Postoperative morbidity adversely impacts oncological prognosis after curative resection for hilar cholangiocarcinoma
2022-03-15ZhiPengLiuWeiYueChenYanQiZhangYanJiangJieBaiYuPanShiYunZhongYunPingZhongZhiYuChenHaiSuDai
Zhi-Peng Liu,Wei-Yue Chen, Yan-Qi Zhang, Yan Jiang, Jie Bai, Yu Pan, Shi-Yun Zhong,Yun-Ping Zhong, Zhi-Yu Chen, Hai-Su Dai
Abstract
Key Words: Hilar cholangiocarcinoma; Morbidity; Surgery; Oncology; Survival; Recurrence
INTRODUCTION
Cholangiocarcinoma is a common malignancy of the liver, second only to hepatocellular carcinoma(HCC) in incidence and accounting for approximately 10% of primary liver tumors[1,2]. Hilar cholangiocarcinoma (HCCA), also referred to as a Klatskin tumor, represents 60% of cholangiocarcinomas[3].The HCCA incidence is increasing, and tumors have a poorer prognosis than any other hepatobiliary tumor, such as HCC, with five-year survival rates of 20% to 40%[4,5]. Radical surgery offers a possible cure for eligible HCCA patients. However, the oncological prognosis after liver resection for HCCA is often uncertain, as the tumor recurs within five years in over 60% of patients[6,7]. Consequently,identifying the risk factors that influence HCCA recurrence is important to improve outcomes.
Previous studies have demonstrated that postoperative morbidity is linked to greater recurrence and lower survival rates than many other gastrointestinal tumors, such as HCC[8], pancreatic[9], gastric[10,11], and colorectal carcinomas[12,13], as well as intrahepatic cholangiocarcinoma[14]. Systemic inflammation may result from postoperative morbidity, which could, in turn, reduce the effectiveness of the immune response against the tumor[15]. This may explain the relationship between poorer prognosis and postoperative morbidity. Regrettably, because HCCA surgery is one of the most complicated operations in hepatobiliary surgery, there is a high incidence of postoperative morbidity, ranging from 30% to 70%[16]. Postoperative morbidity is linked to both surgical factors and patients' underlying diseases[17-19]. From our point of view, surgery should be both safe and effective, avoiding postoperative morbidity to improve oncological prognosis. However, few studies have investigated the effects of postoperative morbidity on oncological prognosis in patients with HCCA after curative resection.
Therefore, this study aimed to determine if there is a link between the presence of postoperative morbidity and oncological prognosis following curative resection for HCCA. Additionally, the study assessed the independent risk factors for the occurrence of postoperative morbidity.
MATERIALS AND METHODS
Patients
The data of patients with HCCA who had undergone curative resection for newly diagnosed HCCA between January 2010 and December 2017 at The First Affiliated Hospital of Army Medical University(Southwest Hospital) in China were collected and analyzed. The HCCA diagnosis was confirmed by postoperative pathological evaluation. Extrahepatic bile duct resection and partial hepatectomy were performed on all patients. Regardless of preoperative computed tomography (CT), magnetic resonance imaging (MRI) or suspicion of lymph node metastasis, all patients underwent locoregional lymphadenectomy. To achieve curative resection, combined pancreaticoduodenectomy and/or vascular resection was conducted, with curative resection classified as complete tumor (both macroscopic and microscopic) removal, with clear resection margins visible on microscopy (R0 resection). Patient exclusion criteria were: (1) Having received adjuvant chemotherapy and radiotherapy; (2) Unresectable tumor at exploration; (3) Having undergone R1 or R2 resection; (4) Recurrent HCCA; (5) Age < 18 years;(6) Postoperative death within 30 d; and (7) Incomplete data. The study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board of the South-West Hospital of Chongqing, China (No. KY2021129). Patients were not required to give informed consent for the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Variables
Variables related to patients and liver pathology included age, sex, American Society of Anesthesiologists (ASA) score, obesity (BMI > 30), diabetes mellitus, cirrhosis, and preoperative drainage.Cirrhosis was verified by postoperative histopathology. Tumor-related variables included preoperative carbohydrate antigen 19-9 (CA19-9) levels, maximum tumor size, macrovascular and microvascular invasion, peripheral nerve invasion, metastasis to lymph nodes, Bismuth type, and poorly differentiated tumors. A preoperative CA19-9 level of 150 U/L was used as a threshold to separate patients into two groups[20]. The operative variables were intraoperative blood loss, blood transfusion, and the extent of hepatectomy (minor or major). Resection of three or more Couinaud liver segments was called major hepatectomy, whereas resection of fewer segments was labeled minor hepatectomy.
