A Viologen-bearing Coordination Polymer with Multi-stimuli Responsive Behaviors①
2022-03-12ANMiaoMiaoCUIJingWangTANBinCHENYunRuiZHANGJie
AN Miao-Miao CUI Jing-Wang TAN Bin CHEN Yun-Rui ZHANG Jie
(School of Chemistry and Chemical Engineering, MOE Key Laboratory of Cluster Science, Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, Beijing Institute of Technology, Beijing 102488, China)
ABSTRACT A viologen-bearing Zn(II) coordination polymer, [Zn(Bpybc)SO4]·H2O, has been successfully synthesized and structurally characterized. The title compound exhibits sunlight and UV-induced photochromism,thermoschromism, and amine-selective chemochromism.
Keywords: viologens, coordination polymer, multi-stimuli response;
1 INTRODUCTION
In recent years, stimuli-responsive materials have drawn intense attention for their extensive applications in various high-tech areas[1,2], such as smart windows, rewritable copy papers, secret writing, sensors, brand protection, and anti-counterfeiting[3-7]. Under this background, chromic materials that possess the advantages of intuitive color change in response to stimuli including light, temperature,pressure, electricity, or chemicals have been explored[8,9].However, slow response rate and single-stimulus response behavior of these materials are still the limitation for their applications[10,11]. Thus, how to obtain a class of rapid multi-stimuli responsive materials is being an important issue for chemists.
Viologen derivatives, famous for their electron-deficient and redox properties, can exhibit chromic phenomena under proper conditions[12,13]. Different from those common chromophore groups that show chromic behaviors only in the case of structural transfomation such as diarylethene, spiropyran, olefin derivatives, and azobenzene which can perform local photochemical reactions including open or closed ring, dimerizationand cis-transisomerization[14-16], viologen derivatives′chromism is mainly attributed to stimuli-induced electron transfer from donor to acceptor. It should be noted that the electron transfer efficiency can be regulated by altering the distance and orientation between donor and acceptor.Therefore, we and other groups have been devoted to making use of viologen derivatives to construct coordination compounds by means of crystal engineering in order to improve chromic performance[17,18]. Even so,considering that most of these chromic materials function in isolation, it is highly desirable to develop viologenbased coordination compounds owning more than two different responsive behaviors.
To fulfill this aim, we selected viologen derivative H2BpybcCl2as a building block to synthesize a coordination polymer [Zn(Bpybc)SO4]·H2O (Zn-Bpy), showing photochromism, thermochromism, and amine-selective chemochromism. Upon irradiation by UV light or sunlight,this compound displays instant color change. Heattreatment at 100~150 °C can also trigger obvious coloration, meaning the occurrence of thermochromism.Zn-Bpy also has good performance in detecting amines and exhibits different color development once fumigated under different amines. Thus, the compound is capable of exhibiting multiple responses to light, heat and chemical stimuli.
2 EXPERIMENTAL
2. 1 Materials and instruments
All chemicals were obtained from commercial sources and used without further purification. IR spectra (KBr pellets)were obtained on a Nicolet iS10 FT-IR spectrometer. The Powder X-ray diffraction (PXRD) pattern was obtained by using a Bruker D8 Advance X-ray diffractometer with CuKαradiation (λ= 1.54056 Å). UV-vis diffuse reflectance spectra were recorded at room temperature by a Perkin-Elmer Lambda 950 spectrometer. Electron paramagnetic resonance(EPR) signal was collected with a JES FA-200 spectrometer.
2. 2 Synthesis of compound Zn-Bpy
H2BpybcCl2was synthesized according to our previous method[19]. Zn-Bpy was synthesized by the reaction of ZnSO4,H2BCbpyCl2, and 1,2,4-triazole under hydrothermal conditions. H2BpybcCl2(0.0125 g, 0.025 mmol),ZnSO4·7H2O (0.0287 g, 0.1 mmol) and 1,2,4-triazole (0.0069 g, 0.1 mmol) were dissolved in ethanol (3 mL) and water (2 mL). The mixture was sealed in a 25 mL glass bottle after ultrasonic oscillation for 10 minutes, then placed in an oven for three days at a constant temperature of 100 °C. Colorless transparent crystals can be obtained by naturally cooling to room temperature. The yield is about 45% based on Zn.
