Determination of volatile oil and non-volatile organic compounds contents and ultrastructure changes in the petals of Dendrobium huoshanense upon Zn application*
2022-03-11ZHUWangshengDAIJunCHENNaidongWANGJiahong
ZHU Wangsheng,DAI Jun,CHEN Naidong,WANG Jiahong
(1.Engineering Technology Research Center for Plant Cell of Anhui Province,West Anhui University,Lu’an 237012,China;2.Department of Food Science and Technology,College of Light Industry and Food Engineering,Nanjing Forestry University,Nanjing 210037,China)
Abstract:The petals of Dendrobium huoshanense have great potential as functional food because of their pleasant fragrance and health values.To obtain high-quality raw materials for functional food production,more attention is being directed toward improving the food quality traits of D.huoshanense petals under field cultivation conditions.In this study,we investigated the effects of Zn application on the volatile oil and non-volatile organic compounds contents in the petals of D.huoshanense.Furthermore,the mechanism was also studied from the perspective of petal ultrastructural changes.Volatile oil constituents were investigated using gas chromatography-mass spectrometry.The contents of non-volatile organic compounds,including soluble sugar,free amino acids,and phenolic compounds,were investigated using chemical analysis methods.Ultrastructural changes were observed via transmission electron microscopy.The results showed that Zn application increased the yield of volatile oils(primarily terpenes and their derivatives)and non-volatile organic compounds from the petals of D.huoshanense.Different levels of Zn treatments changed the production of the above-mentioned volatiles and non-volatile compounds to varying degrees.The maximum rate of increase in the content of volatile oil,soluble sugars,free amino acids,and phenolic compounds was 28.57%,33.53%,28.89%,and 58.41%,respectively.Ultrastructural changes at the cellular level showed that Zn application promoted vacuole fusion and increased the production of mitochondria,starch grains,and lipid droplets.In conclusion,Zn application improves the production of volatile oils and non-volatile organic compounds.Ultrastructural analysis has provided new anatomical insights into the production of volatile oils and non-volatile organic compounds.
Keywords:Dendrobium huoshanense;Non-volatiles;Ultrastructure;Volatile oils;Zn levels
(Orchidaceae),commonly known as ‘soft gold’,is a perennial medicinal plant distributed in Anhui,China(Chen et al.,2020;Lee et al.,2014).In recent years,has been increasingly cultivated as an edible plant or aromatic crop due to its high economic value(Jin et al.,2016;Pan et al.,2013).The petals ofhave great potential to be utilized as functional food,including essential oils and tea products,owing to their pleasant fragrance and health values.To obtain high-quality raw materials for functional food production,more attention is being focused on improving the food quality traits ofpetals under field cultivation conditions by regulating environmental factors such as microelements.
Zinc(Zn)is an essential micronutrient for plants.It has various roles in several biochemical processes,including enzyme activation,photosynthesis,respiration,carbon(C)and nitrogne(N)metabolism,and flower development,etc.(Amiri et al.,2016;Kumar et al.,2019;Olechnowicz et al.,2018;Song et al.,2015;Ullah et al.,2020).In crop production,the low solubility of Zn in the soil often leads to Zn deficiency in edible crops.Zn deficiency can inhibit cell growth and proliferation and reduce the yield and quality of edible crops(Navarro-León et al.,2016;Song et al.,2015).Thus,it is often important to supplement edible crops with Zn fertilizers.However,as with Zn deficiency,an excess of Zn can also cause a decrease in the yield and quality of edible crops by inhibiting electron transfer via photosynthesis,damaging cell membranes,and blocking nutrient absorption(Kaznina et al.,2019;Rezaeieh et al.,2016).Therefore,neither Zn deficiency nor excess Zn level is conducive to the high-yield and high-quality production of edible crops.Therefore,the rational application of Zn in edible crop production has become an interesting topic for research.