Perioperative outcomes
Postoperative morbidity was classified according to the Clavien-Dindo classification[21], with minor morbidity defined as Clavien-Dindo grades I-II and major morbidity defined as grades III-V. The occurrence of postoperative morbidity within 30 d was recorded, as was the postoperative morbidity hospital stay. Morbidity included posthepatectomy liver failure (PHLF); blood, lung, abdominal, and biliary infection; pleural effusion; bile leakage; ascites; intestinal leakage cholangitis; abdominal hemorrhage; delayed gastric emptying; and wound dehiscence, among others. PHLF was recognized by the “50-50 criteria” five days or more after surgery[22]. A severe drop of > 3 g/dL in the postoperative hemoglobin level compared with the preoperative level was indicative of abdominal hemorrhage, with or without the need for transfusion and/or reoperation. Bile leakage was defined as a drain bilirubin concentration of more than three times higher than that of serum. Ascites or pleural effusions requiring diuretic administration or paracentesis were also recognized. Surgical site infection was diagnosed based on the Prevention of the National Nosocomial Infections Surveillance and Centers for Disease Control[23].
Follow-up procedures
The patients were followed up at regular intervals (approximately 1-2 mo) after discharge. A standard protocol was used to evaluate the presence of HCCA recurrence. This included clinical symptoms,physical examinations, laboratory tests (liver function and tumor biomarkers), and abdominal ultrasonography. CT, MRI, or ultrasonic contrast was performed every two months after surgery or when tumor recurrence was suspected. The presence of new lesions seen on MRI or CT was defined as recurrence that was treated by surgery, drugs, or supportive therapy.
Endpoints
The primary endpoint was overall survival (OS), and the secondary endpoint was recurrence-free survival (RFS). OS was considered to be the interval from curative resection to death or last follow-up.For patients with recurrence, RFS was considered to be the interval from curative resection to the diagnosis of tumor recurrence. For patients without recurrence, RFS was taken as the interval from curative resection to death or last follow-up. Until the study's termination on July 15, 2020, all patients were followed up on until death or loss to follow-up.
Statistical analysis

Figure 1 Selection of the study population. HCCA: Hilar cholangiocarcinoma.
Continuous variables are expressed as the means ± SD or medians (range), and categorical variables are expressed as the frequencies and percentages. Student’s t test or the Mann-Whitney U test was used for continuous variables, and Pearson’s chi-square test was used for categorical variables. The Kaplan-Meier method and log-rank test were used to calculate and compare the OS and RFS rates.Variables showing significance levels ofP< 0.1 on univariate analyses were used for multivariate analysis by the Cox proportional hazard model. In univariate and multivariate Cox regression studies,hazard ratios (HRs) and their 95 percent confidence intervals (CIs) were calculated. SPSS®version 26.0(IBM, Armonk, New York, United States) was used for statistical analysis.Pvalues were two-sided, and statistical significance was defined as aPvalue of less than 0.05.
RESULTS
Perioperative outcomes
In our study, 239 patients were included based on established inclusion criteria (Figure 1). All patients performed open surgery. Table 1 presents the perioperative outcomes for the 239 patients. Of these patients, 146 (61.1%) experienced morbidity within 30 d of surgery, with minor morbidity occurring in 78 (32.6%) and major morbidity in 68 (28.5%) patients. The top three causes of morbidity were surgical site infection (36/239, 15.1%), bile leak (32/239, 13.4%), and pleural effusion (24/239, 10.0%).
Patient characteristics
Table 2 shows the comparisons of patients’ clinicopathologic and operative variables between those with and without postoperative morbidity. Notably, obesity, diabetes mellitus, cirrhosis, and intraoperative blood loss > 500 mL were more common in patients with morbidity (P< 0.05).