2. 3 X-ray crystallographic study
Single-crystal X-ray analysis was performed with an Agilent Gemini Ultra diffractometer with graphite-monochromated MoKαradiation (λ= 0.71073 Å) at 293 K. All absorption correction was performed by using the multi-scan program. The structures were solved by direct methods and refined by using a full-matrix least-squares refinement onF2with the SHELXTL-2017 program. All non-hydrogen atoms were refined with anisotropic displacement parameters. The hydrogen atoms attached to C atoms were generated geometrically and refined by a riding mode with isotropic thermal parameters fixed at 1.2 times that of the mother atoms,while the hydrogen atoms of water molecules were placed on calculated positions and refined with isotropic thermal parameters fixed at 1.5 times that of the O atoms.
3 RESULTS AND DISCUSSION
3. 1 Structure description
Single-crystal diffraction analysis reveals that Zn-Bpy crystallizes in the triclinic system, space groupPwitha=10.4577(8),b= 10.8735(10),c= 13.5499(13) Å;α=109.509(9)º,β= 93.392(7)º,γ= 115.307(8)º,V= 1275.6(2)Å3,Z= 2,Dc= 1.572 g·cm-3,F(000) = 620,μ= 1.103 mm-1,S= 1.027,R= 0.0406 andwR= 0.0864 (I> 2σ(I)). The selected bond lengths and bond angles are listed in Table 1. There is one Zn2+, two halves of Bpybc, one SO42-, and one dissociative water molecule in the asymmetric unit. Although 1,2,4-triazole does not appear in Zn-Bpy, its presence is helpful for the crystallization of Zn-Bpy. As depicted in Fig.1a, each Zn2+ion is four-coordinated in a slightly distorted tetrahedral geometry by two O atoms from two SO42-ions and two O atoms from two different Bpybc2+ligands. Two SO42-ions bridge two Zn2+ions to form a dinuclear [Zn2(SO4)2] unit,which is connected by four Bpybc ligands to generate a two-dimensional square-grid coordination network,which contains large edge-sharingparallelogram grids with the approximate size of 23×26 Å as measured between the Zn2+nodes (Fig. 1b). There is no interpenetration in the structure of Zn-Bpy. These two- dimensional laminar networks are further stacked in an -ABCDE- mode along thebaxis to give rise to a three- dimensional stacking architecture (Fig. 1c). No significant hydrogen bonding andπ-πinteractions occur between these laminar networks.
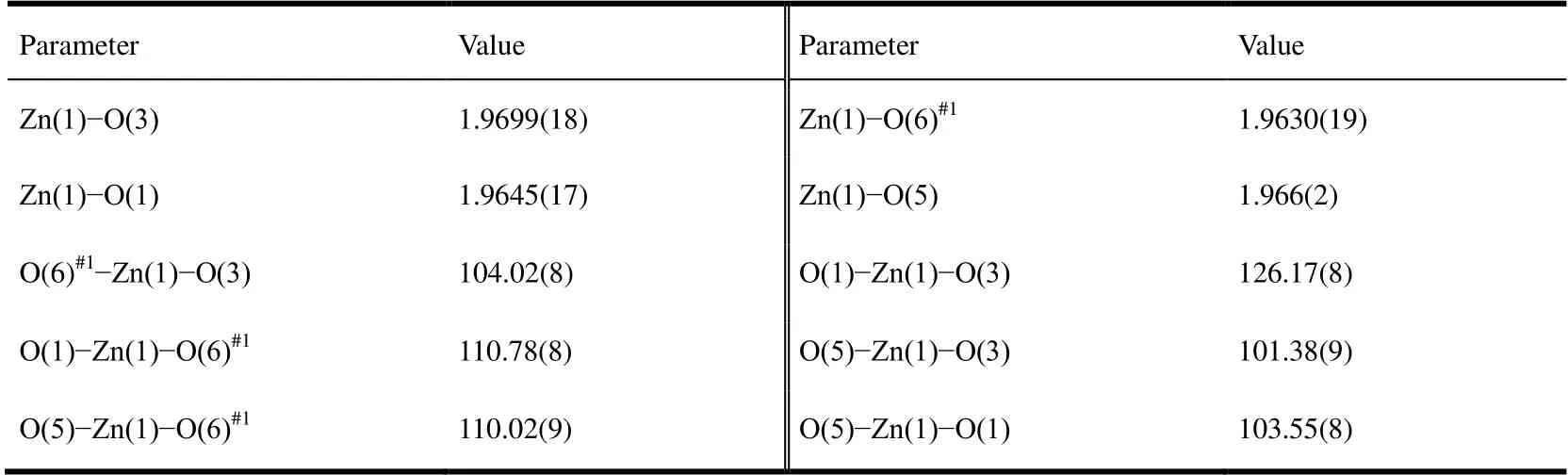
Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
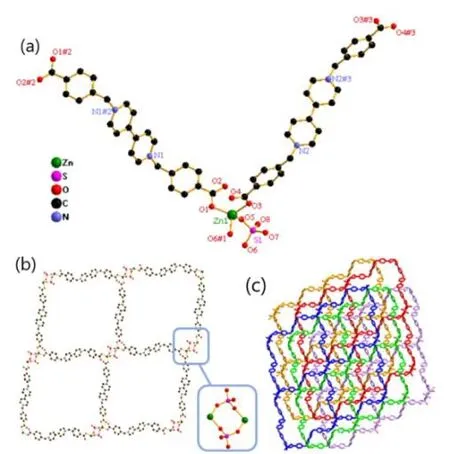
Fig. 1. (a) Coordination environment around the Zn2+ ion in Zn-Bpy. Symmetry codes:#1: 1-x, 1-y, -z; #2: -1-x, -y, 1-z; #3: 2-x, 3-y, 2-z. (b) The 2D layered motif in Zn-Bpy.The enlarged image shows the linkage of a dinuclear Zn2+ unit. (c) The packing structure of Zn-Bpy
3. 2 IR and PXRD spectral analysis
The compound shows a characteristic peak at 1636 cm-1in the infrared spectrum (Fig. S1), corresponding to the stretching vibrations of C=N and C=C of the pyridinium ring.The peaks at 1557 and 1375 cm-1can be attributed tovasandvsstretching vibrations of the carboxyl group. The characteristic vibration of sulfate species appears at 1116 cm-1.Such spectral information agrees with the X-ray crystal structures of the title compound. The powder X-ray diffraction pattern shows that the pattern of the as-synthesized sample is in accordance with the simulated one from the single-crystal structure analysis, confirming its phase purity.
3. 3 Photochromism
Zn-Bpy shows a rapid and obvious photochromic transformation from colorless to dark blue in response to the irradiation of 365 nm light for only 1 s, and the color gradually deepens as the irradiation time extends (The inset of Fig. 2a). From the UV-vis spectra, it can be observed that two new absorption peaks appear at about 410 and 640 nm,respectively, and they gradually increase with prolonged irradiation time (Fig. 2a). The EPR spectrum displays a typical free radical signal atg= 2.0051 after 365 nm light irradiation (Fig. 2b). These results clearly reveal that the photochromic behavior originates from the generation of free radicals via photoinduced electron transfer. No obvious decoloration is observed in the dark at room temperature for two months while the sample can be discolored gradually when heated under an air atmosphere at 70 °C for 7 h.Additionally, the coloration-decoloration process for Zn-Bpy can be repeated several times, confirming the reversibility and recyclability of such a chromic process. Besides rapid photochromism in response to UV light, Zn-Bpy also shows an obvious color change into blue when exposed to sunlight,and a tinge of blue can be observed under ambient light (The inset of Fig. 2b).
