Synthesis, Structures, Luminescence and Catalytic Activity in the Knoevenagel Condensation Reaction of Two Cd(II) Coordination Polymers Based on a Biphenyl-dicarboxylic Acid①
2022-03-12WUJiangGUJinZhong
WU Jiang GU Jin-Zhong
a (Key Laboratory for Tibet Plateau Phytochemistry of Qinghai Province,School of Pharmacy, Qinghai University for Nationalities, Xining 810007, China)
b (College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, China)
ABSTRACT Two cadmium(II) coordination polymers, namely [Cd(μ-dda)(H2biim)]n (1) and [Cd(μ4-dda)(py)]n(2) have been constructed hydrothermally using H2dda (H2dda = 4,4΄-dihydroxybiphenyl-3,3΄-dicarboxylic acid),H2biim (H2biim = 2,2΄-biimidazole), py (py = pyridine), and cadmium chloride at 160 °C. The products were isolated as stable crystalline solids and were characterized by IR spectra, elemental analyses, thermogravimetric analyses (TGA), and single-crystal X-ray diffraction analyses. Single-crystal X-ray diffraction analyses revealed that both compounds crystallize in the monoclinic system, space group C2/c. Compound 1 discloses a 1D chain structure. Compound 2 features a 3D framework. The luminescent properties of compounds 1 and 2 were evaluated.Besides, the catalytic activities in the Knoevenagel condensation reaction of two compounds were investigated.Compound 1 exhibits an excellent catalytic activity in the Knoevenagel condensation reaction at room temperature.
Keywords: coordination polymer, dicarboxylic acid, luminescence, catalytic properties, Knoevenagel condensation reaction; DOI: 10.14102/j.cnki.0254-5861.2011-3250
1 INTRODUCTION
Coordination polymers and derived materials have attracted tremendous attention due to their structural and topological diversity as well as their potential applications as functional materials[1-15]. In the last five years, special organic carboxylate ligands have been widely used in synthesizing coordination polymers and derived materials due to strong coordination ability of the carboxyl group and rich coordination modes[5,6,12,16-19]. Among them, biphenylcarboxylic acids have been extensively applied as versatile building blocks towards the assembly of metal-organic architectures[16,20,21].
The 4,4΄-dihydroxybiphenyl-3,3΄-dicarboxylic acid (H2dda)is a good bridging ligand for the constructing coordination polymers[22-24], under considering structural semi-rigidity,which has multiple coordinate sites involving four carboxylate oxygen atoms andtwo hydroxyl O atoms.
Herein, we report the synthesis, crystal structures,luminescence, and catalysis of two Cd(II) coordination polymers with H2dda ligand.
2 EXPERIMENTAL
2. 1 General procedures
All chemicals and solvents were of AR grade and used without further purification. Carbon, hydrogen and nitrogen were determined using an Elementar Vario EL elemental analyzer. IR spectra were recorded using KBr pellets and a Bruker EQUINOX 55 spectrometer. Thermogravimetric analysis (TGA) was performed under N2atmosphere with a heating rate of 10 K/min on a LINSEIS STA PT1600 thermal analyzer. Excitation and emission spectra were recorded on an Edinburgh FLS920 fluorescence spectrometer using the solid samples at room temperature. Powder X-ray diffraction patterns (PXRD) were measured on a Rigaku-Dmax 2400 diffractometer using Cu-Kαradiation (λ= 1.5406 Å); the X-ray tube was operated at 40 kV and 40 mA. The data were collection in the range of 5~45º. Solution1H NMR spectra were recorded on a JNM ECS 400 M spectrometer.
2. 2 Synthesis of compound 1
A mixture of CdCl2·H2O (0.040 g, 0.2 mmol), H2dda (0.055 g, 0.2 mmol), H2biim (0.027 g, 0.2 mmol), NaOH (0.016 g,0.4 mmol), and H2O (10 mL) was stirred at room temperature for 15 min, and then sealed in a 25 mL Teflon-lined stainless-steel vessel, and heated at 160 ℃ for 3 days,followed by cooling to room temperature at a rate of 10 ℃/h.Yellow block-shaped crystals of 1 were isolated manually, and washed with distilled water. Yield: 47% (based on H2dda).Anal. Calcd. (%) for C20H14CdN4O6: C, 46.31; H, 2.72; N,10.80. Found (%): C, 46.41; H, 2.71; N, 10.72. IR (KBr, cm-1):1628w, 1553m, 1525w, 1473s, 1414s, 1358w, 1278w, 1250w,1175w, 1155w, 1127w, 1087w, 1039w, 992w, 952w, 920w,860w, 828m, 801w, 757m, 677w, 562w.
