Crystal Structure, Fe3+ Luminescence Sensing and Color Tuning of 2D Lanthanide-metal-organic Frameworks Constructed from Tricarboxylic Acid Ligand①
2022-03-08PANMengQiYANGRunQiMUHAMMADYseenCAIKunTongHANFengZHANGHoChenNIUYunQingWANGHo
PAN Meng-Qi YANG Run-Qi MUHAMMAD Yseen CAI Kun-Tong HAN Feng ZHANG Ho-Chen NIU Yun-Qing WANG Ho②
a (Beijing Key Lab of Special Elastomer Composite Materials, College of New Materials and Chemical Engineering, Beijing Institute of Petrochemical Technology, Beijing 102617, China)
b (Institute of Chemical Sciences, University of Peshawar, Peshawar 25120, Pakistan)
ABSTRACT A new lanthanide metal-organic framework (MOF) [Eu(BTB)(phen)(DMF)] DMF (1, DMF =N,N-dimethylformamide) was synthesized using H3BTB (1,3,5-tri(4-carboxyphenyl)benzene) and phen(1,10-phenanthroline) under solvothermal conditions. The structure of the prepared MOF was characterized by single-crystal X-ray diffraction analyses, elemental analysis, fluorescence spectrum, FT-IR spectroscopy, powder X-ray diffraction and thermogravimetric analyses. The structure of 1 can be viewed as a 3-D supramolecular network, which is formed by the stacking of 2D layers through π-π interaction. The luminescence explorations revealed that 1 possesses favorable selectivity and sensitivity for testing Fe3+. Additionally, color tuning was achieved by varying Eu3+:Tb3+ ratios in the reaction mixtures.
Keywords: lanthanide metal-organic frameworks, sensing, luminescence, Fe3+, color tuning;
1 INTRODUCTION
Iron(Ⅲ), as an indispensable trace elements of our life,plays an important role in a number of physiological processes such as DNA synthesis, hemoglobin formation,oxygen transport and storage, and coordination of brain functions[1-5]. However, the presence of a large amount of iron(Ⅲ) is harmful to humans[6,7], causing damages to heart,liver, and other vital organs through the blood circulation, and hence in turn affects the absorption of other elements[8,9].Therefore, detection of iron(Ⅲ) in human body is getting greater attention where luminescent sensing has been marked as an efficient tool to achieve this task based on simplified and convenient operation[10-12].
Recently, metal-organic frameworks (MOFs) as new materials have been widely applied in numerous areas,including gas storage and separation, catalysis, optoelectronics, energy storage and conversion, photocatalysis and luminescence and sensing[13-24]. As a promising subfamily of MOFs, lanthanide based MOFs (Ln-MOFs) have attracted great attention for their significant luminescent properties because of the intrinsic features of lanthanide elements.Ln-MOFs have unique advantages and properties due to the 4felectron configuration of the lanthanide metal, such as strong absorption and inherent luminescence band of lanthanides, large Stokes shift, pure color, and relatively long luminescence lifetime, which make them receive more attention in luminescence sensing[25-33]. Furthermore, the fluorescent properties of Ln-MOFs can be further modulated by both analyte-metal and analyte-ligand interactions since the unique luminescence mechanism involves ligand-to-metal energy transfer (LMET)[34-36]. In particular, Eu3+and Tb3+based complexes have great significance for the luminescent studies because they can emit strong characteristic red or green light when excited by energy transfer from the ligand to the lanthanide ion via UV radiation. Moreover, mixed-Ln-
Received 30 April 2021; accepted 15 July 2021 (CCDC 2079858)
① This project was supported by the High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year
Plan (CIT&TCD201904044) and URT program of Beijing Institute of Petrochemical Technology (2020J00009)② Corresponding author. Wang Hao, E-mail: wangh@bipt.edu.cn MOFs can be obtained through integrating Eu3+and Tb3+into a single crystal of Ln-MOFs, which can effectively combine dual-luminescent centers to generate multicolored emission materials. In general, the changes in the emission color can be effectively modulated by changing the mixing ratios of Eu3+:Tb3+[37,38].
In our previous study, we have reported that some Ln-MOFs show sensitive response to nitrobenzene through fluorescence quenching mechanism[39]. In continuation to that study, herein we report the synthesis of a new Eu-MOF (1) by a solvothermal method using 1,3,5-tri(4-carboxyphenyl)benzene (H3BTB) as ligand for that polycarboxylic acids have rich coordination modes and strong coordination ability[40-43].1 is a two-dimensional (2D) laminar structure, and the three-dimensional (3D) structure was formed by the stacking of the 2Dlayers throughπ-πinteractions. The luminescence explorations revealed that 1 possesses good selectivity and sensitivity for the sensing of Fe3+. Additionally, color tuning in Eu3+/Tb3+systems was achieved by varying lanthanide ions ratios in the reaction mixture.
2 EXPERIMENTAL
2. 1 Materials and instruments
All the chemicals including H3BTB used in this study were of analytical grade and commercially available.
