.
1.1 Pediatric drugs in the priority review
By December 2019,the number of pediatric drugs included in the priority review system was 114,of which 82 applications were for registration of chemical drugs,accounting for 71.92%.There were 4 applications for registration of class 1 chemical drugs (accounting for 3.51%),17 applications for class 2 improved new drugs (accounting for 14.91%).Therefore,the number of applications for registration of new pediatric drugs in China is still not optimistic.Of the 114 pediatric drugs registrations included in the priority review,80 registration applications have been reviewed,accounting for 70.18%.
In terms of therapeutic indications or systemic classification,neurological drugs (22.52%) accounted for the most significant proportion,followed by drugs for endocrine system and metabolism (20.18%),and drugs for immune system ranked third (11.01%).In contrast,drugs for respiratory system,tumor and cardiovascular system accounted for less,as detailed in Table 1.
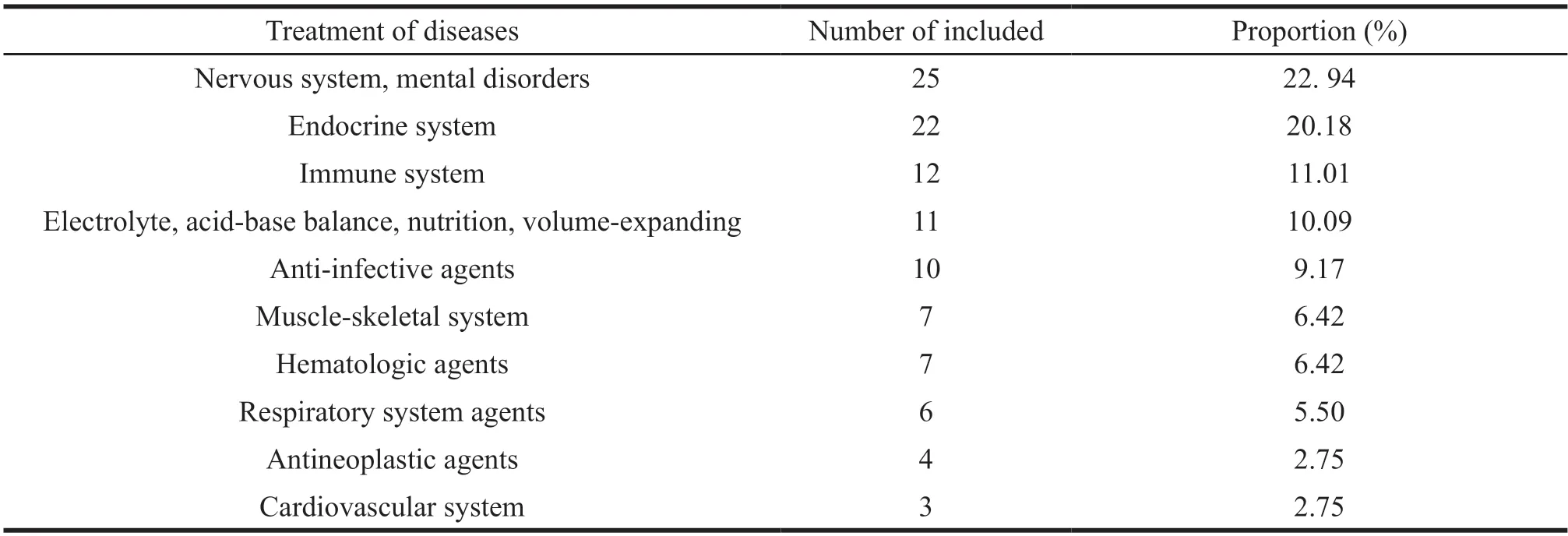
Table 1 Pediatric drugs included in the priority review
1.2 Analysis of the encouragement policy for pediatric drug registrations
To guide enterprises to develop pediatric drugs,the former National Health and Family Planning Commission and other six departments organized pediatric clinical and pharmacological experts to study the specifications of suitable pediatric drugs listed abroad but not yet registered and listed in China in accordance with clinical needs,and then the“List of Pediatric Drugs for Research and Development Declaration”was issued[3-5].From 2016 to 2019,three batches of the list were issued,involving 105 varieties,of which five varieties,including tacrolimus pellets,ataluren pellets,midazolam oral solution,propranolol oral solution,and hydrated chloral have applied for registration,accounting for 4.63%.While China has successfully introduced the policy to encourage the priority review of children’s drugs,it has not been able to solve the problem of insufficient motivation for the development of these drugs.Besides,the number of registrations of pediatric drugs has not been rising yet.The registration and declaration of the listed species are shown in Table 2.