2. 3 Synthesis of compound 2
Synthesis of 2 was similar to 1 except using py (0.5 mL, 6.3 mmol) instead of NaOH. Colourless block-shaped crystals of 2 were isolated manually, and washed with distilled water.Yield: 50% (based on H2dda). Anal. Calcd. (%) for C19H13CdNO6: C, 49.21; H, 2.83; N, 3.02. Found (%): C,49.45; H, 2.81; N, 3.00. IR (KBr, cm-1): 1628w, 1599w,1539w, 1520m, 1478w, 1450w, 1416s, 1341w, 1294w, 1243m,1220w, 1154w, 1064w, 1036w, 1008w, 952w, 906w, 877w,821m, 745w, 704m, 637w, 554w.
2. 4 Structure determination
Two single crystals of the title compounds were mounted on a Bruker CCD diffractometer equipped with a graphitemonochromatic Cu-Kα(λ= 1.54178 Å) radiation using aφ-ωscan mode at 293(2) K. The structures were solved by direct methods with SHELXS-97[25]and refined by full-matrix least-squares techniques onF2with SHELXL-97[26]. All non-hydrogen atoms were refined anisotropically. All hydrogen atoms (except those bound to water molecules)were placed in the calculated positions with fixed isotropic thermal parameters and included in structure factor calculations in the final stage of full-matrix least-squares refinement. Compound 1 crystallizes in monoclinic system,space groupC2/cwitha= 18.4567(4),b= 7.05850(10),c=28.5221(6) Å,V= 3710.03(12) Å3,Z= 8, C20H14CdN4O6,Mr= 518.75,Dc= 1.857 g/cm3,F(000) = 2064, the finalR=0.0231 andwR= 0.0560 for 3440 observed reflections (I>2σ(I)). Compound 2 crystallizes in monoclinic system, space groupC2/cwitha= 18.4249(11),b= 11.6325(5),c=7.7798(4) Å,V= 1604.40(15) Å3,Z= 4, C19H13CdNO6,Mr=463.70,Dc= 1.920 g/cm3,F(000) = 920, the finalR= 0.0273 andwR= 0.0691 for 1491 observed reflections (I> 2σ(I)).The selected important bond parameters are given in Table 1.The hydrogen bonds in crystal packing of compounds 1 and 2 are listed in Tables 2 and 3.
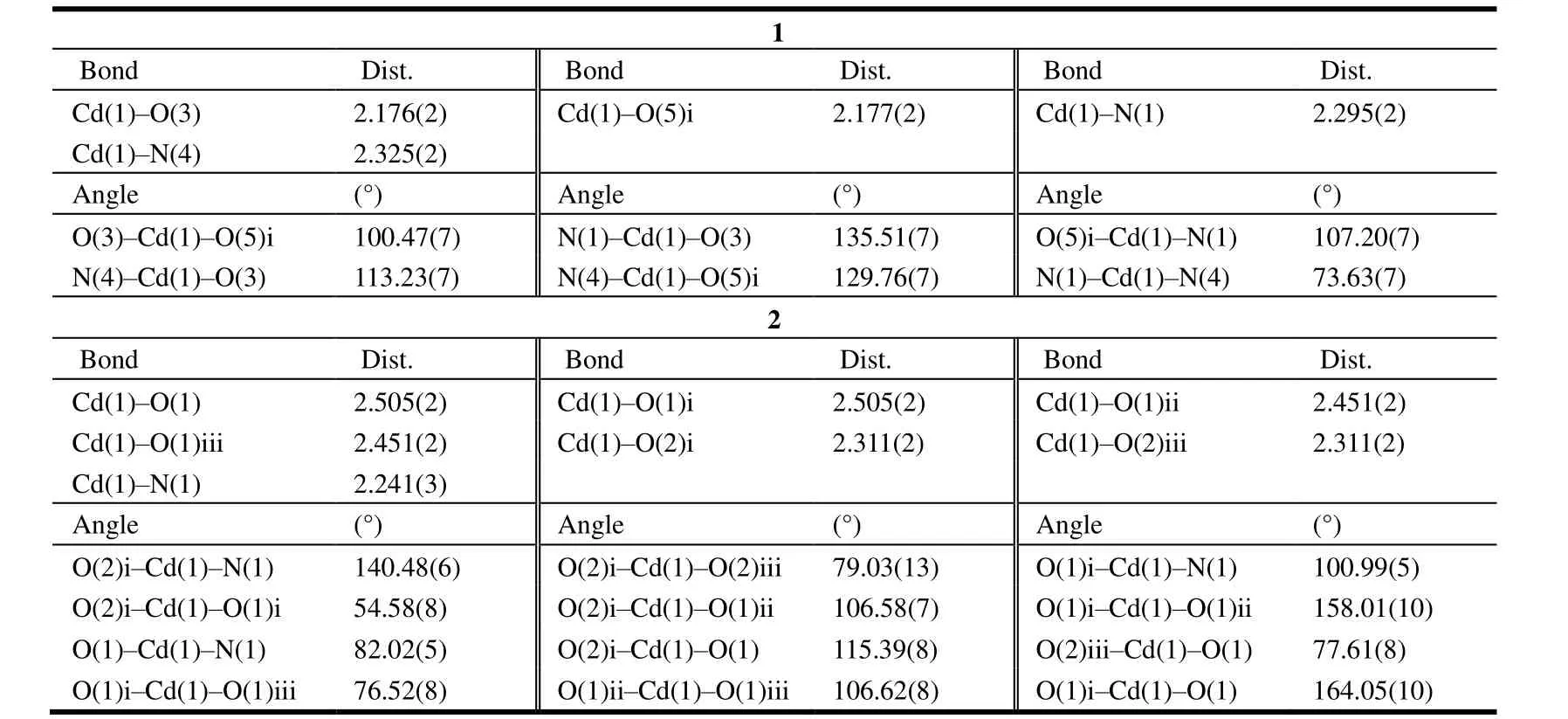
Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for 1 and 2

Table 2. Geometrical Parameters of Hydrogen Bonds for 1

Table 3. Geometrical Parameters of Hydrogen Bonds for 2
2. 5 Catalytic the Knoevenagel condensation reaction of aldehydes
