Synthesis, Crystal Structure, Fungicidal Activities and Molecular Docking of Acyl Urea Derivatives Containing 2-Chloronicotine Motif①
2022-03-08SUNGuoXiangWANGQiaoMINLiJingHANLiangLIUXingHai
SUN Guo-Xiang WANG Qiao MIN Li-Jing HAN Liang LIU Xing-Hai②
a (School of Chemistry and Chemical Engineering,Yancheng Institute of Technology, Yancheng, Jiangsu 224051, China)
b (College of Life Science, Key Laboratory of Vector Biology and Pathogen Control of Zhejiang Province, Huzhou University, Huzhou 313000, China)
c (College of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310014, China)
ABSTRACT Eighteen new acyl urea derivatives containing 2-chloronicotine moiety were synthesized using 2-chloronicotinic acid as starting material via 4 steps conveniently. These 2-chloronicotine acyl urea structures were confirmed by 1H NMR, 13C NMR and HRMS. Compound 4r was further confirmed by X-ray diffraction. It crystallizes in the orthorhombic system, space group Pbca with a = 7.2960(3), b = 14.8546(6), c = 25.2840(11) Å,V = 2740.3(2) Å3, Z = 8, the final R = 0.0442 and wR = 0.1033 for 4028 observed reflections with I > 2σ(I). The antifungal activity results demonstrate that some of these compounds possessed good activity against B. cinerea,G. zeae, P. piricola, and P. Capsici at 50 ppm. Further molecular docking results indicated that the key group is urea bridge and pyridine ring.
Keywords: acyl urea compounds, crystal structure, synthesis, antifungal activity, docking;
1 INTRODUCTION
Nitrogen containing heterocycle is an important nucleus in synthetic compounds or natural products because of diver sity activity[1-5]. Among the nitrogen containing heterocycles,pyridine ring possessed various activityies in synthetic small molecules or natural products, such as plant growth regulatory activities[6], anti-HIV activity[7], herbicidal activity[8,9], fungicidal activity[10,11], anti-inflammatory activity[12], immunosuppressive activity[13], antioxidant activity[14], anticancer activity[15], and so on. Some of pyridine compounds had been commercialized as pesticides or drugs. 2-Chloro-N-(4΄-chloro-[1,1΄-biphenyl]-2-yl)nicotinamide, whose commercial name is boscalid (SDH inhibitor), is an famous fungicide to protect crops,vegetables and fruits. In this fungicide, pyridine ring and amide group are the key groups. Urea group also had the substrate of amide, which is always found in synthetic or natural compounds[16-19]. For instance, the chitin synthetase inhibitor dimilin contains urea group, which is an insect growth regulatory.
In our previous work, lots of heterocyclic compounds with pesticidal activity or medicinal activity were designed and synthesized[20-30], including the pyridine derivatives. A series of fluorine substituted pyridine acyl urea derivatives were synthesized from boscalid and some compounds exhibited moderate fungicidal activity[31]. In this work, the fluorine substituted group was replaced by other non-fluorine substitution (Fig. 1). Some of the title pyridine acyl urea derivatives exhibited good fungicidal activity. The SAR was also studied using molecular docking method.
2 EXPERIMENTAL
2. 1 Instruments
Melting points (M.P.) were measured by an X-4 apparatus and uncorrected.1H NMR and13C NMR spectra were tested on a Bruker AV III-500 instrument. ESI-HRMS was measured on a JOEL AccuTOF instrument. Single crystal diffraction was done on a Bruker CCD area detector diffractometer.
2. 2 General procedure
The intermediates 1, 2 and 3 were synthesized according to the reported work[32-35].
Syntheses of target compounds 4a~4r
To a solution of intermediate 3 (0.36 g, 2 mmol) in dichloromethane (10 mL), the substituted aniline (2.2 mmol)was added dropwise and stirred at 20oC for overnight. TLC monitor and then solvent dichloromethane were removed,and the crude products were purified by using flash chromatograph to give compounds 4a-4r.