Risk factors for postoperative morbidity
In the multiple logistic regression model using significant (P< 0.1) factors shown in Table 2, cirrhosis[odds ratio (OR): 2.867; 95%CI: 1.207-6.810;P= 0.017], intraoperative blood loss > 500 mL (OR: 2.240;95%CI: 1.162-4.318;P= 0.016), diabetes mellitus (OR: 3.395; 95%CI: 1.082-10.651;P= 0.036), and obesity(OR: 3.694; 95%CI: 1.197-11.394;P= 0.023) were identified as independent risk factors for postoperative morbidity (Table 3).
Survival outcomes
Table 4 shows the relationships between the patient survival outcomes and perioperative morbidity.Over the median 19.0-mo follow-up period, tumor recurrence and death were apparent in 76.7%(112/146) and 71.9% (105/146), respectively, of patients with postoperative morbidity and in 64.5%(60/93) and 59.1% (55/93), respectively, of patients who did not experience morbidity (recurrence,P=0.041; death,P= 0.041). The median OS and RFS were significantly lower in the patients with postoperative morbidity, as shown in Figure 2 (OS: 18.0 movs31.0 mo,P= 0.003; RFS: 16.0 movs26.0 mo,P= 0.002).

Table 1 Postoperative outcomes of 239 patients who underwent curative resection for hilar cholangiocarcinoma
Prognostic factors for survival
Tables 5 and 6 present the results of the univariate and multivariate Cox regression analyses,respectively, for survival prediction. The multivariate analysis identified postoperative morbidity was independently associated with decreased OS (HR: 1.557, 95%CI: 1.119-2.167,P= 0.009) and RFS (HR:1.535, 95%CI: 1.117-2.108,P= 0.008). Furthermore, preoperative CA19-9 > 150 U/L, maximum tumor size > 3 cm, lymph node metastasis, macrovascular invasion, and poor tumor differentiation were also observed to be risk factors for both OS and RFS.
Furthermore, based on the severity of postoperative morbidity, major morbidity was associated with both lower OS and RFS, as shown in Figure 3 (OS: HR: 2.175; 95%CI: 1.470-3.216,P< 0.001; RFS: HR:2.054; 95%CI: 1.400-3.014,P< 0.001).
DISCUSSION
It is difficult to verify and define the quality of surgery, whether assessed on the level of outcome,process, or system[24]. As a tumor-related surgical quality measure, postoperative morbidity has been an increasingly interesting topic. In assessing a potential link between postoperative complications and outcomes in cancer patients, it is necessary to determine the factors leading to postoperative morbidity and the level of morbidity that may result in an unfavorable outcome[25]. Postoperative morbidity,therefore, is an indication of the quality of the surgery and may also act as a reliable prognostication of outcomes with the potential for therapeutic application. Thus, to reduce the incidence of perioperative morbidity, it is important to identify its contributory factors.
Here, we examined the prognostic impacts of 30-d morbidity in 239 HCCA patients after curative resection. The findings showed that perioperative morbidity negatively impacted both OS and RFS,indicating the value of reducing postoperative morbidity to improve patient outcomes. In this study,postoperative morbidity occurred in 146 (61.1%) patients, of which 68 (28.5%) experienced major morbidity. These findings support those of other studies. Hasegawaet al[19] observed a major postoperative complication (grade 3 or more) rate of 46.8%[26]. Gerhardset al[27] described a postoperative morbidity rate of 65.0% in patients following hemihepatectomy[27], while Daret al[28]observed complications in approximately 66.7% of patients within 90 d[28]. In addition, a study from Japan observed that 21 patients (35.0%) had remarkable postoperative complications, while the presence of complications predicted worse outcomes in intrahepatic cholangiocarcinoma patients[14]. A large-sample multicenter study from China indicated that 758 (35.1%) out of 2161 HCC patients experienced morbidity within 30 d, and the median OS and time-to-recurrence in these patients were poorer (48.1 movs91.6 mo and 19.8 movs46.1 mo, respectively)[8]. However, no studies have investigated the link between postoperative morbidity and prognosis in HCCA patients.

Table 2 Comparisons of patients’ clinicopathologic and operative variables between those with and without postoperative morbidity

Table 3 Univariable and multivariable logistic regression analyses of risk factors associated with postoperative morbidity following curative resection for hilar cholangiocarcinoma

Table 4 Comparisons of survival outcomes between patients with and without postoperative morbidity
Both clinicopathological and operative variables were found to differ significantly in relation to postoperative morbidity, including obesity, diabetes mellitus, cirrhosis, and intraoperative blood loss >500 mL. Many previous studies have used propensity score matching to balance the intergroup baseline features in evaluating the effect of postoperative complications on outcomes[29,30]. However, as postoperative 30-d morbidity is itself a short-term outcome, it is not appropriate to adopt this statistical approach, which may increase, rather than decrease, selection bias between the groups. In contrast,classical statistical approaches are appropriate to determine the link between postoperative morbidity and outcomes with adjustment for confounding factors.