In a typical test, a suspension of an aromatic aldehyde(0.50 mmol, benzaldehyde as a model substrate),malononitrile (1.0 mmol), and catalyst (typically 2 mol%)in methanol (1.0 mL) was stirred at room temperature.After a desired reaction time, the catalyst was removed by centrifugation, followed by an evaporation of the solvent from the filtrate under reduced pressure to give a crude solid. This was dissolved in CDCl3and analyzed by1H NMR spectroscopy for quantification of the products (Fig. 1). To perform the recycling experiment, the catalyst was isolated by centrifugation, washed with dichloromethane, dried at room temperature, and reused. The subsequent steps were performed as described above.
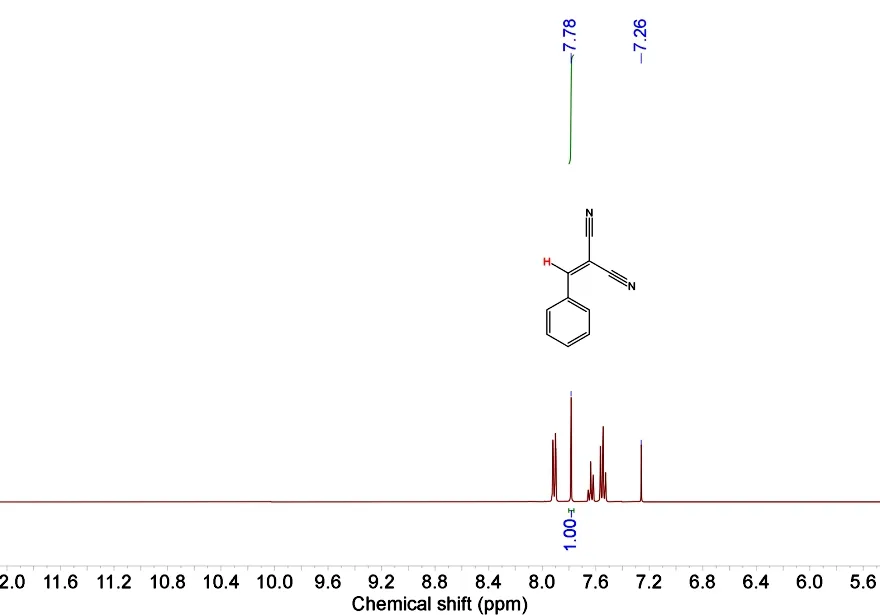
Fig. 1. Example of integration in the 1H-NMR spectrum for the determination of the Knoevenagel condensation reaction products (Table 4, Entry)
3 RESULTS AND DISCUSSION
3. 1 Crystal structure of 1
X-ray crystallography analysis reveals that compound 1 crystallizes in the monoclinic system, space groupC2/c. As shown in Fig. 2, the asymmetric unit of 1 bears one crystallographically unique Cd(II) atom (Cd(1)), oneμ-dda2-block, and one H2biim moiety. The tetra-coordinate Cd(1)atom exhibits a distorted tetrahedral {CdN2O2} environment,which is occupied by two carboxylate O donors from two differentμ-dda2-blocks and two N atoms from the H2biim moiety. The Cd-O and Cd-N bond distances are 2.176(2)~2.177(2) and 2.295(2)~2.325(2) Å, respectively; these are within the normal ranges observed in related Cd(II)compounds[12,20,27]. In 1, the dda2-ligand adopts the coordination mode I (Scheme 1) with two COO-groups being monodentate. The H2biim moiety takes the terminal coordination fashion. In the deta4-ligand, a dihedral angle(between two aromatic rings) is 25.17°. Theμ-dda2-blocks connect adjacent Cd atoms to give a 1Dchain (Fig. 3). This 1Dcoordination polymer features double chains built from the 4-connected Cd andµ4-dda2-nodes (Fig. 4). As a result,the chains represent a uninodal 4-connected network with a SP 1-periodic net (4,4)(2,2) topology. The neighboring chains are assembled into a 2Dsheet through the N-H···O hydrogen bonds (Table 2 and Fig. 5).