The luminescence spectra and sensing properties for the powdered solid samples were measured on an Edinburgh FS5 spectrophotometer under identical experimental conditions.Fourier transform infrared (FTIR) spectra were obtained on a Tensor 27 OPUS (Bruker) FT-IR spectrometer in a wavenumber range of 400~4000 cm-1. Thermogravimetric(TG) analysis was carried out on a Rigaku standard TG-DTA analyzer at a heating rate of 10 ℃ min-1from ambient temperature to 800 ℃ using an empty Al2O3crucible as a reference. The powder X-ray diffraction spectra (PXRD)were recorded on a Rigaku D/Max-2500 diffractometer at 40 kV, 100 mA for a Cu-target tube, and a graphite monochromator. Simulation of the PXRD pattern was carried out by the single-crystal data and diffraction-crystal module of the Mercury (Hg) program version 1.4.2. Elemental analyses (C,H and N) were performed on a Perkin-Elemer240C analyzer.
2. 2 Synthesis and crystallization
2. 2. 1 Synthesis of [Eu(BTB)(phen)(DMF)] DMF (1)
1 was synthesized using solvothermal method. A mixture of Eu(NO3)3·6H2O, H3BTB, 1,10-phenanthroline, and 5 mL DMF:H2O (v:v= 1:1) was added to a glass bottle, heated at 95 ℃ for 3 days, and then cooled to room temperature. The heterogeneous solution was separated from the solid phase and the crystals were washed with ethanol and dried at room temperature. Colorless block crystals were obtained. The net yield of various components was: 25%. Anal. Calcd. for C45H37EuN4O8(%): C, 59.14; H, 4.05; N, 6.13. Found (%): C,59.06; H, 4.15; N, 6.10. FT-IR (KBr pellets, cm-1): 3444 w,1650 m, 1582 s, 1520 s, 1409 s, 1182 w, 1104 w, 1014 w, 963 w, 859 w, 787 s, 730 w.
2. 2. 2 Synthesis of [EuxTb1-x(BTB)(phen)(DMF)] DMF (2~5)
EuxTb1-x-MOF were synthesized according to a similar procedure to that of 1 except doping a certain amount of Tb(NO3)3⋅6H2O instead of Eu(NO3)3⋅6H2O. The percentage of Tb(NO3)3⋅6H2O was 90.91% (2), 98.04% (3), 99.01% (4)and 100% (5), respectively. Complex 2: yield: 23%. Anal.Calcd. for C45H37Eu0.0909Tb0.9091N4O8: C, 58.74%; H, 4.05%;N, 6.09%. Found: C, 58.82%; H, 4.12%; N, 6.15%. FT-IR(KBr pellets, cm-1): 3440 m, 3444 w, 1650 m, 1606 w, 1581 m, 1531 s, 1411 s, 1182 w, 1105 w, 1014 w, 860 m, 786 s,730 m, 667 w, 470 m. Complex 3: Yield: 24%. Anal. Calcd.for C45H37Eu0.0196Tb0.9804N4O8: C, 58.71%; H, 4.05%; N,6.09%. Found: C, 58.66%; H, 4.01%; N, 6.02%. FT-IR (KBr pellets, cm-1): 3436 m, 2925 w, 2350 w, 1650 m, 1581 s, 1531 m, 1384 s, 1182 w, 1105 w, 1014 w, 860 m, 788 s, 730 w, 673 m, 470 m. Complex 4: yield: 21%. Anal. Calcd. for C45H37Eu0.0009Tb0.9901N4O8: C, 58.79%; H, 4.06%; N, 6.09%.Found: C, 58.71%; H, 4.13%; N, 6.15%. FT-IR (KBr pellets,cm-1): 3471 m, 3060 w, 2925 w, 1650 m, 1581 m, 1527 m,1425 s, 1182 w, 1014 w, 860 m, 813 w, 786 s, 730 m, 671 m,468 m. Complex 5: yield: 25%. Anal. Calcd. for C45H37TbN4O8: C, 58.70%; H, 4.05%; N, 6.09%. Found: C,58.66%; H, 4.11%; N, 6.17%. FT-IR (KBr pellets, cm-1):3430 m, 3060 w, 2925 w, 1650 m, 1581 m, 1529 m, 1421 s,1182 w, 1105 w, 1014 w, 850 m, 706 s, 730 m, 671 m, 468 m.
2. 3 X-ray crystallography
X-ray single-crystal diffraction data for 1 were collected on a Rigaku SCX-mini diffractometer at 293(2) K with Mo-Kαradiation (λ= 0.71073 Å) by anωscan mode. The crystal data were solved by direct methods and refined by full-matrix least-squares technique onF2using the SHELXS and SHELXL. More details on the crystallographic studies as well as atomic displacement parameters are given in the CIF file. All carbon-bonded hydrogen atoms were added theoretically, riding on the concerning atoms and refined with fixed thermal factors. Crystal data for 1: triclinic system,P1 space group witha= 9.0694(6),b= 14.7630(9),c=15.9047(7) Å,α= 69.049(5)°,β= 81.891(5)°,γ= 83.602(5)°,V= 1964.5(2) Å3,Z= 2, C45H37EuN4O8,Mr= 913.75,Dc=1.545 g/cm3,F(000) = 924,GOOF= 0.93. Selected bond lengths and bond angles of 1 are given in Table 1.