It is important to identify the risk factors for postoperative morbidity to reduce its incidence. Here,we specifically investigated the independent risk factors for morbidity and identified obesity, diabetes mellitus, cirrhosis, and intraoperative blood loss > 500 mL. These findings are significant for guiding clinical practice. Similar conclusions have been reported; for example, a major morbidity rate of 40%was observed after liver resection in obese or overweight patients[31,32]. There is evidence to explain this phenomenon, namely, hepatic steatosis associated with obesity may adversely affect the regeneration of liver remnants and thus influence morbidity[33]. During the perioperative period, obese patients should be instructed by dieticians to adjust their dietary habits and properly match their nutritional structure. It is known that obesity is closely related to chronic liver diseases, such as steatosis, nonalcoholic steatohepatitis, and other comorbidities, including diabetes[34]. Moreover, the presence of diabetes mellitus is known to be linked to postoperative complications after HCCA surgery[35]. For severe diabetes, the clinician needs to effectively control blood glucose levels before surgery with the assistance of endocrinologists. In addition to the above two risk groups related to metabolism,for patients with cirrhosis, due to their worse liver function, surgeons should evaluate the remaining liver volume and reserve function more carefully before surgery and pay more attention to the prevention of complications, including PHLF, pleural effusion, abdominal hemorrhage, and biliary infection. Notably, cirrhosis may cause poor blood coagulation, making it more difficult to control the amount of bleeding during surgery[36]. For patients with poor liver function and coagulation dysfunction, intraoperative infusion of plasma or cryoprecipitate may help to reduce intraoperative bleeding. For patients with severe liver cirrhosis, the surgeon should use Pringle’s maneuver to obstruct the temporary hilar of the liver for hepatectomy. In addition, the anesthetist should ensure low central venous pressure to reduce the amount of bleeding during the surgery. Moreover, the vast majority of intraoperative bleeding occurs during liver resection. With new medical advances, many kinds of instruments can be used for liver resection: Ultrasonic knife, electrocautery (bipolar, monopolar, or water sealed bipolar), and radiofrequency-assisted liver resection. However, which can better prevent intraoperative bleeding may be related to the patient's liver condition and the operator's habits, and it is worthy of further study. As this study showed that postoperative morbidity (especially major morbidity) can affect the oncological prognosis of HCCA after curative resection, adjusting the above risk factors can reduce complications and also improve the prognosis of patients. In our opinion, only through multidisciplinary treatment can we reduce the postoperative morbidity of patients who undergo curative HCCA resection.