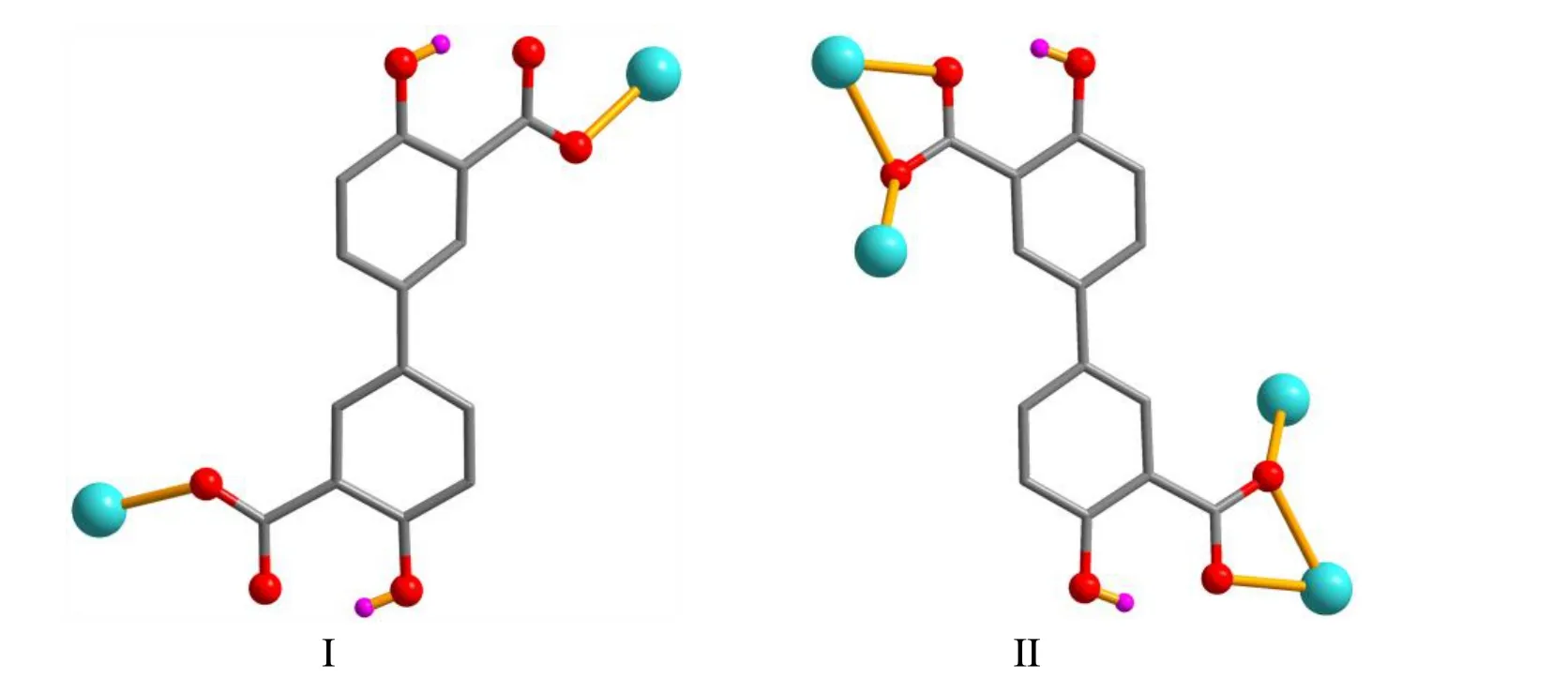
Scheme 1. Coordination modes of the dda2- ligands in compounds 1 and 2
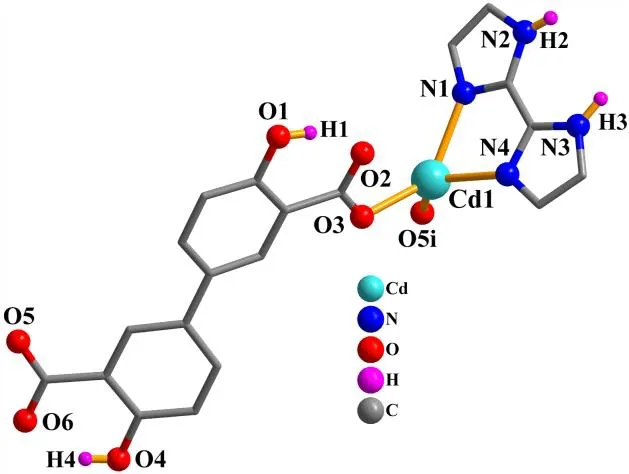
Fig. 2. Coordination environments of the Cd(II) atom in compound 1. The hydrogen atoms are omitted for clarity except in OH and NH groups (Symmetry code: i: -x+2, y+1, -z+1/2)
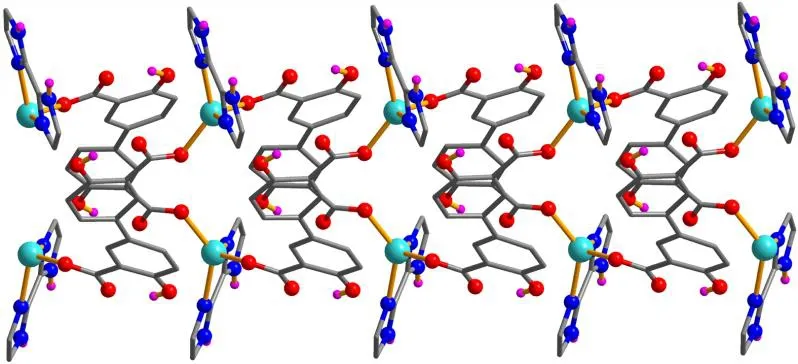
Fig. 3. Perspective view of the 1D chain along the c axis
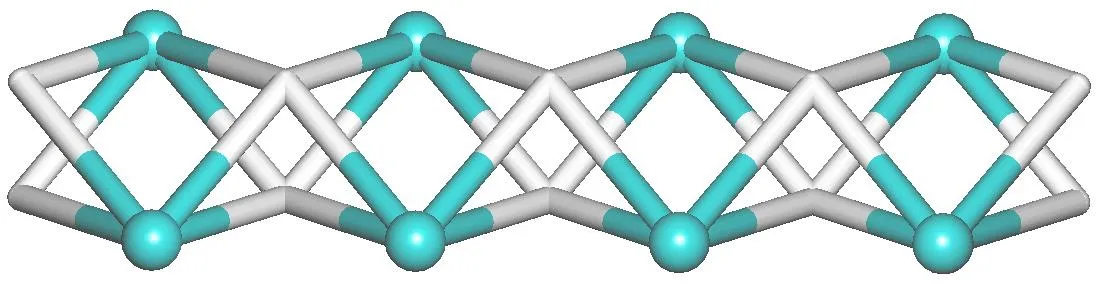
Fig. 4. Topological representation of a uninodal 4-connected network with a SP 1-periodic net (4,4)(2,2) topology;view along the c axis; 4-linked Cd nodes (green balls), centroids of 4-connected μ4-dda2- nodes (gray)
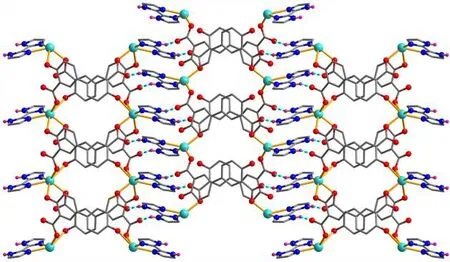
Fig. 5. Perspective view of the 2D sheet along the bc plane in 1 (Blue dashed lines present H-bonds)
3. 2 Crystal structure of 2
The asymmetric unit of compound 2 contains one crystallographically unique Cd(II) atom (Cd(1) with half occupancy), a half ofμ4-dda2-block, and a half of py moiety.As depicted in Fig. 6, the Cd(1) center is seven-coordinated and displays a distorted pentagonal bipyramidal {CdNO6}geometry. It is taken by six carboxylate O atoms from four individualμ4-dda2-blocks and one N donor from the py ligand. The bond lengths of Cd-O are in the 2.311(2)~2.505(2) Å range, while the Cd-N bond is 2.241(3) Å, being comparable to those found in some reported Cd(II)compounds[20,27,28]. In 2, the dda2-block acts as aμ4-linker(mode II, Scheme 1), in which two carboxylate groups adopt a tridentate mode. Besides, two aromatic rings are coplanar in theμ4-dda2-ligand. Theμ4-dda2-blocks multiply interconnect the neighboring Cd1 centers to generate a 3Dmetal-organic framework (Fig. 7). This structure is assembled from the 4-connected Cd andµ4-dda2-nodes, which are arranged into a binodal 4,4-linked net with a pts [PtS, Cooperite] topology and a point symbol of (42.84) (Fig. 8).