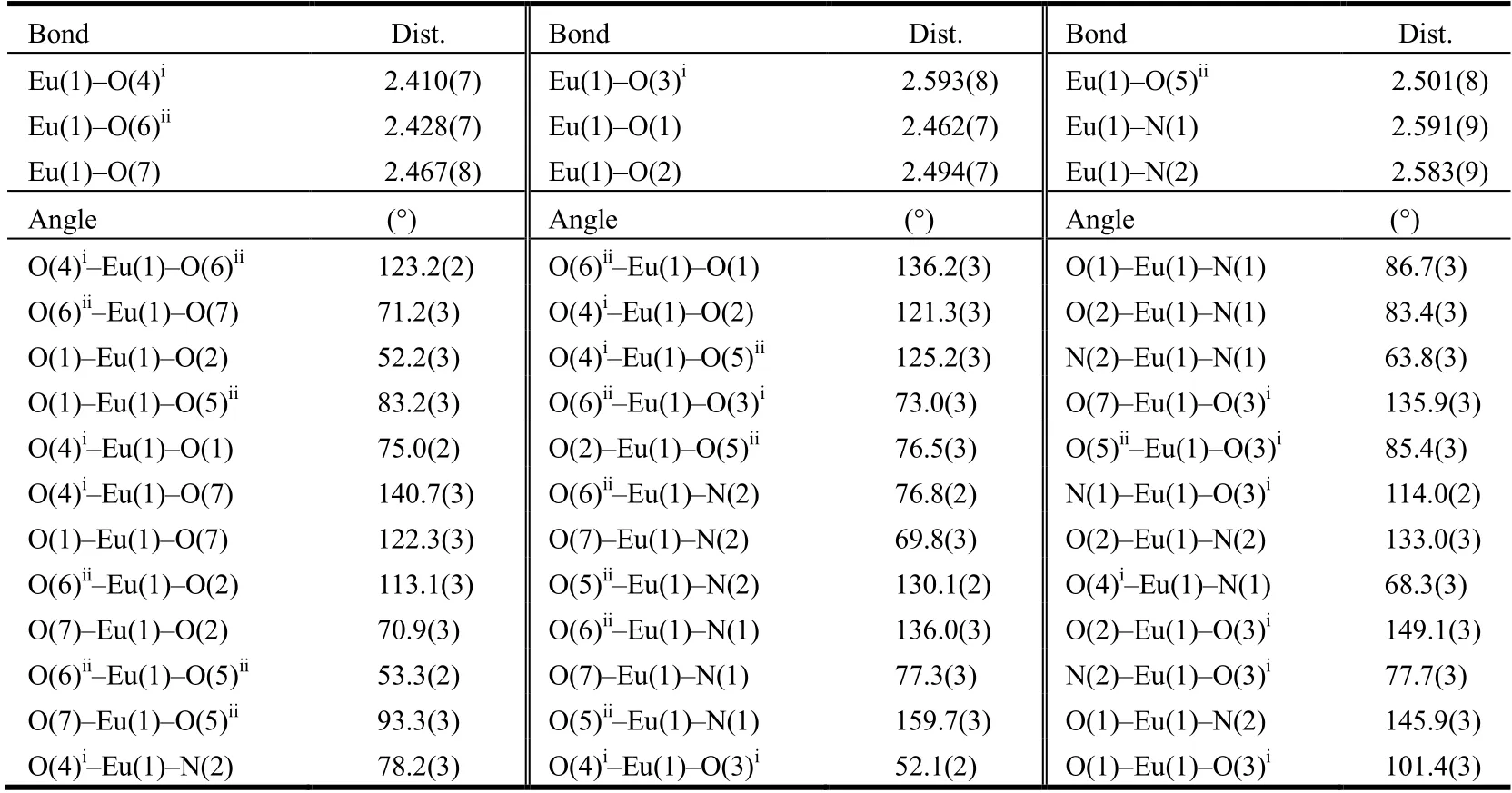
Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for 1
3 RESULTS AND DISCUSSION
3. 1 Description of the crystal structure
Single-crystal X-ray diffraction analysis reveals that 1 crystallizes in the triclinic system with space groupP1. In the asymmetric unit, there are one Eu3+ion, one BTB3-ligand,one 1,10-phenanthroline (phen) and one coordinated DMF as well as a free DMF. The central Eu3+ion exhibits a nine-coordinate environment forming a twisted triangular prism with six oxygen atoms from three BTB3-, two nitrogen atoms from a phen and an oxygen atom from a coordinated DMF (Fig. 1a). Each BTB3-connects three Eu3+ions through chelating coordination of carboxylic acids (Fig. 1b). As shown in Fig. 1c, Eu3+cations are connected as a node through three BTB3-to form a 2Dplane structure. Each 2Dstratified structure is stacked into a 3Dsupramolecular structure throughπ-πinteractions with the shortest centroidto-centroid distance of 3.646 Å (Fig. 1d). Topologically, Eu3+and BTB3-can be simplified as 3-connected nodes, and the 2Dplane as ahcbnet (Fig. 1e).