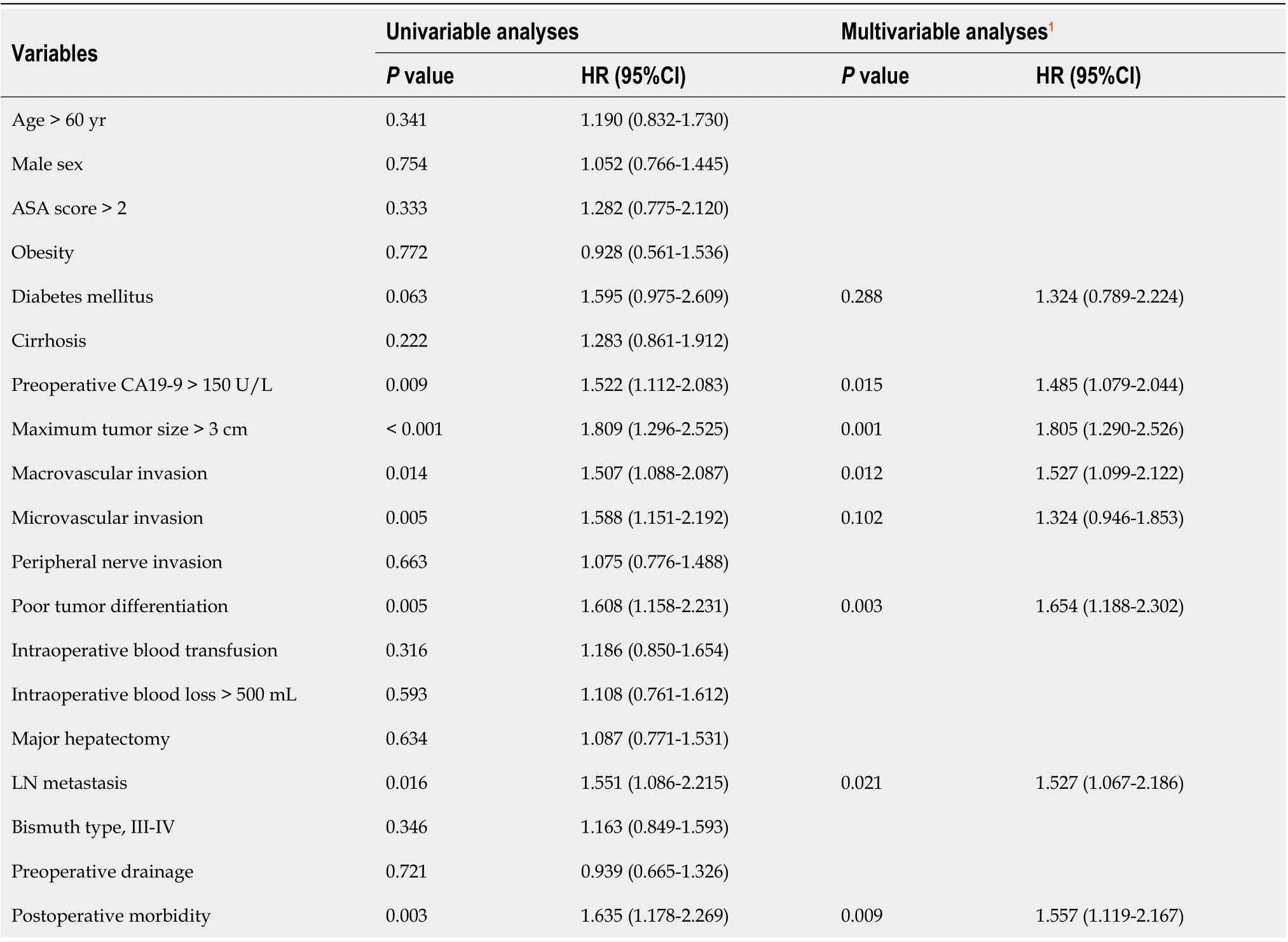
Table 5 Univariable and multivariable Cox regression analyses of risk factors associated with overall survival following curative resection for hilar cholangiocarcinoma
In other cancers, postoperative morbidity may be an independent predictor of poor prognostic outcome, including colorectal liver metastasis[37], HCC[8,38,39], pancreatic cancer[40], and esophageal cancer[41]. Although the precise association between postoperative morbidity and unfavorable prognostic outcomes remains to be elucidated, there are several possible explanations. Previous studies have shown that major surgery can induce systemic inflammation, with increased secretion of inflammatory cytokines, including interleukin-1 and interleukin-6, which contributes to cancer angiogenesis,proliferation, growth, and metastases[42-44]. In addition, severe systematic inflammation caused by postoperative morbidity may lead to an immunosuppressive condition and state, which can regulate the reduction of tumor monitoring and may lead to both metastasis and disease-specific death[15,42].Notably, the postoperative stress response can inhibit cell-mediated immune function. Consequently,during the period of postoperative morbidity and relative immunosuppression caused by postoperative stress, residual malignant cells may proliferate[45]. Therefore, postoperative 30-d morbidity may negatively impact long-term oncological outcomes.
There are several limitations to this study. Specifically, it was a single-institution study with a retrospective design. Despite this, the database was established by standardized surgical techniques and perioperative management, thus preventing some limitations of multicenter, population-based, or national studies. Nevertheless, the impact of postoperative morbidity on the prognosis of HCCA patients still requires evaluation using a larger prospective study. In addition, in this study, patients who received adjuvant chemotherapy and radiotherapy were excluded. Some previous studies have demonstrated a benefit of prognosis for patients following surgery who received postoperative adjuvant therapy[46,47]. However, adjuvant therapy cannot be administered immediately when morbidities occur after surgery. As a result, we believed it was better to exclude patients who received adjuvant therapy to more accurately reflect the impact of postoperative complications on prognosis.