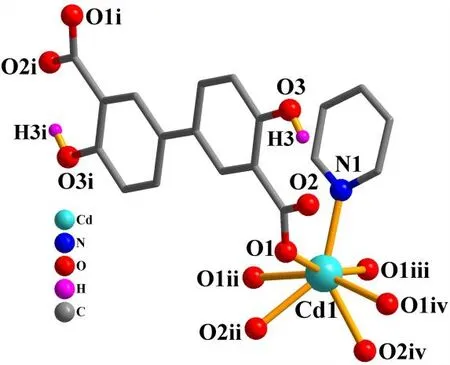
Fig. 6. Coordination environments of the Cd(II) atom in compound 2. The hydrogen atoms are omitted for clarity except in OH groups(Symmetry codes: i: -x+1/2, -y+1/2, -z+2; ii: x, -y, z+1/2; iii: -x, y, -z+3/2; iv: -x, -y, -z+1)

Fig. 7. View of 3D metal-organic framework along the ab plane. The py ligands are omitted for clarity
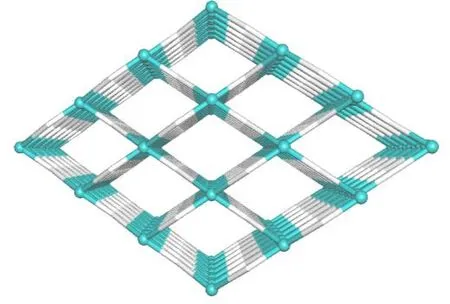
Fig. 8. Topological representation of a binodal 4,4-connected network with a ptstopology; view along the ab plane; 4-linked Cd nodes (green balls),centroids of 4-connected μ4-dda2- nodes (gray)
Compounds 1 and 2 were assembled under similar conditions except for the type of auxiliary ligand used(H2biim for 1 and py for 2). The difference in their structures,1Dchain in 1vs. 3Dsheet in 2, indicates that the assembly process is dependent on the type of auxiliary ligand.
3. 3 Thermal analysis
To determine the thermal stability of polymers 1 and 2,their thermal behaviors were investigated under nitrogen atmosphere by thermogravimetric analysis (TGA). As shown in Fig. 9, compounds 1 and 2 do not contain solvent of crystallization or H2O ligands and remain stable up to 245 or 274 ℃, followed by a decomposition on further heating. CdO is expected as a final decomposition product of 1 (exptl.25.1%, calcd. 24.8%) and 2 (exptl. 28.0%, calcd. 27.7%).
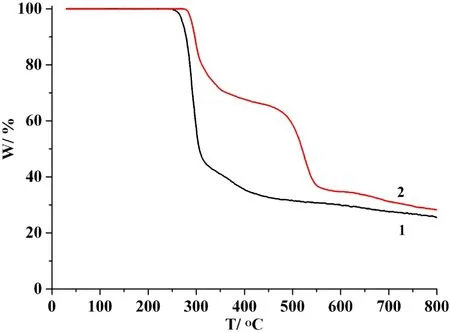
Fig. 9. TGA curves of compounds 1 and 2
3. 4 Luminescent properties
The excitation and emission spectra of 4,4΄-dihydroxybiphenyl-3,3΄-dicarboxylic acid (H2dda) and polymers 1 and 2 were measured in the solid state at room temperature (Figs. 10 and 11). The uncoordinated H2dda shows a weak photoluminescence with an emission maximum at 482 nm (λex= 366 nm). In contrast, compounds 1 and 2 display significantly more intense emission bands with the maxima at 455 and 444 nm (λex= 348 nm), respectively. The emissions of compounds 1 and 2 are blue-shifted relative to that of the free H2dda ligand, which can be assigned to the ligand-to-metal charge transfer (LMCT)[27,29]. The luminescence enhancement in the coordination compounds can be attributed to the binding of ligands to the metal centers, which effectively increases the rigidity of the ligand and reduces the loss of energy by radiationless decay[27,28]. The quantum yield and life time were measured (1.36%, 0.1 ms for 1 and 5.88%, 0.3 ms for 2).
3. 5 Catalytic Knoevenagel condensation reaction
Given the potential of cadmium(II) coordination compounds to catalyze the organic reactions[30-32], we explored the application of 1 and 2 as heterogeneous catalysts in the Knoevenagel condensation reaction of benzaldehyde as a model substrate to give 2-(phenylmethylene)-propanedinitrile.Typical tests were carried out by reacting a mixture of benzaldehyde, malononitrile, and a Cd catalyst in methanol at room temperature (Scheme 2, Table 4). Such effects as reaction time, catalyst loading, solvent composition, catalyst recycling, and finally substrate scope were investigated.