So far,many studies have been conducted on how to improve the effects of Zn on edible crop production.These studies focused on the application methods and doses of Zn.The types of edible crops concentrated on are primarily rice(),wheat(),mung beans(),etc.(Kaznina et al.,2019;Lajayer et al.,2017;Moreno-Moyano,et al.,2016;Samreen et al.,2017).However,no similar studies have been conducted on,and especially on the volatile oils and non-volatile organic compounds in the petals.
Volatile oils and non-volatile organic compounds are important indicators for the quality evaluation of functional food,such as essential oils and tea products(Aisala et al.,2020;Ayseli et al.,2016).Their production efficiency in plant cells can be evaluated based on the anatomical features of the plants(Gill et al.,2015).For example,the petal epidermis and parenchyma cells are the places where volatile oils are released and nonvolatiles are stored.The production of volatile and nonvolatile organic compounds can be characterized by specific changes in vacuoles and related organelles in petal cells(Konarska,2017;Maiti et al.,2017).Virtually no ultrastructural analysis was performed onpetals to determine cell specificity in terms of volatile oils and non-volatile organic compound production.
In research onpetals for use as essential oils,tea products,or other functional food,we focused our attention on whether different Zn fertilizer rates promote volatile oil production inpetals under field cultivation conditions.Furthermore,we investigated whether the Zn fertilizer treatments mentioned above increased the content of non-volatile organic compounds such as soluble sugar,free amino acids,and phenolic compounds.Finally,we performed ultrastructural studies to provide new anatomical insights into the production of volatile oils and non-volatile organic compounds.
1 Materials and methods
1.1 Plant material and Zn treatments
Healthy 3-year-oldplants were used in this study.Theplants were planted in a greenhouse(31°76′ N,116°48′ E,47.68 m above sea level).The greenhouse roof was covered with a sunshade net with 70% light transmittance of natural light and a humidity level of 40%-60% was automatically controlled under a diurnal temperature from -5 to 40 ℃.Fully fermented pine bark was used as a cultivation medium(pH 6.05,organic carbon 50.89 g·kg,total nitrogen 1.35 g·kg,total phosphorus 0.16 g·kg,total potassium 32.31 g·kg,Zn 0.012 g·kg).Subsequently,a split-plot experiment was designed.The uniformly grownplants were divided into four main plots with four levels of ZnSO·7HO aqueous solutions[0,2.9,5.8 and 11.6 mg·(100 mL)]being applied to the main plots.The control group was sprayed with 100 mL of 0 mg·(100 mL)ZnSO·7HO aqueous solution(distilled water)(named Zn0)by foliar fertilization every 6 days for one month,for a total of five applications.Similarly,the experimental groups were sprayed with 100 mL of 2.9,5.8,11.6 mg·(100 mL)ZnSO·7HO aqueous solution(named Zn2.9,Zn5.8,and Zn11.6),with total Zn levels of 134,268,and 536 g·hm,respectively.No surfactant was added to the Zn fertilizer solution.At full blooming stage,petal samples of the Zn0-Zn11.6 treatments were collected for analysis(Fig.1A-D).

Fig.1 Representative samples of petals of Dendrobium hu-oshanense treated with different levels of Zn.A(Zn0):0 mg·(100 mL)-1 ZnSO4·7H2O;B(Zn 2.9):2.9 mg·(100 mL)-1 ZnSO4·7H2O;C(Zn5.8):5.8 mg·(100 mL)-1 ZnSO4·7H2O;D(Zn11.6):11.6 mg·(100 mL)-1 ZnSO4·7H2O.
1.2 Analysis of volatile oils
petals(100 g)were added to 800 mL distilled water for the separation of volatile oil components by hydro distillation.Hydro distillation continued until no more volatile oils were obtained.Before chemical analysis,the volatile oils were stored in sealed vials in the dark at -20 ℃.Gas chromatography-mass spectrometry(GC-MS,Thermo Fisher Trace ISQ)was used to analyze the volatile oil components.The yield of volatile oils was estimated based on fresh weight.