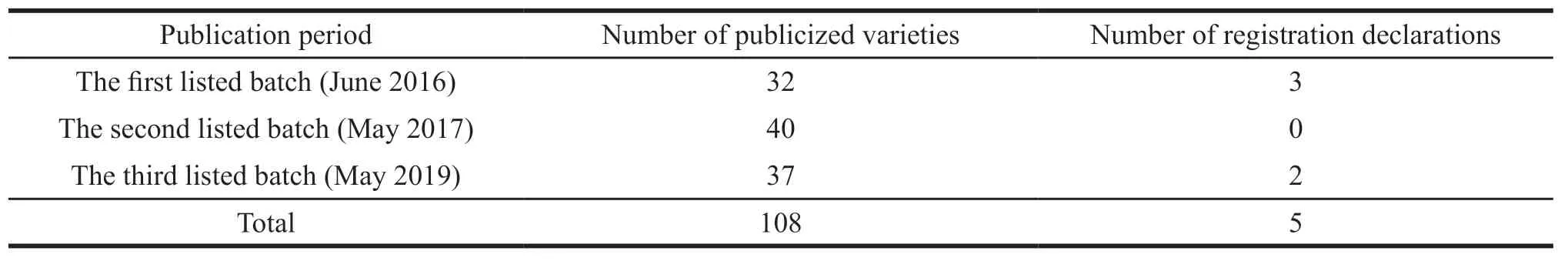
Table 2 Status of registration declarations for listed species
1.3 Data analysis of pediatric drug clinical trials
Clinical trial data of pediatric drugs is an essential supporting basis for the marketing of pediatric drugs.Generally,new pediatric drugs must pass three phases of clinical trials to prove their safety and effectiveness.The government requires that clinical trials for pediatric drugs should be conducted in drug clinical testing institutions with corresponding qualifications.According to the data issued by NMPA,as of January 2020,there are 1 802 drug clinical trial institutions in China,including 144 institutions with pediatric drug clinical trial qualifications.Pediatric drug clinical trial institutions are mostly distributed in economically developed areas and cities.However,in remote areas such as Qinghai,Tibet,Ningxia provinces,there are few pediatric drug clinical trial institutions.In terms of institutional qualifications,the number of qualified institutions for pediatric respiratory,pediatric hematology,pediatric neurology,and other specialties is high (> 40).In contrast,the number of qualified institutions for pediatric infection,pediatric ophthalmology,and pediatric anesthesia are low (1 each).
Through the drug clinical trial registration and information disclosure platform,the keywords“children”“pediatric”and“infant”were searched.Statistical analysis was conducted by the year of the registration number.The results showed that,as of December 2019,a total of 12 948 drug clinical trials had been registered in China (according to registration number),including 437 drug clinical trials for pediatric drugs.There were 260 drug clinical trials for special pediatric preparations (accounting for 2.0%).In terms of trial types,the majority of pediatric drug clinical trials in China are chemical drugs and biological products,accounting for 85.38%.Meanwhile,the proportion of traditional Chinese medicine or natural medicine,which is strongly supported by the government,is the lowest,accounting for 14.62%.Besides,it is difficult to recruit subjects for pediatric drug clinical trials.There are 109 completed projects,accounting for 42.08% of the total.There are 51 projects with a recruitment period longer than 3 years,accounting for 16.69%.The registration of drug clinical trials is shown in Table 3.