Figure 2 Overall survival and recurrence-free survival curve comparisons between patients without and with postoperative morbidity. A:Overall survival, P = 0.003; B: Recurrence-free survival, P = 0.002.
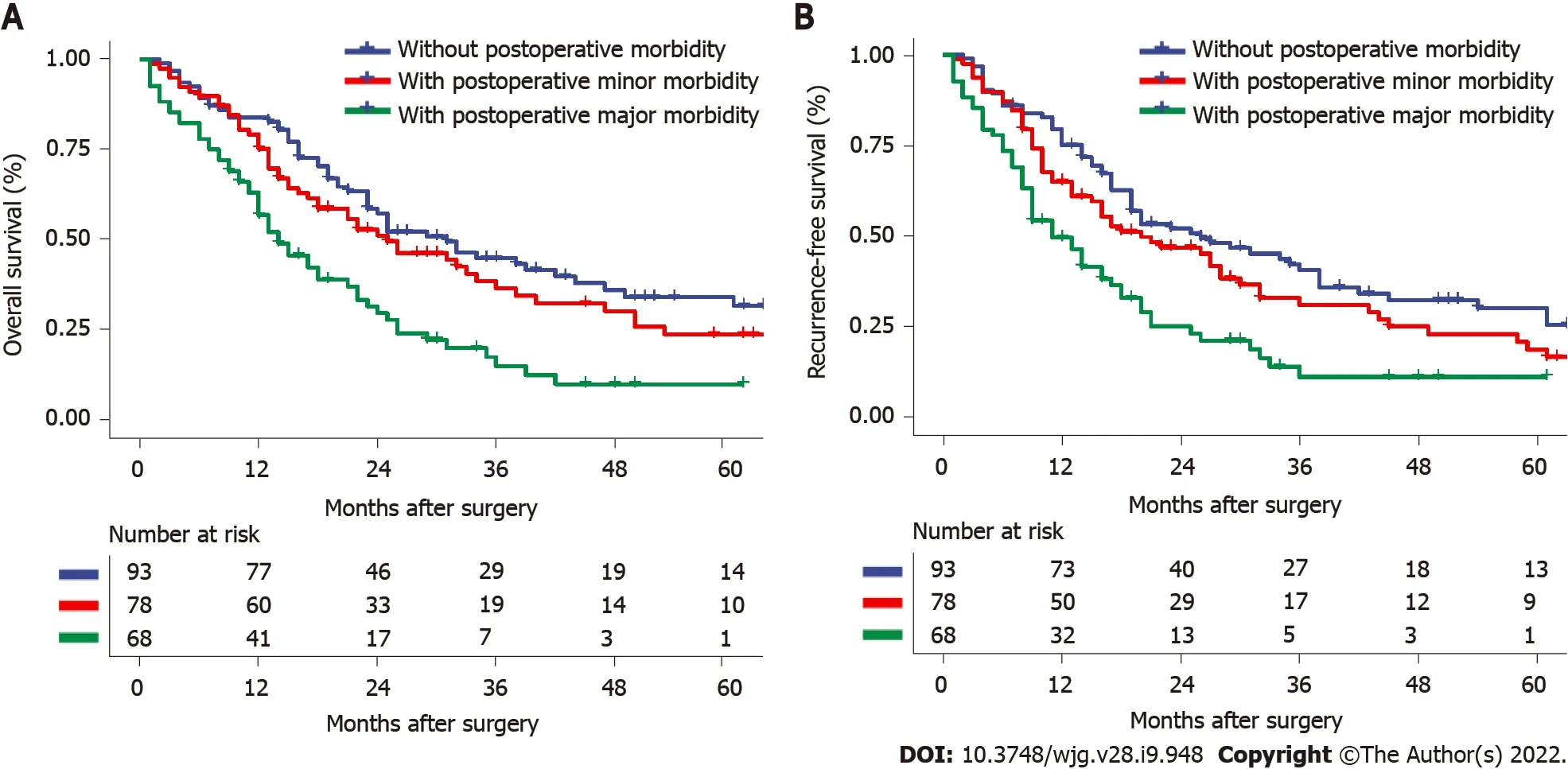
Figure 3 Overall survival and recurrence-free survival curve comparisons among patients without postoperative morbidity, with minor postoperative morbidity, and with major postoperative morbidity. A: Overall survival, P = 0.231 (with minor postoperative morbidity vs without postoperative morbidity), P < 0.001 (with major postoperative morbidity vs without postoperative morbidity); B: Recurrence-free survival, P = 0.132 (with minor postoperative morbidity vs without postoperative morbidity), P < 0.001 (with major postoperative morbidity vs without postoperative morbidity).