The pure volatile oils obtained by hydro distillation were diluted with dichloromethane to a 1% solution.Next,1 μL 1% solution was injected into the gas chromatograph for analysis.The gas chromatograph was equipped with a flame ionization detector and an HP-5MS column(30 m × 0.25 mm × 0.25 μm).The gas chromatograph operating conditions were as follows:the carrier gas was hydrogen(flow rate:1.0 mL·min);oven temperature was programmed to the initial temperature at 40 ℃ for 3 min,ramped to 150 ℃ at 5 ℃·min,and then increased to 250 ℃ at 10 ℃·minand held steady for 5 min.The percentage composition was obtained via normalization.
MS analysis was performed using an MS detector coupled to a gas chromatograph.The MS detector was set at an EI ionization source temperature of 250 ℃,transmission line temperature of 250 ℃,ionization voltage of 70 EV,and a mass scanning range of 35-450 m·z.The oven temperature and injection program followed the gas chromatograph operation described above.The carrier gas was helium,and its flow rate was 1.0 mL·min.
Identification of volatile oil components was performed by comparing their mass spectra with those from the standard NIST library and by comparing their linear retention indices with those from the literature.
To reveal the relationship among petal samples treated with Zn0-Zn11.6 according to the volatile oil composition,and to identify the main constituents influencing variability,the composition data matrix of four samples(51 variables × 4 samples = 204 data)was analyzed using PCA with Origin software(Origin 17.0).A two-dimensional PCA biplot was generated for four levels of Zn and volatile oil compounds.
1.3 Analysis of non-volatile organic compounds
Four grams of fresh petals were harvested,ground,and suspended in 100 mL hydrochloric acid(0.01 mol·L).The mixed suspension was thoroughly mixed at room temperature and allowed to settle for 30 min.Next,15 mL supernatant was centrifuged for 30 min.The supernatant obtained by centrifugation was filtered through a filtration membrane(0.22 μm)and analyzed using a capillary electrophoresis method,as described by Bell et al.(Bell et al.,2017).Subsequently,soluble sugars,including glucose,fructose,sucrose,and galactose,were quantified using an external standard method.
Five grams of fresh petals were added to 50 mL acetonitrile(25%)and homogenized in hydrochloric acid(0.01 mol·L).The homogenate was then centrifuged for 30 min.The supernatant from the homogenate was filtered through a polyvinylidene fluoride membrane(0.22 μm,Millipore,USA).The filtrate(100 μL)was diluted in 900 μL distilled HO.According to the method,as described by Elmore et al.(Elmore et al.,2005),free amino acids were analyzed and quantified.
Two grams of fresh petals were chopped and extracted with 95% methanol(20 mL).At room temperature,extraction was carried out in a shaking incubator for 24 h.After extraction,the extracts were centrifuged at 11 000×g for 30 min(4 ℃).The centrifugation supernatant was filtered using Whatman paper.The phenolic compound content was determined according to the method of Nam et al.(Nam et al.,2019).
1.4 Transmission electron microscopy(TEM)
The fresh petals were placed immediately in a TEM fixative solution(2.5% glutaraldehyde-0.1 mol·Lcacodylate buffer,pH 7.2,4 ℃)for 24 h.The next day,the samples were washed in cacodylate buffer and post-fixed in 1% osmium tetraoxide-0.1 mol·Lcacodylate buffer(pH 7.4,22 ℃)for 1 h.Subsequently,ultrathin sections were prepared according to the method of Marques et al.(Marques et al.,2016).The resulting ultrathin sections were examined using a transmission electron microscope(Hitachi TEM system,Japan).
1.5 Statistical analysis
SPSS software(version 17.0)was used for statistical analysis of the data.One-way ANOVA and Tukey’ s test were used to compare mean differences.Differences were considered statistically significant when the-value was 0.05.