2-Chloro-N-((2,6-dichlorophenyl)carbamoyl)-nicotinamide 4a
White solid, yield 71.2%, m.p. 185~187 °C;1H NMR(CDCl3, 500 MHz),δ: 7.25 (t,J= 6.4 Hz, 1H, Ph),7.40~7.42 (m, 1H, Py), 7.42 (d,J= 6.4 Hz, 2H, Ph),8.19~8.21 (m, 1H, Py), 8.57~8.59 (m, 1H, Py), 9.26 (s, 1H,NH), 10.09 (s, 1H, NH); HRMS (ESI) for C13H8Cl3N3O2m/z:calculated, 343.9755; found, 343.9768 [M+H]+.
2-Chloro-N-((3-isopropylphenyl)carbamoyl)-nicotinamide 4b
White solid, yield 65.6%, m.p. 205~208 °C;1H NMR(CDCl3, 500 MHz),δ: 2.36 (s, 1H, CH3), 7.16 (d,J= 6.6 Hz,2H, Ph), 7.38 (d,J= 6.7 Hz, 2H, Ph), 7.42~7.45 (m, 1H, Py),8.10~8.12 (m, 1H, Py), 8.60~8.62 (m, 1H, Py), 9.39 (s, 1H,NH), 10.37 (s, 1H, NH); HRMS (ESI) for C14H12ClN3O2m/z:calculated, 290.0691; found, 290.0690 [M+H]+.
2-Chloro-N-((2,6-dimethoxyphenyl)carbamoyl)nicotinamide 4c
White solid, yield 60.5 %, m.p. 138~141 °C;1H NMR(CDCl3, 500 MHz),δ: 1.27 (d,J= 5.6 Hz, 6H, CH3),2.88~2.95 (m, 1H, CH), 7.04 (d,J= 6.1 Hz, 1H, Ph), 7.25 (d,J= 6.0 Hz, 1H, Ph), 7.29 (s, 1H, Ph), 7.35 (t,J= 3.6 Hz, 1H,Ph), 7.41~7.44 (m, 1H, Py), 8.08~8.10 (m, 1H, Py),8.59~8.60 (m, 1H, Py), 9.83 (s, 1H, NH), 10.47 (s, 1H, NH);HRMS (ESI) for C16H16ClN3O2m/z: calculated, 318.1004;found, 318.1017 [M+H]+.
2-Chloro-N-((2-methyl-4-nitrophenyl)carbamoyl)-nicotinamide 4d
White solid, yield 68.7%, m.p. 239~240 °C;1H NMR(CDCl3, 500 MHz),δ: 3.71 (s, 3H, CH3), 3.84 (s, 3H, CH3),6.64~6.66 (m, 1H, Ph), 7.01 (d,J= 6.8 Hz, 1H, Ph),7.56~7.58 (m, 1H, Py), 7.86 (s, 1H, Ph), 8.12~8.14 (m, 1H,Py), 8.55~8.56 (m, 1H, Py), 10.81 (s, 1H, NH), 11.44 (s, 1H,NH); HRMS (ESI) for C15H14ClN3O4m/z: calculated,336.0746; found, 336.0751 [M+H]+.
2-Chloro-N-((2,4-dichlorophenyl)carbamoyl)-
nicotinamide 4e
White solid, yield 63.4%, m.p. 203~206 °C;1H NMR(CDCl3, 500 MHz),δ: 2.43 (s, 3H, CH3), 7.58~7.61 (m, 1H,Py), 8.14~8.17 (m, 2H, Ph), 8.21 (s, 1H, Ph), 8.37~8.38 (m,1H, Py), 8.57~8.59 (m, 1H, Py), 10.73 (s, 1H, NH), 11.74 (s,1H, NH); HRMS (ESI) for C14H11ClN4O4m/z: calculated,335.0542; found, 335.0572 [M+H]+.