CONCLUSION
In summary, the results of this study clearly show that postoperative morbidity both lessens long-term survival and raises tumor recurrence in HCCA patients following curative resection. Independent risk factors for postoperative morbidity included diabetes, obesity, liver cirrhosis, and intraoperative blood loss > 500 mL. Clinicians should further optimize preoperative management, surgical procedures, and perioperative care to prevent complications and thus improve both short-term and long-term oncological prognoses.
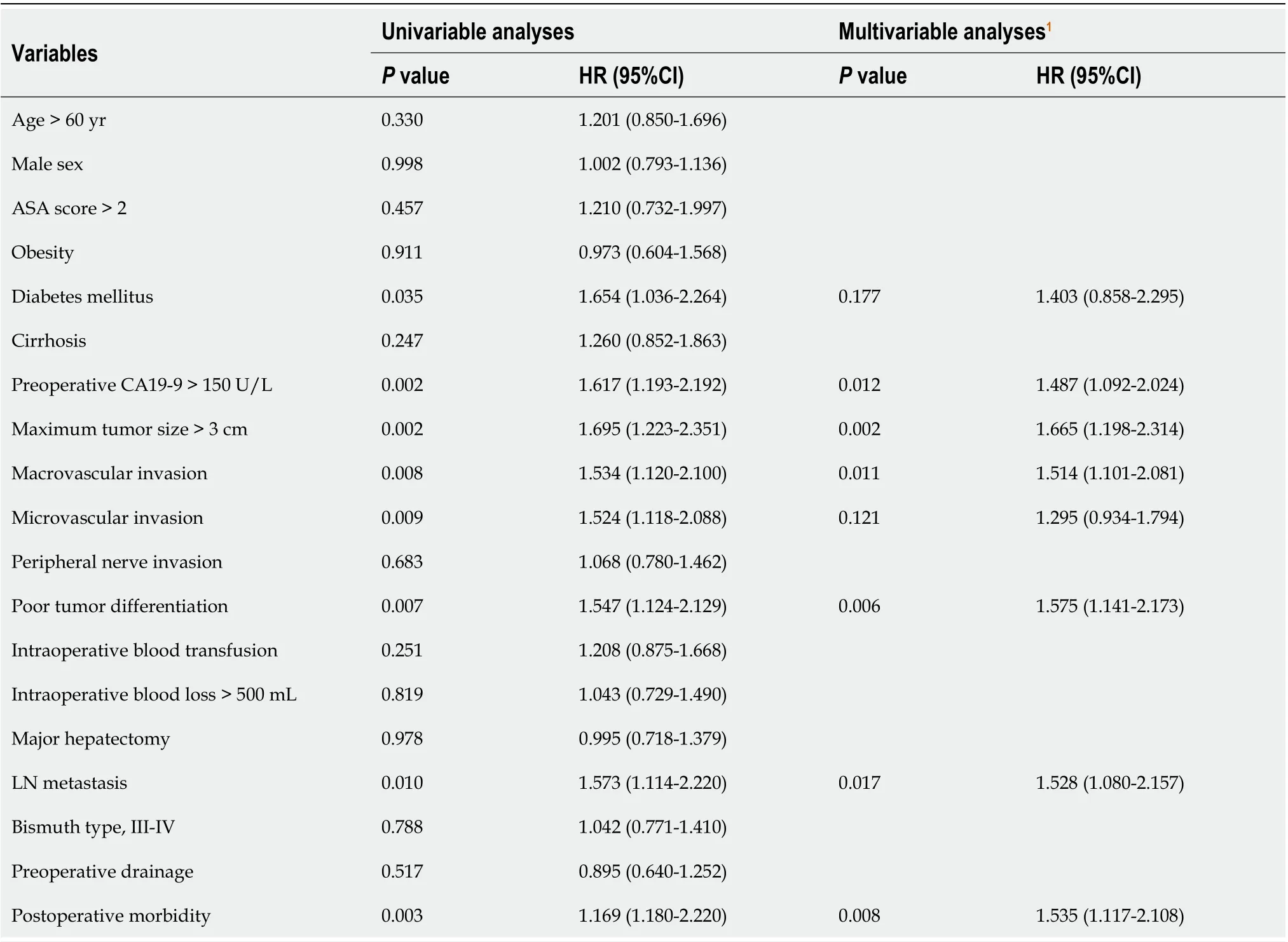
Table 6 Univariable and multivariable Cox regression analyses of risk factors associated with recurrence-free survival following curative resection for hilar cholangiocarcinoma
ARTICLE HIGHLIGHTS

Research results
Postoperative complication occurred in 146 out of 239 patients (61.1%). Multivariate logistic regression revealed that cirrhosis, intraoperative blood loss > 500 mL, diabetes mellitus, and obesity were independently associated with postoperative complication. And, postoperative complication was associated with decreased OS and RFS (OS: 18.0 movs31.0 mo, respectively,P= 0.003; RFS: 16.0 movs26.0 mo, respectively,P= 0.002). Multivariate Cox regression analysis indicated that postoperative morbidity was independently associated with decreased OS [hazard ratios (HR): 1.557, 95% confidence interval (CI): 1.119-2.167,P= 0.009] and RFS (HR: 1.535, 95%CI: 1.117-2.108,P= 0.008). Moreover, major morbidity was independently associated with decreased OS (HR: 2.175; 95%CI: 1.470-3.216,P< 0.001)and RFS (HR: 2.054; 95%CI: 1.400-3.014,P< 0.001) after curative resection for HCCA.
Research conclusions
Postoperative complication (especially major complication) may be independently associated with poor prognosis in HCCA patients following curative resection.
Research perspectives
Clinicians should further optimize preoperative management, surgical procedures, and perioperative care to prevent complications and thus improve both short-term and long-term oncological prognoses.
FOOTNOTES