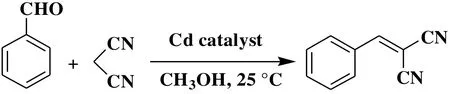
Scheme 2. Cd-catalyzed the Knoevenagel condensation reaction of benzaldehyde (model substrate)
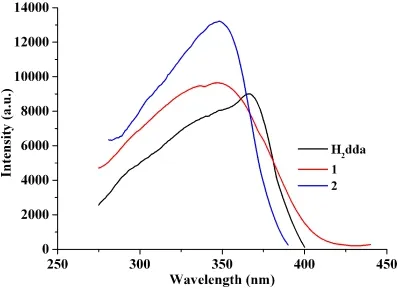
Fig. 10. Solid-state excitation spectra of H2dda, and compounds 1 and 2 at room temperature

Fig. 11. Solid-state emission spectra of H2dda and compounds 1 and 2 at room temperature
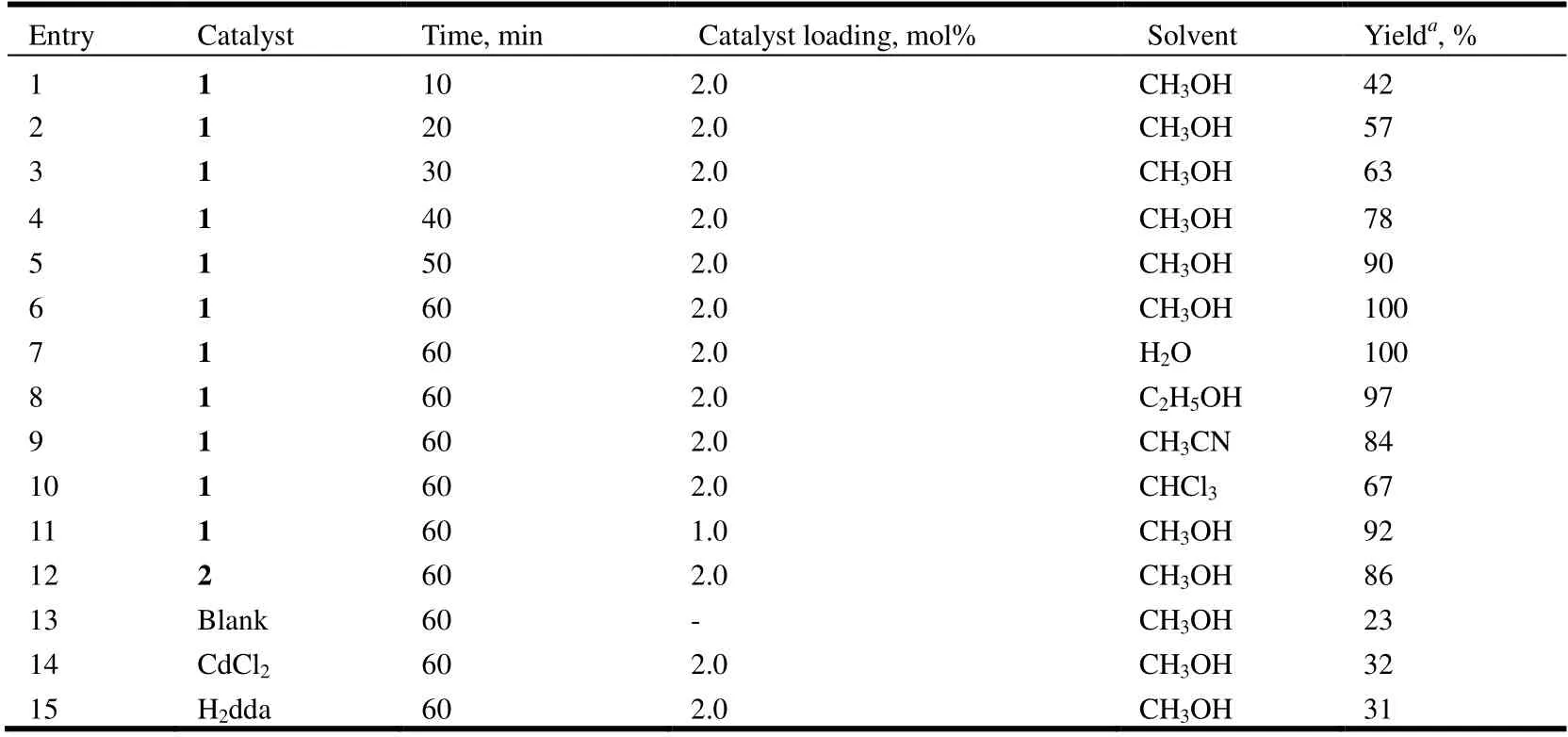
Table 4. Co-catalyzed Knoevenagel Condensation Reaction of Benzaldehyde with Malononitrile
Upon using compound 1 as the catalyst (2 mol%), a high conversion of 100% of benzaldehyde into 2-(phenylmethylene)-propanedinitrile was reached after 60 min in methanol at room temperature (Table 4, entry 6).
The results show that compound 1 is more active than compound 2. Although the relationship between structure and catalytic activity in the present study can not be clearly established, the highest conversation shown by compound 1 may eventually be associated to its 1Dstructure for easily accessible metal centers, together with the presence of the open metal sites[21,30].