2-Chloro-N-(phenylcarbamoyl)nicotinamide 4f
White solid, yield 55.9%, m.p. 207~210 °C;1H NMR(CDCl3, 500 MHz),δ: 7.47~7.49 (m, 1H, Ph), 7.57~7.60 (m,1H, Py), 7.74~7.76 (m, 1H, Ph), 8.15~8.17 (m, 1H, Py), 8.31(d,J= 6.4 Hz, 1H, Ph), 8.57~8.58 (m, 1H, Py), 10.95 (s, 1H,NH), 11.70 (s, 1H, NH); HRMS (ESI) for C13H8Cl3N3O2m/z:calculated, 343.9755; found, 343.9775 [M+H]+.
2-Chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide 4g
White solid, yield 58.9%, m.p. 166~169 °C;1H NMR(CDCl3, 500 MHz),δ: 7.17 (t,J= 5.9 Hz, 1H, Ph), 7.36 (t,J= 6.4 Hz, 2H, Ph), 7.43~7.45 (m, 1H, Py), 7.51 (d,J= 6.4 Hz, 2H, Ph ), 8.11~8.13 (m, 1H, Py), 8.60~8.62 (m, 1H, Py),9.34 (s, 1H, NH), 10.45 (s, 1H, NH); HRMS (ESI) for C13H10ClN3O2m/z: calculated, 276.0534; found,276.0527[M+H]+.
N-((3-(tert-butyl)phenyl)carbamoyl)-2-chloronicotinamide 4h
White solid, yield 48.9%, m.p. 176~179 °C;1H NMR(CDCl3, 500 MHz),δ: 7.09~7.12 (m, 1H, Ph), 7.31~7.32(m,1H, Py), 7.44~7.47 (m, 2H, Ph), 8.19~8.21 (m, 1H, Py),8.24~8.26 (m, 1H, Ph), 8.61~8.62 (m, 1H, Py), 9.24 (s, 1H,NH), 11.00 (s, 1H, NH); HRMS (ESI) for C13H9Cl2N3O2m/z:calculated, 310.0145; found, 310.0162[M+H]+.
N-((2,6-diethylphenyl)carbamoyl)nicotinamide 4i
Yellow solid, yield 62.3%, m.p. 183~186 °C;1H NMR(CDCl3, 500 MHz),δ: 1.34 (s, 9H, CH3), 7.34~7.36 (m, 1H,Py), 7.37 (s, 1H, Ph), 7.40~7.43 (m, 3H, Ph),8.07~8.09 (m,1H, Py), 8.59~8.61 (m, 1H, Py), 9.90 (s, 1H, NH), 10.41 (s,1H, NH); HRMS (ESI) for C17H18ClN3O2m/z: calculated,332.1160; found, 332.1172[M+H]+.
Not being able to imagine what could be the cause of so much obstinacy35 the King began to fear, lest, in spite of all his precautions, she might have heard of the charms of the Prince his son, whose goodness, youth and beauty, made him adored at Court
2-Chloro-N-((4-methyl-2-nitrophenyl)carbamoyl)nicotinamide 4j
White solid, yield 61.3%, m.p. 193~195 °C;1H NMR(CDCl3, 500 MHz),δ: 1.24 (t,J= 6.1 Hz, 6H, CH3),2.64~2.69 (m, 4H, CH2), 7.16 (d,J= 6.1 Hz, 2H, Ph), 7.28 (t,J= 6.0 Hz, 1H, Ph), 7.32~7.35 (m, 1H, Py), 8.11~8.13 (m,1H, Py), 8.54~8.55 (m, 1H, Py), 9.33 (s, 1H, NH), 9.76 (s,1H, NH);13C NMR (CDCl3, 125 MHz)δ: 14.93, 24.96,123.51, 125.68, 127.97, 131.91, 133.05, 138.67, 141.73,146.49, 151.52, 151.90, 167.86; HRMS (ESI) for C17H18ClN3O2m/z: calculated, 332.1160; found,332.1157[M+H]+.