Author contributions:Dai HS had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analyses; Liu ZP, Chen ZY, Zhang YQ, Chen WY, Bai J, and Jiang Y contributed to the study concept and design; Liu ZP, Chen ZY, Zhang YQ, Chen WY, Zhong SY, Zhong YP, and Pan Y contributed to the acquisition, analyses, or interpretation of data; Liu ZP, Dai HS, Chen ZY, and Zhang YQ drafted the manuscript;Dai HS, Chen ZY, and Zhang YQ contributed to the critical revision of the manuscript for the important intellectual content; Liu ZP, Dai HS, and Zhang YQ performed the statistical analyses; Dai HS contributed to the study supervision.
Supported byNational Natural Science Foundation of China, No. 81874211; and Personalized Training of Key Support Objects for The Talent People of The Army Medical University, No. XZ-2019-505-014.
Institutional review board statement:The study was approved by the Institutional Review Board of the South-West Hospital of Chongqing, China, No. KY2021129.
Conflict-of-interest statement:Nothing to disclose.
Data sharing statement:Technical appendix, statistical code, and dataset available from the corresponding author at daihaisu@163.com. Participants gave informed consent for data sharing.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Zhi-Peng Liu 0000-0001-7652-1783; Wei-Yue Chen 0000-0001-6922-8888; Yan-Qi Zhang 0000-0001-9438-0953; Yan Jiang 0000-0003-4628-7150; Jie Bai 0000-0003-3031-8434; Yu Pan 0000-0003-3827-2418; Shi-Yun Zhong 0000-0001-7148-8777; Yun-Ping Zhong 0000-0001-9027-6104; Zhi-Yu Chen 0000-0002-1321-1793; Hai-Su Dai 0000-0001-9896-6249.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
杂志排行
World Journal of Gastroenterology的其它文章
- Dualistic role of platelets in living donor liver transplantation: Are they harmful?
- Cystic fibrosis transmembrane conductance regulator prevents ischemia/reperfusion induced intestinal apoptosis via inhibiting PI3K/AKT/NF-κB pathway
- Applications of endoscopic ultrasound elastography in pancreatic diseases: From literature to real life
- Relationship between clinical remission of perianal fistulas in Crohn’s disease and serum adalimumab concentrations: A multi-center cross-sectional study