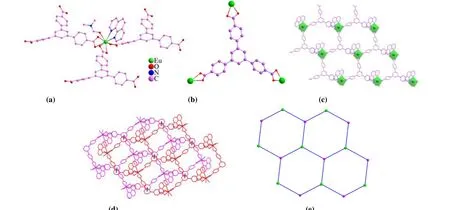
Fig. 1. (a) Coordinated environments of Eu3+; (b) Coordinated environments of BTB3-; (c) 2D framework of 1; (d) 3D supramolecular structure through π-π interactions (black dashed lines for π-π interactions); (e) hcb net of 2D plane
3. 2 PXRD and thermogravimetric analysis
The purity of the bulky crystalline samples of 1 was confirmed by PXRD at room temperature. As shown in Fig. 2,the PXRD pattern of the as-synthesized 1 corresponds well with the simulated one based on the single-crystal diffraction data, which confirms the good purity of 1.
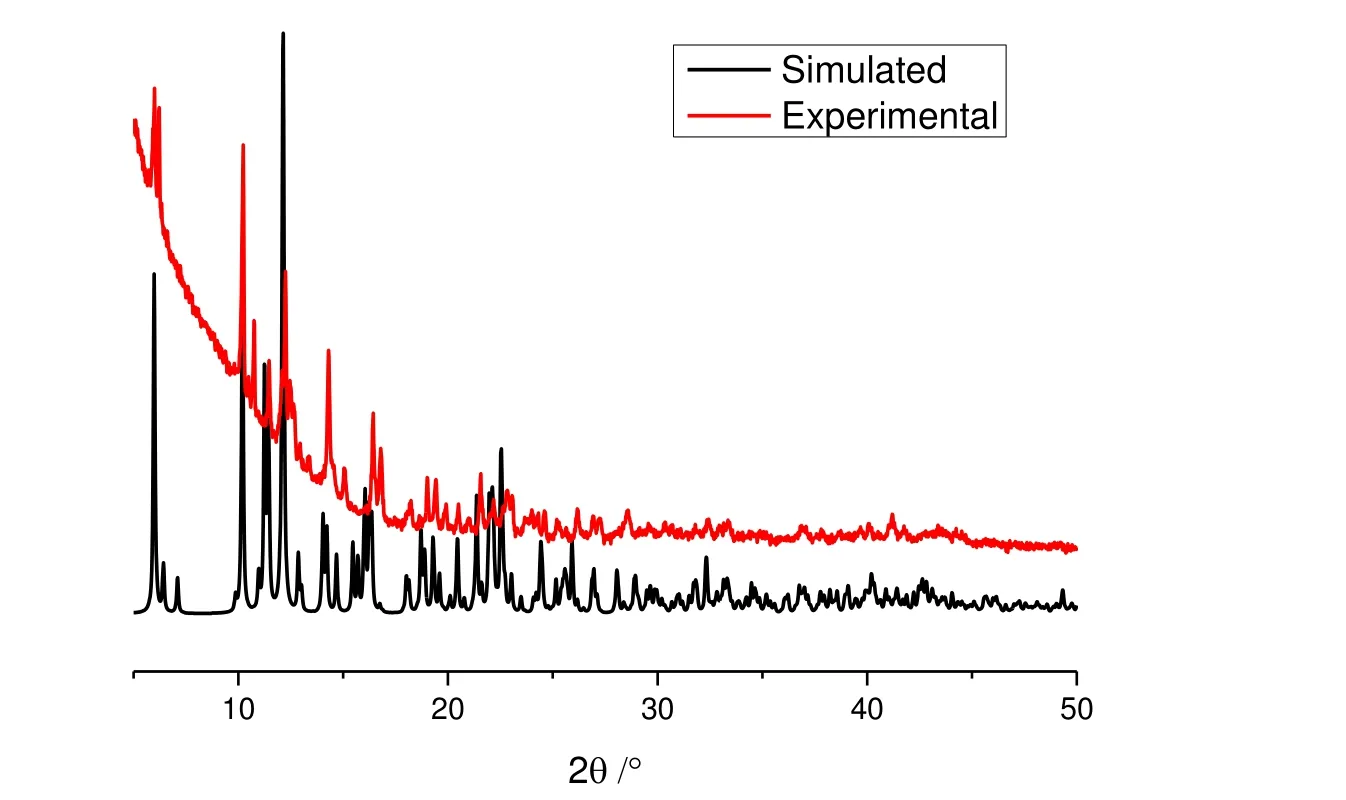
Fig. 2. PXRD patterns of 1
The thermal stability of the complex was evaluated by TG experiment and the results shown in Fig. 3 suggest that the first weight loss of about 16.5% (calculated 16.0%) occurred from room temperature to 500 ℃, corresponding to the removal of DMF molecules. Subsequently, there is a quick mass loss at 500~700 ℃, which may correspond to the decomposition of the framework.