2-Chloro-N-((2-methyl-3-nitrophenyl)carbamoyl)nicotinamide 4k
White solid, yield 57.1%, m.p. 215~218 °C;1H NMR(CDCl3, 500 MHz),δ: 2.44 (s, 3H, CH3), 7.47~7.49 (m, 1H,Py), 7.50 (d,J= 5.6 Hz, 1H, Ph), 8.04 (s, 1H, Ph), 8.29~8.31(m, 1H, Py), 8.50 (d,J= 6.8 Hz, 1H, Ph), 8.61~8.62 (m, 1H,Py), 8.78 (s, 1H, NH), 12.32 (s, 1H, NH); HRMS (ESI) for C14H11ClN4O4m/z: calculated, 335.0542, found,335.0572[M+H]+.
Methyl 4-(3-(2-chloronicotinoyl)ureido)benzoate 4l
White solid, yield 49.2%, m.p. 195~198 °C;1H NMR(CDCl3, 500 MHz),δ: 2.51 (s, 3H, CH3), 7.39 (t,J= 6.5 Hz,1H, Ph), 7.47~7.50 (m, 1H, Py), 7.65 (d,J= 6.2 Hz, 1H, Ph),8.21~8.23 (m, 1H, Py), 8.26 (d,J= 6.1 Hz, 1H, Ph),8.63~8.64 (m, 1H, Py), 9.00 (s, 1H, NH), 10.64 (s, 1H, NH);HRMS(ESI) for C14H11ClN4O4m/z: calculated, 335.0542,found, 335.0557[M+H]+.
2-Chloro-N-((2,5-dichlorophenyl)carbamoyl)nicotinamide 4m
2-Chloro-N-((3,4-dimethylphenyl)carbamoyl)nicotinamide 4n
White solid, yield 65.7%, m.p. 168~171 °C;1H NMR(CDCl3, 500 MHz),δ: 2.40 (s, 3H, CH3), 7.09~7.12 (m, 1H,Ph), 7.23 (t,J= 6.2 Hz, 2H, Ph), 7.42~7.44 (m, 1H, Py),7.91 (t,J= 6.4 Hz, 1H, Ph), 8.13~8.15 (m, 1H, Py),8.60~8.61 (m, 1H, Py), 9.45 (s, 1H, NH), 10.41 (s, 1H, NH);HRMS (ESI) for C14H12ClN3O2m/z: calculated, 290.0691,found, 290.0648[M+H]+.
2-Chloro-N-((4-phenoxyphenyl)carbamoyl)nicotinamide 4o
White solid, yield 67.9%, m.p. 189~191 °C;1H NMR(CDCl3, 500 MHz),δ: 2.26 (s, 3H, CH3), 2.27 (s, 3H, CH3),7.10 (d,J= 6.5 Hz, 1H, Ph), 7.23 (d,J= 6.4 Hz, 1H, Ph),7.27 (s, 1H, Ph), 7.40~7.42 (m, 1H, Py), 8.09~8.11 (m, 1H,Py), 8.59~8.61 (m, 1H, Py), 9.49 (s, 1H, NH), 10.35 (s, 1H,NH); HRMS (ESI) for C15H14ClN3O2m/z: calculated,304.0847; found, 304.0837[M+H]+.
2-Chloro-N-((4-ethylphenyl)carbamoyl)nicotinamide 4p
White solid, yield 65.0%, m.p. 172~175 °C;1H NMR(CDCl3, 500 MHz),δ: 7.01~7.03 (m, 4H, Ph), 7.13 (t,J=6.0 Hz, 1H, Ph), 7.34~7.38 (m, 2H, Ph), 7.43~7.45 (m, 1H,Py), 7.47~7.49 (m, 2H, Ph), 8.12~8.14 (m, 1H, Py),8.59~8.60 (m, 1H, Py), 9.24 (s, 1H, NH), 10.61 (s, 1H, NH);HRMS (ESI) for C19H14ClN3O3m/z: calculated, 368.0796;found, 368.0775 [M+H]+.