2 Results
2.1 Volatile oil constituents in petals under different Zn treatment conditions
The volatile oils hydro distilled from the petals oftreated with different levels of Zn are reported in Table 1 and Fig.2.A total of 51 components were identified in the volatile oils.They were primarily characterized by terpenes,with β-caryophyllene and βocimene being the most abundant components.The second most abundant group was alcohols,with 1-hexanol and(E,E)-farnesol being the relatively abundant components.The third group was ketones,with 3-octanone,geranyl acetone,andβ-dihydro-β-ionone being the representative components.Finally,esters with tiglic acid ethyl ester as the main component were present in a certain proportion,whereas benzenes,aldehydes,and other volatile compounds were present in small amounts.

Fig.2 Total ion chromatogram of petal volatile oils of Dendrobium huoshanense treated with different levels of Zn application.A:0 mg·(100 mL)-1 ZnSO4·7H2O treatment;B:2.9 mg·(100 mL)-1 ZnSO4·7H2O treatment;C:5.8 mg·(100 mL)-1 ZnSO4·7H2O treatment;D:11.6 mg·(100 mL)-1 ZnSO4·7H2O treatment.

Table 1 Constituents of volatile oils from the petals of Dendrobium huoshanense treated with different levels of Zn
When comparing the volatile oils obtained frompetals treated with different levels of Zn,we found that the Zn2.9-Zn11.6 treatments increased the oil yield compared with the control(Zn0 treatment).Moreover,under the Zn11.6 treatment,the highest increase in oil yield was reached at 28.57%.These differences were primarily due to the relatively higher level of terpenes,including α-farnesene,β-ocimene,β-caryophyllene,and a higher quantity of alcohols,including 1-hexanol in the volatile oils.
PCA further evidenced distinct separations in the volatile oil constituents under different Zn treatments.The two principal components(PC1 and PC2)in the PCA explained 80.0% of the sample variance,indicating that the analysis was effective(Fig.3).The score analysis revealed that Zn0-Zn11.6 treatments were well sep-arated from each other depending on the volatile oil constituents.This indicated that there was an evident difference among the effects of different Zn treatments on volatile oils,and Zn application generally promoted the production of volatile oils.The loading analysis confirmed that the variability in volatile oil constituents was collectively generated mostly by αfarnesene,β-ocimene,β-caryophyllene,1-hexanol,tiglic acid ethyl ester,3-octanone,(E,E)-farnesol,and geranyl acetone.

Fig.3 Bi-plot(score plot and loading plot)of the PCA of the volatile oil constituents in petals of Dendrobium huoshanense asinfluenced by different levels of Zn application.Zn0:0 mg·(100 mL)-1 ZnSO4·7H2O;Zn2.9:2.9 mg·(100 mL)-1 ZnSO4·7H2O;Zn5.8:5.8 mg·(100 mL)-1 ZnSO4·7H2O;Zn11.6:11.6 mg·(100 mL)-1 ZnSO4·7H2O.No.1-51represents the corresponding volatile oil components in Table 1.
2.2 Contents of non-volatile organic compounds in petals under different Zn treatment conditions
The soluble sugars contents were determined in the petals oftreated with different levels of Zn.The total and individual soluble sugar contents are reported in Table 2.All Zn treatments increased the contents of four soluble sugars.Moreover,Zn5.8 and Zn11.6 treatments significantly(<0.05)increased the contents of total soluble sugars,fructose,and glucose.The maximum rate of increase in the total soluble sugars contents was 33.53%.
The contents of total and individual free amino acids in the petals oftreated with different levels of Zn are reported in Table 3.The results showed that the contents of total free amino acids under Zn2.9-Zn11.6 treatments were significantly higher than that of the control.The maximum rate of increase in the total free amino acids content was 28.89%.For individual amino acids,their contents varied significantly under different Zn treatments.Overall,Zn treatments decreased the contents of lysine,leucine,and valine but increased the contents of glutamic acid,asparagine,alanine,proline,and threonine.Under Zn5.8-Zn11.6 treatments,the contents of the above-mentioned five individual amino acids were significantly increased compared with the control.