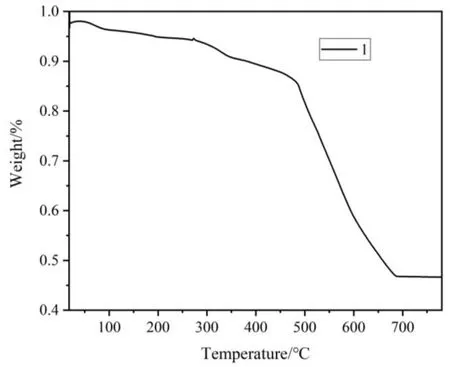
Fig. 3. TGA curve of 1
3. 3 Fluorescence properties
3. 3. 1 Solid-state photoluminescent spectra
The luminescent spectra of 1 were determined in the solid state at room temperature and the results are shown in Fig. 4.When excited at 300 nm, the emission peaks at 579, 588, 594,615, 618, 650, 683 and 692 nm correspond to the transition from5D0→7FJ(J= 0~4) of Eu3+ions. The most intense emissions in the luminescent spectra around 615 and 618 nm are ascribed to the electric dipolar5D0→7F2transition,which is responsible for the red emission observed for these MOFs[44]. The splitting emission band for the5D0→7F1,5D0→7F2and5D0→7F4transition indicates only one type of coordination environment for the Eu3+cation in 1[44]. The results are in good agreement with the single-crystal X-ray analyses.
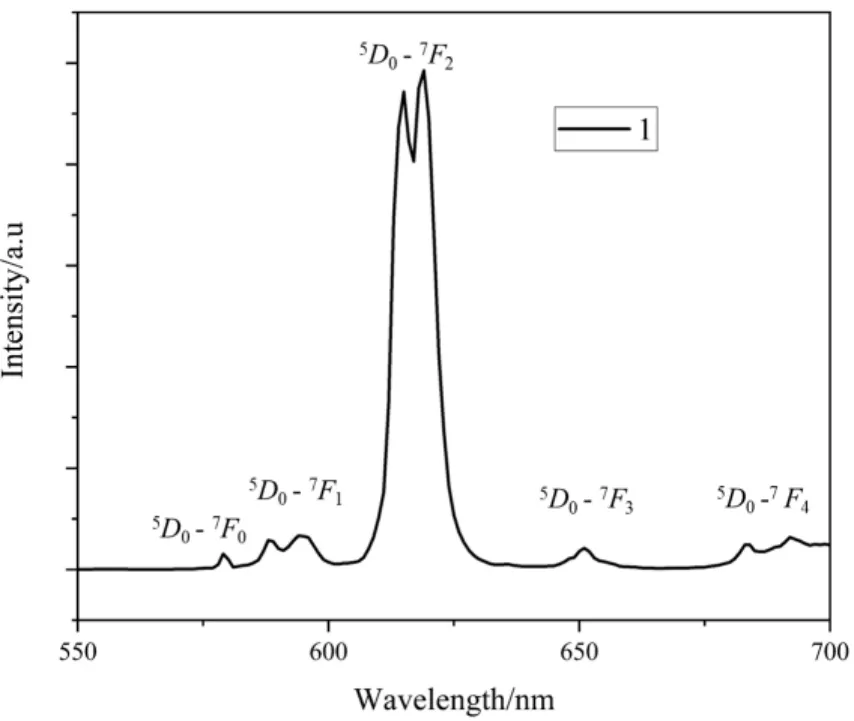
Fig. 4. Solid-state emission spectra of 1
3. 3. 2 Fluorescence sensing properties of 1
The fluorescence properties of 1 in different metal ion solutions were also investigated. The corresponding MOF/Mm+was prepared by introducing 1 (3 mg) powder into 3 mL ethanol solution of MNOx(Mm+= K+, Cd2+, Mg2+, Mn2+,Ni2+, Ca2+, Zn2+, Cu2+, Fe3+) with each concentration to be 1 mmol/L, which was uniformly dispersed under ultrasonication for 5 min. A series of luminescent responses were recorded and compiled in Fig. 5a, which suggests that at 615 nm, 1 exhibited luminescence intensities for different metal ions with great degree of variation in their bursting effect.Interestingly, Fig. 5b shows that 1 exhibits a drastic quenching effect in the Fe3+ion solution, which can be of great help in practical application for the sensing of Fe3+,which was further evaluated in the subsequent experiments.Due to the coexistence of multiple metal ions in real solution system, competing experiments were conducted with respectively adding various metal ions to the suspension of 1,followed by the treatment of equivalent amount of Fe3+. As can be seen from Fig. 5c, the presence of other metal ions has almost no significant effect on the selectivity of 1 to Fe3+,further indicating the high fluorescence selectivity of 1 to Fe3+.