We also compared the activities of catalyst 1 in the reactions of other substituted aromatic aldehydes with malononitrile, and the corresponding yields fall in the range of 55~100% (Table 5). Aryl aldehydes bearing strong electron-withdrawing substituents (e.g., nitro and chloro)exhibited higher reactivities (Table 5, entries 2~5), which may be related to an increase in the electrophilicity of the substrate. Aldehydes containing electron-donating groups(e.g., methyl) showed lower reaction yields (Table 5, entries 6~8), as expected.
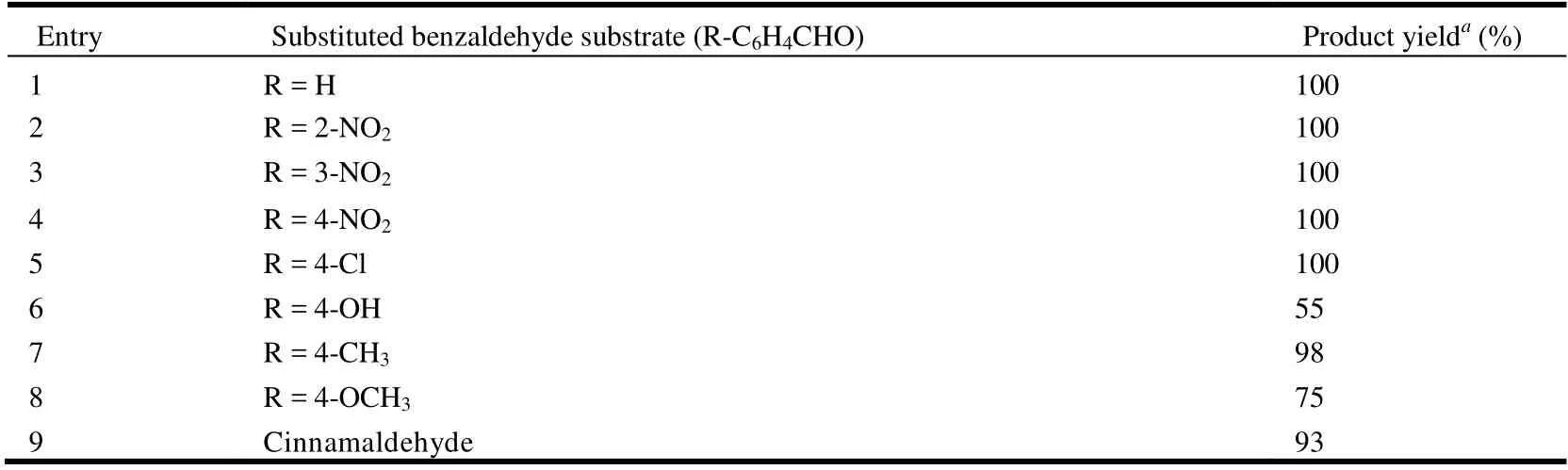
Table 5. Knoevenagel Condensation Reaction of Various Aldehydes with Malononitrile Catalyzed by 1
To examine the stability of 1 in the catalytic reaction, we tested the recyclability of this heterogeneous catalyst. For this purpose, upon completion of a reaction cycle, we separated the catalyst by centrifugation, washed it with CH2Cl2, and dried it at room temperature before further use. We recycled catalyst 1 repeatedly, and the catalytic system maintained the higher activity over at least five consecutive cycles (the yields are 100, 100, 99, and 98% for the second to fifth runs,respectively). According to the PXRD data (Fig. 12), the structure of 1 is essentially preserved after five catalytic cycles.

Fig. 12. PXRD patterns for 1: simulated (red), before (black) and after (blue) catalysis
4 CONCLUSION
In summary, we have synthesized two Cd(II) coordination polymers based on a dicarboxylate ligand. Compound 1 discloses a 1Dchain structure, and compound 2 features a 3Dframework. The catalytic properties of both compounds were investigated. Compound 1 revealed an excellent catalytic activity in the Knoevenagel condensation reaction at room temperature.
杂志排行
结构化学的其它文章
- A Terbium(III)-organic Coordination Polymer:Synthesis, Characterization and Its Luminescence
- Structural and Magnetic Characterization of Two New Coordination Compounds Based on a Fluorene Derivative Ligand①
- A Viologen-bearing Coordination Polymer with Multi-stimuli Responsive Behaviors①
- Synthesis, Crystal Structure and DNA-Binding Property of a New Cu(II) Complex Based on 4-(Trifluoro-methyl)nicotinic Acid①
- Crystal Structure, Fe3+ Luminescence Sensing and Color Tuning of 2D Lanthanide-metal-organic Frameworks Constructed from Tricarboxylic Acid Ligand①
- Drug Design, Molecular Docking, and ADMET Prediction of CCR5 Inhibitors Based on QSAR Study①