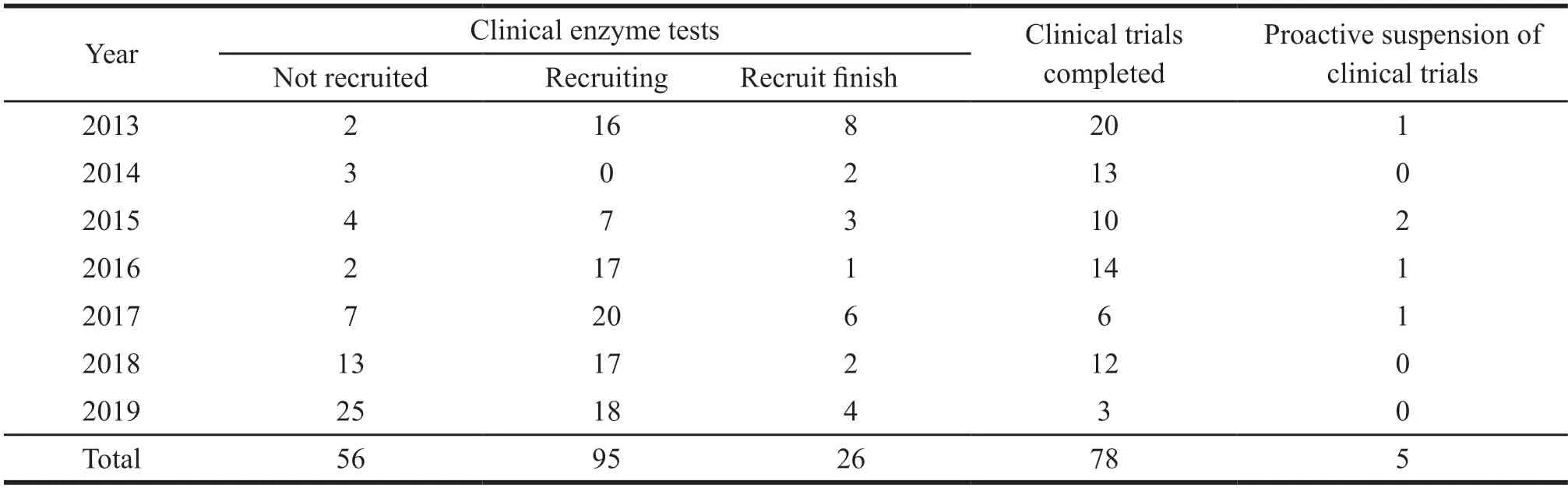
Table 3 Recruitment of subjects in pediatric drug clinical trials
In summary,in the registration application,the number of pediatric drug registration applications in China is much lower than that of adult drugs.Among them,the number of innovative pediatric drug applications that the government encourages enterprises to develop accounts for less than 5%.As to the pediatric drug clinical trials,there are problems such as long clinical trial cycle,difficulty in recruiting subjects,a small number of pediatric drug clinical trial institutions,and uneven distribution of pediatric certification.
2 Problems in the development of pediatric drugs in China
2.1 Enterprises are less motivated to develop pediatric drugs
According to the registration of pediatric drugs in China,the number of applications is low,especially the pediatric drugs and class 1 new drugs which have the policy support from the government.In the meantime,the applications for registration of imported pediatric drugs are equivalent to domestic ones,indicating that the domestic enterprises have low R&D enthusiasm.Although pediatric drugs were included in the priority review in February 2016,and the“Drug Administration Law of the People’s Republic of China”was implemented on December 1,2019[6],the legal documents only mentioned the keywords of“encouragement”,“support”and“priority approval”,consistent with the pediatric drug regulations previously issued.Therefore,it lacks preferential policies at the economic levels.
2.2 Difficulties in conducting clinical trials of pediatric drugs
2.2.1 Clinical trials are more difficult
Children are not a microcosm of adults.The structure and physiological functions of their organs are different from those of adults.In addition,there are differences in their physical and psychological characteristics at different ages[7].This difference increases the risk of pediatric drug clinical trials,so the quality control management of institutions,researchers proficiency and their ability to respond to emergency must be better than that of normal drug trials.In addition,the small number of pediatric drug clinical trial institutions and the uneven distribution of pediatric certification can lead to the clustering of pediatric drug clinical trials.These factors are not conducive to the pediatric drug clinical trials.