Table 2 Soluble sugar contents(mean±SE)in the petals of Dendrobium huoshanense treated with different levels of Zn mg·g-1(FW)

Table 3 Free amino acid contents(mean±SE)in the petals of Dendrobium huoshanense treated with different levels of Zn μg·g-1(FW)
The phenolic compound contents in the petals oftreated with different levels of Zn are shown in Table 4.All Zn treatments significantly increased the contents of individual and total phenolic compounds inpetals,and the higher the levels of Zn,the higher the content of individual and total phenolic compounds.The maximum rate of increase in the total phenolic compound content was 58.41%.
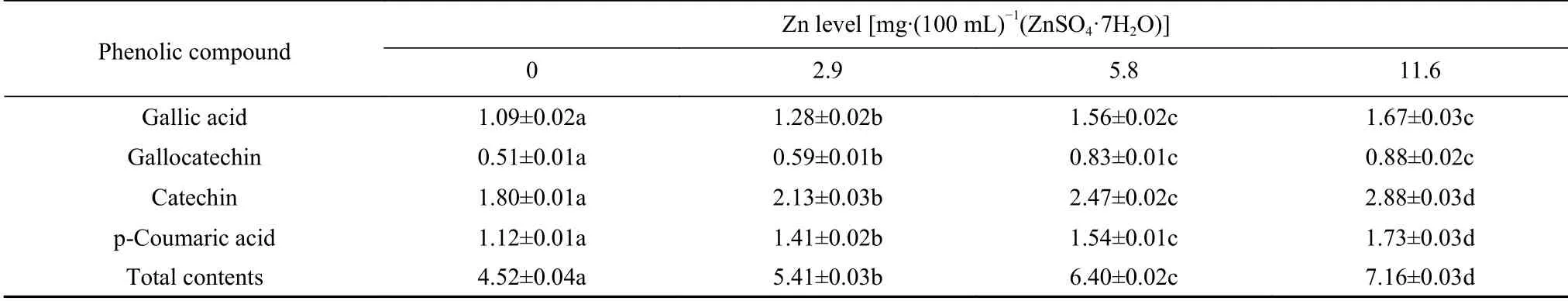
Table 4 Phenolic compound contents(mean±SE)in the petals of Dendrobium huoshanense treated with different levels of Zn g·kg-1(FW)
2.3 Ultrastructure characteristics of petals under different Zn treatment conditions
To explain the relationship between the production of volatiles and non-volatiles and ultrastructural changes,TEM analysis was carried out to observe changes in the submicroscopic structure of petal cells.In the petal samples ofthat were not treated with Zn(control),more scattered vacuoles were observed in the cells(Fig.4A).There were few mitochondria in the cytoplasm(Fig.4I).Moreover,only a few starch grains and lipid droplets were observed in the plastids(Fig.4E).After Zn treatment,TEM studies of petal cells revealed certain features of enhanced cell metabolism(Fig.4B-D,F-H,J-L).Under the Zn2.9 and Zn5.8 treatments,petal cells of the sample showed a trend of vacuole fusion,and vacuoles with long strips or connected vesicles were observed(Fig.4B-C).The number of mitochondria seemed to increase,and more mitochondria were visible in the cytoplasm(Fig.4J-K).At this point,more starch grains and lipid droplets were also visible in the plastids(Fig.4F-G).Under the Zn11.6 treatment,much of the petal cell volume was occupied by a central vacuole(Fig.4D).Mitochondria appeared in large numbers(Fig.4L),and many starch grains and lipid droplets in the plastids were also visible(Fig.4H).