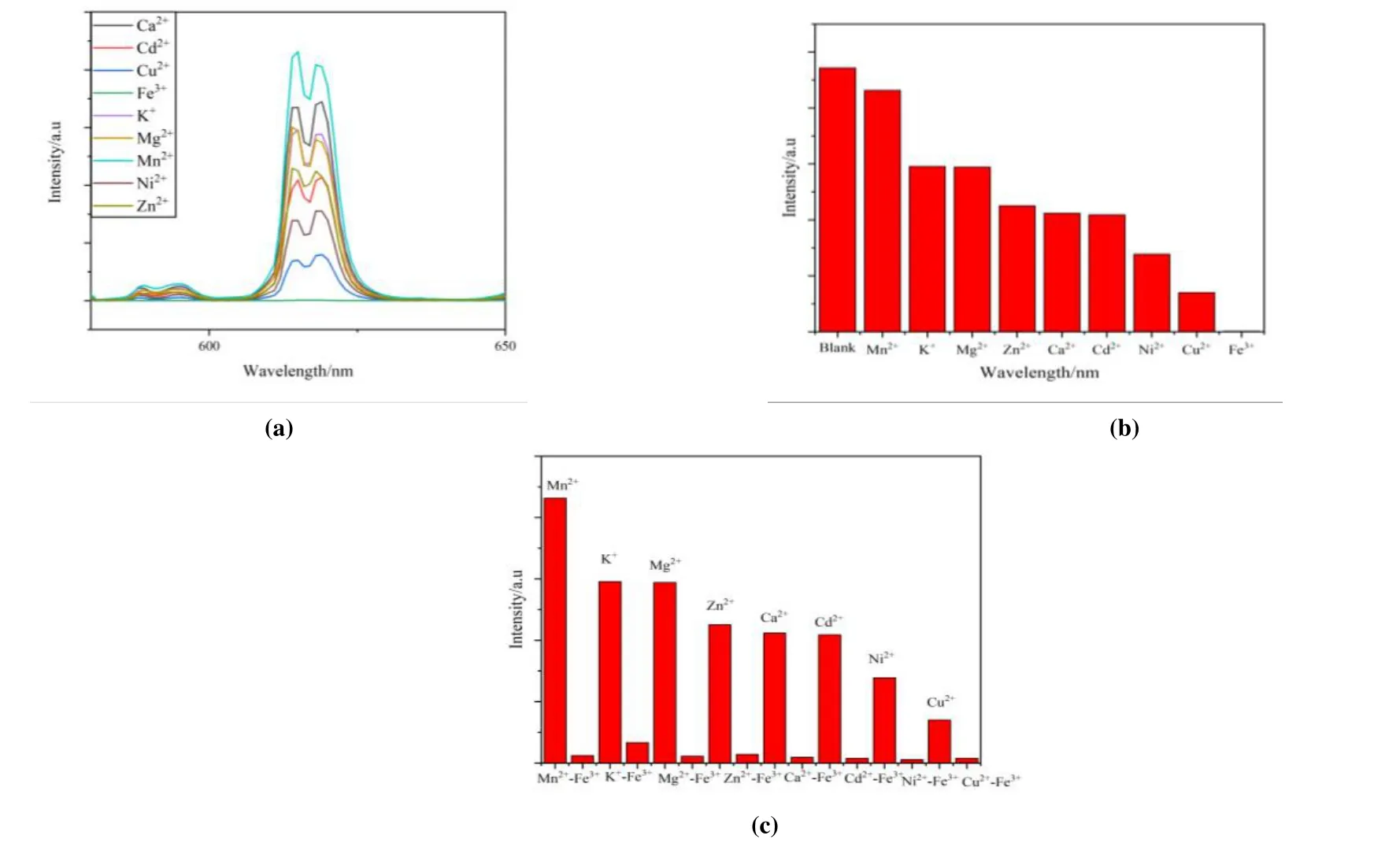
Fig. 5. (a) Luminescence spectra of 1 dispersed in ethanol solutions of different metal ions; (b) Maximum emission intensity of 1 in multiple metal cation-ethanol solution; (c) Luminescence intensity (615 nm) of 1 dispersed in EtOH with the addition of different ions (Mg2+, Cd2+, Zn2+, Mn2+, K+, Ni2+, Ca2+, Cu2+) (1 mmol/L) and Fe3+ incorporated systems (1 mmol/L)
To better understand the sensing sensitivities of 1 for Fe3+ions, a series of titrating experiments were carried out in ethanol solutions with variable concentrations of Fe3+ions. 1(3 mg) was sonicated in EtOH (3 mL) followed by the addition of different volumes of Fe3+(1 mmol/L) and the emission spectra were measured as displayed in Fig. 6. It is noted that the fluorescence intensity of 1 is completely quenched at Fe3+ion concentration of 0.1 mM (300µL). The relationship between the quenching effect and Fe3+concentration was further investigated through the Stern-Volmer equationi.e.I0/I= 1 +KSV[M], whereI0andIrepresent the initial luminescence intensity and luminescence intensity after adding analyte, respectively, [M] is the concentration of analyte, andKSVstands for the Stern-Volmer constant[45]. The Stern-Volmer plot of 1 in Fig. 7 shows that at higher Fe3+ion concentration, there is a nonlinear upward curvature, indicating that the mechanism of luminescence quenching may be a combination of dynamic quenching and static quenching[32]. TheKSVvalue was found to be 3.91 × 104M-1for 1 at low concentrations of Fe3+ions. The value is comparable with those reported for MOF-based sensor materials in the literatures (Table 2)[46-49]. The limits of detection (LOD) can be determined byLOD= 3σ/k(kis the slope of the linear curve andσis the standard deviation of the blank sample)[50]. With the slope of the fitting line and the measurement error of the intensity with blank samples, the detection limit for Fe3+was calculated to be 1.7μM. On the other hand, the quenching efficiency of 1 can be maintained above 80% through five cycles of experiments (Fig. 8).