2.2.2 Difficulty in recruiting subjects
The basic condition for a child to participate in a clinical trial is to obtain his consent or the consent of his guardian.In clinical trials of pediatric drugs,it is difficult for the guardian to allow his child to participate due to the risks.In the case of children’s consent,his poor understanding of the content of the consent form makes him reluctant to participate in the clinical trial.In addition,the design of a clinical trial,the characteristics of the drug and the rights of the subject granted by the sponsors also affect the enrollment of subjects in the clinical trial.
3 Reflections and insights on the registration and marketing of pediatric drugs
3.1 Improving incentives policy for pediatric drugs
Generally,it is more difficult to develop pediatric drugs than normal drugs for adults because of the high cost and technology.Besides,these drugs have much lower economic returns than adult drugs.The huge difference between input and revenue hinders enterprises from developing pediatric drugs.Some European countries and the United States have also faced the problem of improving the enthusiasm of enterprises.To solve this problem,FDA of the United States encourages pharmaceutical companies to conduct pediatric clinical trials with the policy of“six months of pediatric exclusivity protection”through the implementation of the“Best Pharmaceuticals for Children Act”.This policy increased the proportion of clinical trials for pediatric drugs,which significantly improved their safety and effectiveness.China can learn from the experience of the developed countries to give policy support to enterprises for producing new pediatric drugs or clinically-needed pediatric drugs.For instance,the government can make preferential tax policies or establish a special fund to mobilize the enthusiasm of enterprises for pediatric drug research and development.
3.2 Prompting clinical trials of pediatric drugs
The following measures can be taken to promote the development of clinical trials of pediatric drugs.(1) Relevant incentive policies for the clinical trial institution filing system should be made.At present,the number of filings of pediatric drug clinical trial institutions in China is small,which is difficult to meet the clinical needs of pediatric drugs.Therefore,tax relief policy should be given to the registered pediatric drug clinical trial institutions to promote the filings.(2) The publicity of clinical trials of pediatric drugs should be increased.The difficulties in recruiting subjects for clinical trials of pediatric drugs are mainly due to parents’ fear of clinical trials.Therefore,the relevant drug clinical trial institutions should increase the publicity on pediatric drug clinical trials.Besides,the consent form should be designed in simple and clear language to facilitate the understanding of clinical trials by parents and the children.(3) For high-risk clinical trials of pediatric drugs,the ethics committee should strengthen the review to protect the rights and interests of the subjects.The sponsors may be required to establish a data monitoring committee(DMC) for clinical trials of pediatric drugs.At the same time,insurance is necessary to protect the rights and interests of the subjects.
4 Conclusions
The development of the priority review system for pediatric drugs in China has improved the efficiency of reviewing,sped up the drug marketing,and enriched the choice of pediatric drugs.Still,there is the problem of insufficient motivation for research and the development of pediatric drugs in Chinese enterprises.In this article,the situation of pediatric drug registration in China is analyzed,and the problems in the laws and regulations are found.Some recommendations are made in the context of China’s situation.With the improvement of pediatric drug regulations and the drug clinical trial environment,the development of pediatric drugs can be promoted greatly,which in return,will guarantee the safe use of pediatric drugs.
杂志排行
亚洲社会药学杂志的其它文章
- Research and Suggestions on the Development of Smart Hospital -Taking Hospital A in Liaoning for Example
- The Development Opportunities and Dilemmas of Telemedicine-Base on the Perspective of Medical Resource Distribution
- Development Status and Enlightenment of Precision Medicine in China
- A Review of the Development and Effect of Contraceptive Counseling After Abortion
- Design of Pharmaceutical Care Process for Retail Pharmacies Based on Pareto Analysis
- Study on the Application of Quality Risk Management on Drug Collinear Production