Fig.4 Transmission electron microscopic images showing the submicroscopic structure characteristic of petal cells of Dendrobium huoshanense treated with different levels of Zn.There is an obvious difference in vacuole morphology(AD),starch and lipid accumulation(E-H),and the number of mitochondria(I-L)in petal cells among the Zn0-Zn11.6 treatments,respectively.Fig.A,E,and I show the Zn0 treatment[0 mg·(100 mL)-1 ZnSO4·7H2O];fig.B,F,and J show Zn2.9 treatment[2.9 mg·(100 mL)-1 ZnSO4·7H2O];fig.C,G,and K show Zn5.8 treatment[5.8 mg·(100 mL)-1 ZnSO4·7H2O];fig.D,H,and L show Zn11.6 treatment[11.6 mg·(100 mL)-1 ZnSO4·7H2O].“CW”cell wall,“CN”cell nucleus,“V”vacuole,“SG”starch grain,“P”plastid,“M”mitochondria,“→”lipid droplet.
3 Discussion
The proper Zn level has a positive effect on metabolite production in plants.However,when the Zn level is too low or excessive,it has a negative effect(Montalvo et al.,2016;Ullah et al.,2020).In general,as a microelement,the range of Zn level in fertilizer is 2.5 -15 mg·(100 mL),which stimulates various physiological processes of plants into achieving optimal effects.Values less than two or more than 40 mg·(100 mL)will cause symptoms of Zn deficiency or toxicity in plants(Kumar et al.,2019;Zhao et al.,2017).In this study,the 2.9-11.6 mg·(100 mL)Zn fertilizer treatments showed positive effects,which promoted the production of volatile oils and non-volatile organic compounds in the petals of.The ultrastructure of the petals also showed anatomical features consistent with positive changes in the above-mentioned volatile and non-volatile compounds.These changes were consistent with the expected research hypothesis.
3.1 Effects of Zn treatments on volatile oils
The yield of volatile oils from plants is not only affected by internal factors,such as genotype and ontogeny,but also by environmental factors such as microelements.The positive effects of micronutrients on volatile oil yields have been reported by many researchers(Alvarenga et al.,2015;Lajayer et al.,2017;Rezaeieh et al.,2016).In this study,Zn treatment promoted the yield of volatile oils inpetals(Table 1,Fig.2),and there was an evident concentration-effect among different levels of Zn(Fig.3).This has also been confirmed in other plants,such as(Lajayer et al.,2017).The difference in the positive effects of Zn on volatile oil production may be related to enhanced photosynthesis in plants.Previous studies have shown that Zn has an important effect on photosynthesis(Amiri et al.,2016;Zhao et al.,2017).Zn is a component of carbonic anhydrase which is an important enzyme affecting COfixation and glucose production during photosynthesis(Ogée et al.,2018;Rezaeieh et al.,2016).In volatile oil production,COand glucose are suitable precursors for terpenoid biosynthesis.Our study showed that volatile oils frompetals were primarily composed of terpenes and their derivatives(Table 1).Therefore,the concentration effect of Zn may cause differences in the photosynthesis rate and the number of terpenoid precursors,resulting in different yields of volatile oils inpetals.
3.2 Effects of Zn treatments on non-volatile organic compounds
Similar to volatile oils,non-volatiles are also organic metabolites from plants,and their accumulation in plants can also be affected by microelements.Several studies have shown that different levels of Zn have different effects on the content of carbohydrates,amino acids,and phenolic compounds in plants by changing carbon,nitrogen metabolism,and related biochemical processes(Amiri et al.,2016;Navarro-León et al.,2016;Ullah et al.,2020).
Soluble sugars,including fructose,glucose,galactose,and sucrose are important factors affecting flavor quality(Kelly et al.,2016).Soluble sugars content in the reproductive organs can be altered by different levels of Zn by affecting carbon metabolism and transport in plants.At an appropriate level,Zn can catalyze the hydration of carbon dioxide,enhance the intensity of photosynthesis,and improve the metabolic rate of carbohydrates(Saadati et al.,2013).Moreover,it can also promote and strengthen the transport of carbohydrates,especially soluble sugar,to reproductive organs(Razzaq et al.,2013;Song et al.,2015).Therefore,there should be an important correlation between the soluble sugar content and Zn levels.In this study,the soluble sugar content inpetals treated with different levels of Zn increased to varying degrees(Table 2).This was also demonstrated in research conducted on other plants.For instance,Song et al.reported that the soluble sugar content in the berry ofcv.Merlot was also increased by different levels of Zn application(Song et al.,2015).