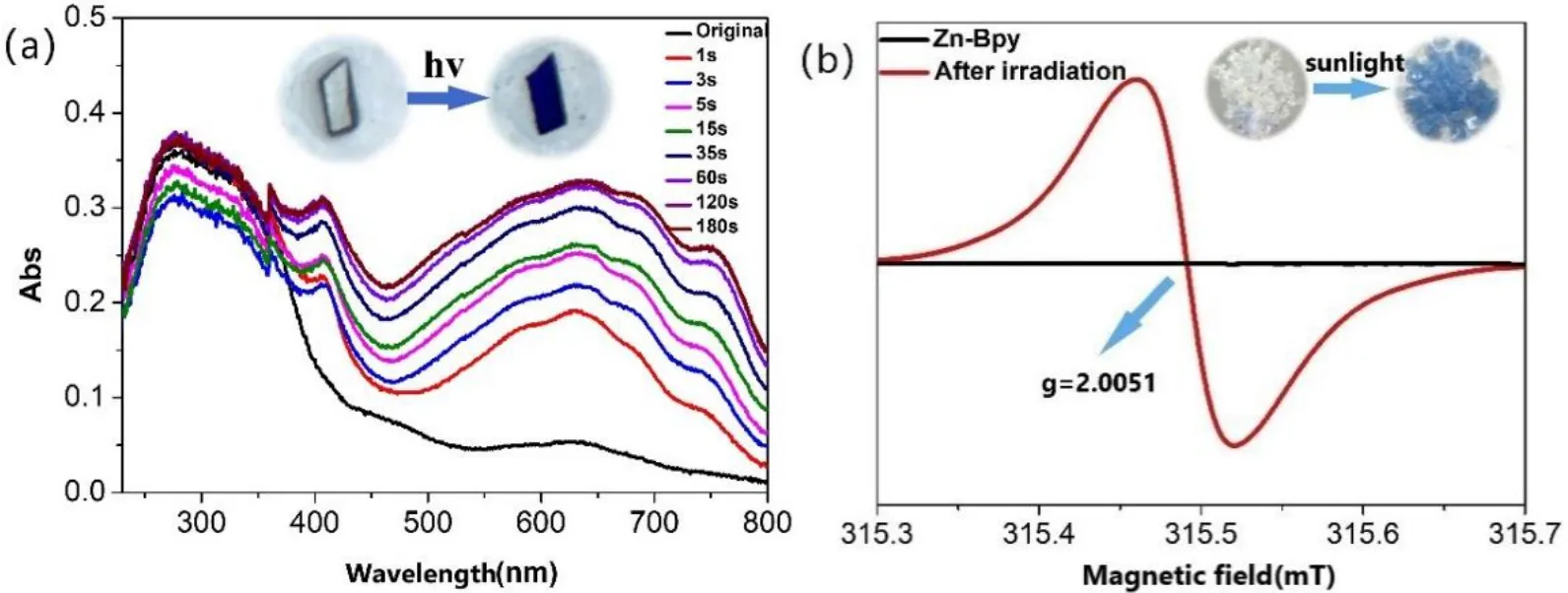
Fig. 2. (a) Time-dependent UV-vis adsorption spectra of Zn-Bpy under 365 nm irradiation. Inset: photochromic phenomenon of Zn-Bpy before and after UV light irradiation. (b) The EPR spectra of Zn-Bpy before and after 365 nm irradiation. Inset: sunlight-induced photochromic phenomenon of Zn-Bpy
3. 4 Thermochromism
Besides photochromism, Zn-Bpy also exhibits remarkable thermochromic behavior in the air atmosphere. Upon heat-treatment under 100 °C just for 3 s, Zn-Bpy turns light green, and as the temperature rises to 150 °C, the crystals become dark green. Heated continuously to 170 °C, it starts to undergo a decoloration process (Fig. 3a, top). The PXRD spectra of Zn-Bpy at 150 °C change a little as compared with that of the as-synthesized sample, indicating the formation of a new phase and a tiny transformation in the structure induced by loss of water molecules and color change (Fig. 3b,bottom).