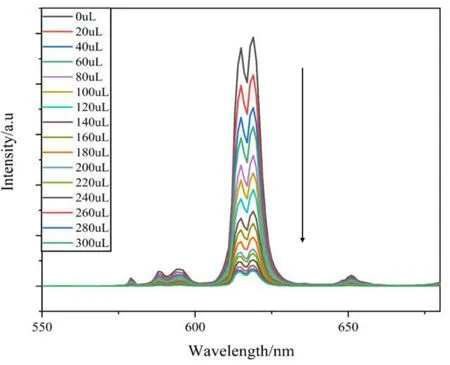
Fig. 6. Emission spectra of 1 dispersed in EtOH upon increasing addition of Fe3+
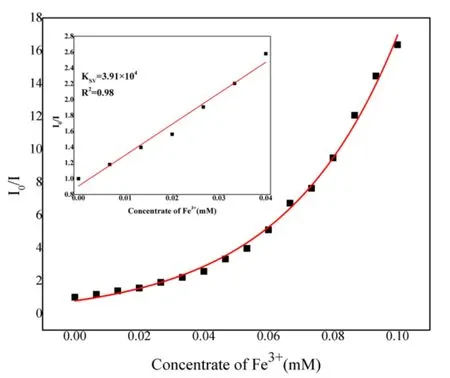
Fig. 7. Stern-Volmer plots of 1; the inset demonstrates the quenching linearity relationship at low concentration of Fe3+ ion

Table 2. KSV Values for Fe3+ in Comparison with Literatures
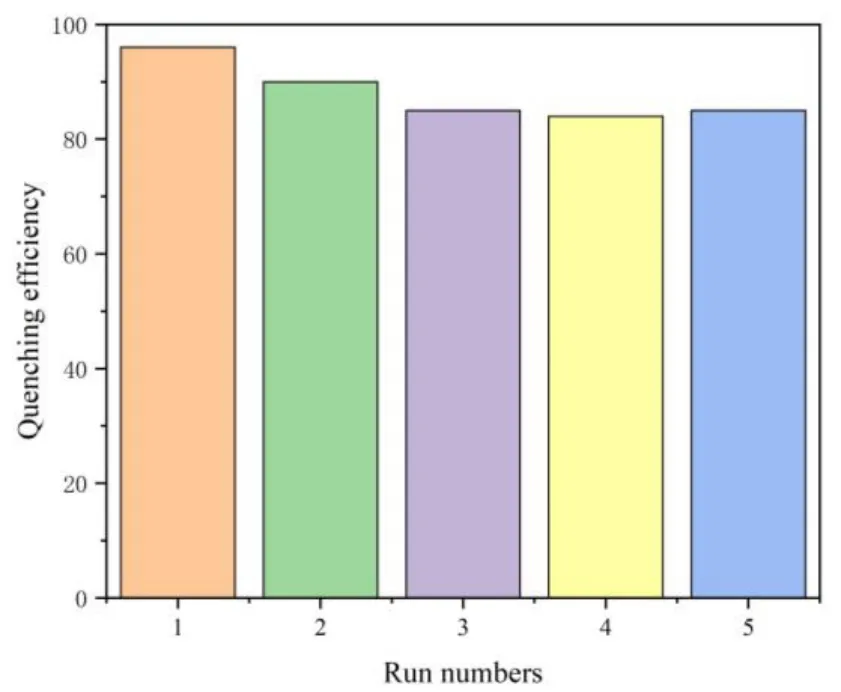
Fig. 8. Quenching efficiency of 1 dispersed in EtOH with the addition of 0.1 mM Fe3+ for 5 runs
3. 4 Color tuning
Because of the similar c oordination environment and coordination number of the Ln3+ions, different Ln ions can be combined in the same MOF. Using an analogous synthetic protocol to that used for the preparation of 1, various ratios of Eu3+and Tb3+were introduced to the solutions of H3BTB in DMF: water and various complexes of EuxTb1-xwere synthesized (complexes 2~5). After isolation of the crystalline products, PXRD were performed to analyze the structural analogy of these complexes to homometallic 1 (Fig. 9). The solid samples were then studied by fluorescence spectroscopy and the effect of altering the lanthanide ion ratios in EuxTb1-xwas manifested in the variation of the emission spectra (Fig.10). It was noted that as the molar ratio of Eu3+increased from 0.0009 to 0.0909, the relative emission intensity of the strongest emission of Tb3+at 544 nm gradually weakened,while that of the Eu3+ion at 615 nm gradually enhanced. The general shift in color can be safely assumed giving the trend of the spectra, and chromaticity coordinates were calculated and plotted in the CIE coordinate diagram to more clearly present the colors of emission (Fig. 11).