Free amino acids have various important biological activities.Their production may be affected by different levels of Zn which affects nitrogen metabolism.Navarro-León et al.reported that Zn deficiency negatively affected nitrogen metabolism(including NOreduction and NH
assimilation),and changed the free amino acid profile inandplants in different states of Zn deficiency(Navarro-León et al.,2016).According to our results,Zn application increased the content of free amino acids inpetals(Table 3).This may be related to the concentration effect of Zn in promoting nitrogen metabolism.Several studies have reported that appropriate levels of Zn significantly increase the nitrogen uptake of crop seedlings and the contents of nine amino acids in grains(Barrameda-Medina et al.,2017).
Phenolic compounds are ubiquitous metabolites in photosynthesizing organisms(Okatan,2018).In higher plants,their synthesis is primarily related to carbon and nitrogen that are produced through the shikimic acid pathway(Elmastaş et al.,2017).As mentioned above,Zn can change the carbon and nitrogen metabolism.Thus,in theory,Zn can also affect the production of phenolic compounds,and there should also be differences in the concentration effect.Many studies have confirmed this finding.For example,Kumar et al.reported that Zn application significantly increased the concentration of phenolic compounds,and there was a significant difference among the contents of p-hydroxybenzoic acid,gallic acid,salicylic acid,syringic acid,protocatechuic acid,and vanillic acid inunder different levels of Zn treatment(Kumar et al.,2019).Similarly,in our study,different levels of Zn increased the content of phenolic compounds to varying degrees inpetals(Table 4).
3.3 Ultrastructure changes associated with volatile oils and non-volatile organic compounds
The accumulation of metabolites in plants is related to the intensity of metabolism.The intensity of metabolism is reflected in plant morphology and anatomy,which has been confirmed by studies in many types of plants(Gill et al.,2015;Guimarães et al.,2016;Maiti et al.,2017).Ultrastructural changes inpetals were observed under different Zn treatments.The TEM analysis in this study showed that the Zn2.9-Zn11.6 treatments promoted vacuole fusion of petal cells(Fig.4A-D).As the Zn level increased,the degree of vacuole fusion became more evident(Fig.4AD).It is well known that small vacuoles in mature plant cells are fused into larger vacuoles,which play an important role in promoting substance absorption,transport,and accumulation(Rodriguez-Furlan et al.,2019;Zhang et al.,2014).Simultaneously,the number of mitochondria,starch grains,and lipid droplets(Fig.4E-L)also gradually increased in petal cells with the Zn level,from low to high.Mitochondria are involved in cell energy metabolism.It is generally believed that the probability of mitochondrial appearance increases with the increasing intensity of cell activity(Maiti et al.,2017).In addition,an increase in starch grains and lipid droplets indicates an acceleration of organic matter metabolism(Boehlein et al.,2019;Chen et al.,2016;Manan,et al.,2016).Regrettably,changes in chloroplast ultrastructure that are closely related to the metabolism of organic matter have not been observed.However,despite this,ultrastructural changes in the above-mentioned organelles have presented an anatomical feature of enhanced cell metabolism,which is manifested by an increase in the production of volatile oils and non-volatile compounds in the petals ofunder different levels of Zn treatments.
4 Conclusions
This study demonstrated that Zn application increased the production of volatile oils and non-volatile organic compounds in the petals of.Furthermore,ultrastructural changes in petal cells were observed.The vacuoles in the cells of petals after Zn treatment presented a fusion trend,and the number of mitochondria increased.Moreover,there were more starch grains and lipid droplets in the plastids of petal cells.It can be concluded that Zn application increased the production of volatile oils and non-volatile organic compounds,probably by promoting vacuole fusion and increasing the number of mitochondria,starch grains,and lipid droplets.Ultrastructural analysis has provided new anatomical insights into the production of volatile oils and non-volatile organic compounds.