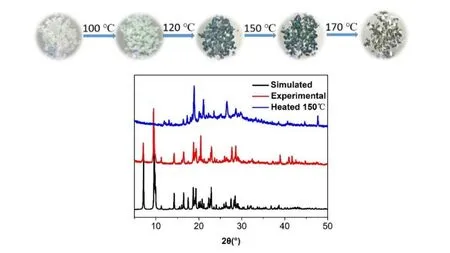
Fig. 3. Top: the color change of Zn-Bpy at different temperature.Bottom: comparison for PXRD patterns of Zn-Bpy before and after heat-treatment at 150 °C
3. 5 Chemochromism
In addition to physical stimuli such as light and heat, there are also many chemical stimuli in nature. Volatile amine vapors are one of the most common chemical stimuli and have received widespread attention because they are commonly harmful to humans and the environment, and are usually colorless and difficult to detect. By using contact with ammonia/amine vapor to produce color changes that are visible to the naked eye, it is possible to develop a more convenient and economical method for fast and intuitive detection. Zn-Bpy turns purple-blue within 10 seconds when exposed to ammonia vapor, and the colored sample can be bleached to the initial state in the air within 3 h owing to the quenching effect of oxygen. However, no color change is observed when exposed to the vapor of the ammonia-water binary mixture, suggesting an inhibition role of water molecules in the generation of bipyridinium radicals. Besides,fumigation in ethylamine, ethanediamine, 1,2-propanediamine, or diethylamine causes the compound to turn to blue,purple, or yellow-green, respectively (Fig. 4a, top). The response rate of Zn-Bpy to these amines is different. More rapid response to ammonia and ethanediamine can be observed, while the relatively slow response rate to ethylamine and 1,2-propanediamine, and the lowest response rate to diethylamine. Strikingly, these colored samples maintain crystal form and can be bleached in the air due to the quenching effect of oxygen. Remarkably, there is also a fast decoloration when fumigated with water vapor. This phenomenon may well explain why Zn-Bpy discolors when exposed to ammonia vapor but no color change under the vapor atmosphere of the ammonia-water binary mixture. The UV-vis diffuse reflectance spectra of Zn-Bpy show new broad absorption peaks between 450 and 750 nm after fumigation with pure ammonia vapor, ethylamine,ethanediamine, diethylamine and 1,2-propanediamine vapors.These spectral traces correspond with the characteristic absorption of bipyridinium radicals (Fig. 4b, bottom),demonstrating that the coloration response originates from the electron transfer from the amine electron donors to viologen electron-acceptor units. Despite intense color changes observed for most amine-response in the crystalline state, the absorption intensity of the viologen radicals is relatively weak in UV-vis diffuse reflectance spectra because the surface area of the sample is greatly increased when the sample is dispersed on a barium sulfate substrate. A more rapid oxygen quenching might occur during the measurement process.
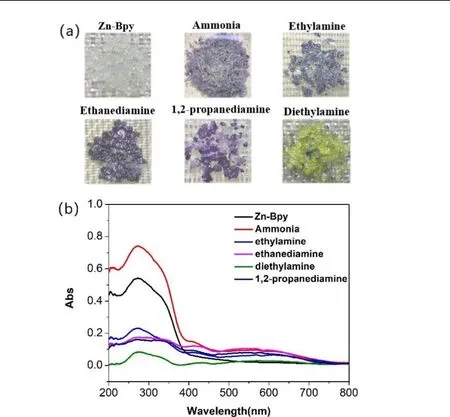
Fig. 4. (a) Color change of Zn-Bpy after fumigation with different amine vapors.(b) UV-vis spectra of Zn-Bpy before and after exposure to different amine vapors
4 CONCLUSION
In conclusion, a novel viologen-based coordination polymer has been synthesized and structurally characterized.The title compound possesses multiple-in-one chromic behaviors which can recognize various external stimuli such as UV light, sunlight, heat, ammonia and different types of volatile organic amine vapors. Such multiple response behaviors illustrate the potential of Zn-Bpy in serving as a convenient multifunctional sensing material. The current results also provide a new insight into the design and synthesis of multi-stimuli responsive smart materials in the future.
杂志排行
结构化学的其它文章
- A Terbium(III)-organic Coordination Polymer:Synthesis, Characterization and Its Luminescence
- Structural and Magnetic Characterization of Two New Coordination Compounds Based on a Fluorene Derivative Ligand①
- Synthesis, Crystal Structure and DNA-Binding Property of a New Cu(II) Complex Based on 4-(Trifluoro-methyl)nicotinic Acid①
- Synthesis, Structures, Luminescence and Catalytic Activity in the Knoevenagel Condensation Reaction of Two Cd(II) Coordination Polymers Based on a Biphenyl-dicarboxylic Acid①
- Crystal Structure, Fe3+ Luminescence Sensing and Color Tuning of 2D Lanthanide-metal-organic Frameworks Constructed from Tricarboxylic Acid Ligand①
- Drug Design, Molecular Docking, and ADMET Prediction of CCR5 Inhibitors Based on QSAR Study①