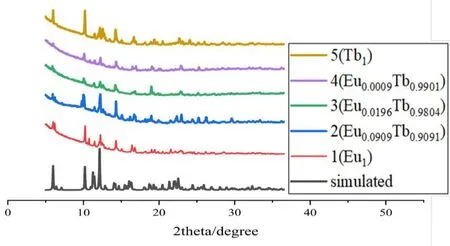
Fig. 9. PXRD pattern of complexes 1~5
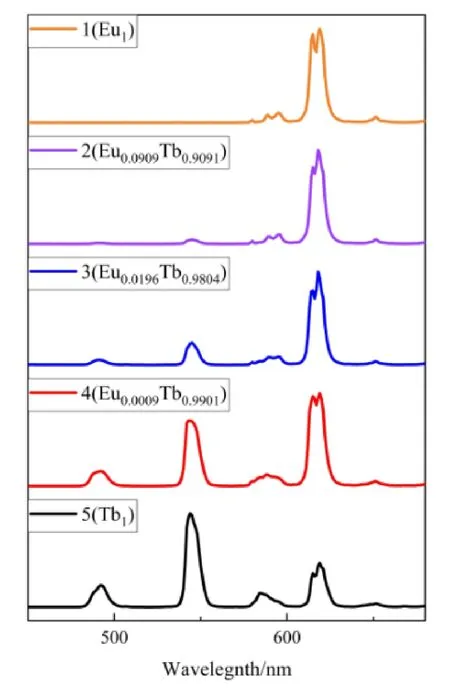
Fig. 10. Emission spectra of complexes 1~5
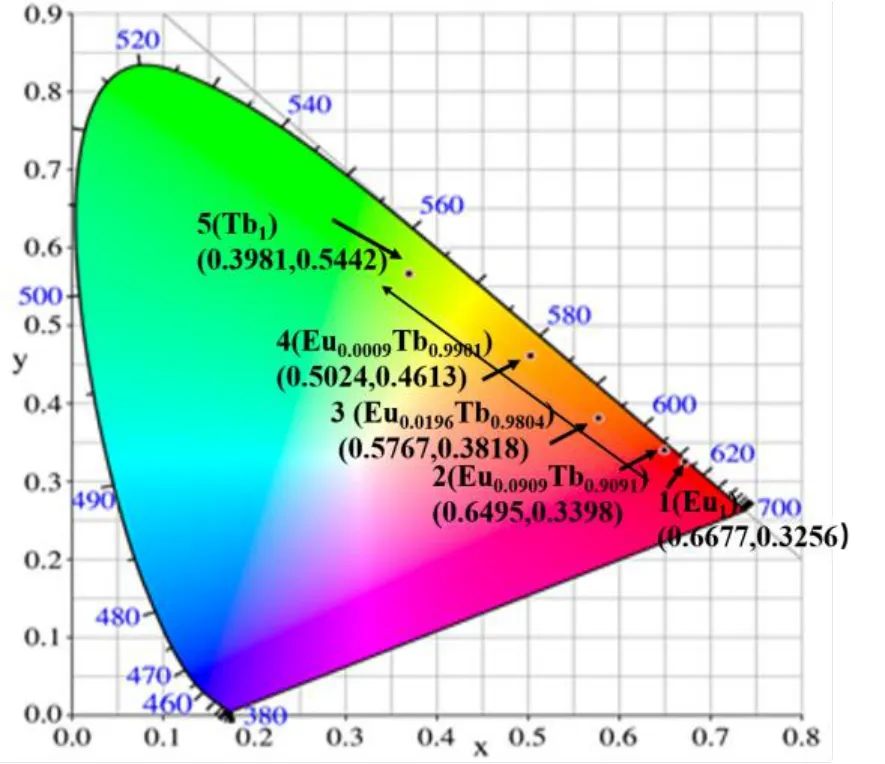
Fig. 11. CIE chromaticity coordinates of complexes 1~5 displaying a green shift in the color of emission
4 CONCLUSION
In summary, a novel 2DEu-MOF (1) was successfully synthesized under solvothermal conditions using H3BTB as the ligand. X-ray single-crystal analysis demonstrated that 1 is a 3Dsupramolecular network formed by the stacking of 2Dlayers throughπ-πinteractions. The luminescence explorations revealed that 1 possesses high selectivity and sensitivity for the sensing of Fe3+by quenching mechanism withKSV= 3.91 × 104M-1. Furthermore, a series of co-doped Ln-MOFs with tunable luminescence were also fabricated and tested, which showed that their luminescent color can be effectively tuned from red to green by controlling the ratios of Eu3+:Tb3+. This study provides important guidelines for the fabrication of Eu-MOF for the highly efficient sensing of Fe3+in practical applications.
杂志排行
结构化学的其它文章
- Synthesis, Crystal Structure and Fungicidal Activity of 3,4-Dichloro-5-(6-chloro-9-(4-fluorobenzyl)-9H-purin-8-yl)isothiazole①
- Synthesis, Crystal Structure and Antifungal Activity of New Furan-1,3,4-oxadiazole Carboxamide Derivatives①
- Syntheses, Crystal Structures, Anticancer Activities and DNA-Binding Properties of the Dibutyltin Complexes Based on Benzoin Aroyl Hydrazone①
- Synthesis, Crystal Structure, Fungicidal Activities and Molecular Docking of Acyl Urea Derivatives Containing 2-Chloronicotine Motif①
- A New Borate-phosphate Compound CsNa2Lu2(BO3)(PO4)2:Crystal Structure and Tb3+ Doped Luminescence①
- Drug Design, Molecular Docking, and ADMET Prediction of CCR5 Inhibitors Based on QSAR Study①