2-Chloro-N-(mesitylcarbamoyl)nicotinamide 4q
White solid, yield 65.7% , m.p. 133~136 °C;1H NMR(CDCl3, 500 MHz),δ: 1.25 (t,J= 6.1 Hz, 3H, CH3),2.63~2.68 (m, 2H, CH2), 7.18 (d,J= 6.7 Hz, 2H, Ph), 7.42(d,J= 7.2 Hz, 2H, Ph), 7.43~7.45 (m, 1H, Py), 8.11~8.13 (m,1H, Py), 8.60~8.62 (m, 1H, Py), 9.29 (s, 1H, NH), 10.37 (s,1H, NH);13C NMR (CDCl3, 125 MHz)δ: 16.10, 28.02,120.46, 123.47, 128.68, 131.79, 135.46, 138.79, 139.91,146.52, 150.66, 151.60, 167.73; HRMS (ESI) for C15H14ClN3O2m/z: calculated, 304.0847; found,304.0871[M+H]+.
2-Chloro-N-((2,5-dichlorophenyl)carbamoyl)nicotinamide 4r
Yellow solid, yield 54.9%, m.p. 172~175 °C;1H NMR(CDCl3, 500 MHz),δ: 2.25 (s, 6H, CH3), 2.31 (s, 3H, CH3),6.93 (s, 2H, Ph), 7.34~7.36 (m, 1H, Py), 8.10~8.12 (m, 1H,Py), 8.54~8.55 (m, 1H, Py), 9.40 (s, 1H, NH), 9.68 (s, 1H,NH);13C NMR (CDCl3, 125 MHz)δ: 18.49, 20.96, 123.50,128.87, 130.00, 131.74, 135.26, 136.31, 138.70, 146.51,151.30, 151.50, 167.69; HRMS (ESI) for C16H16ClN3O2m/z:calculated, 318.1004; found, 318.1007[M+H]+.
2. 3 Structure determination
A colorless crystal suitable for X-ray diffraction study was cultivated in the test tube from EtOH by self-volatilization. A crystal with dimensions of 0.28mm × 0.22mm × 0.14mm was mounted on a Bruker APEX-II CCD diffractometer equipped with a graphite-monochromatic MoKαradiation (λ= 0.71073 Å). Intensity data were collected at 296(2) K by using a multi-scan mode in the range of 5.48≤θ≤60.23°with the following index ranges: –10≤h≤10, –20≤k≤20 and –35≤l≤35. A total of 58508 reflections were collected and 4028 were independent (Rint= 0.0532). The crystal structure was solved by direct methods with SHELXS-97[44]and refined by full-matrix least-squares refinements based onF2with SHELXL-97. All non-hydrogen atoms were refined anisotropically, and all hydrogen atoms were located in the calculated positions and refined with a riding model.The final refinement converged atR= 0.0442 andwR=0.1033.
2. 4 Fungicidal activity
Antifungal activities of 2-chloronicotine acyl urea compounds 4a~4r were determined according to our previous work[36-38].
2. 5 Molecular docking
Molecular docking was carried out by using DS 2.5. The active sites were generated from the crystal of SDH (PDB code: 2FBW). The detailed method was according to our previous work[39-42].
3 RESULTS AND DISCUSSION
3. 1 Synthesis and spectra analysis
The synthetic route of acyl urea compounds containing nicotinic motif is illustrated in Scheme 1. In the first step,SOCl2is used as solvent and reactant. A gas absorption device was used in this reaction in order to avoid environment pollution. During the second synthesis step, the diluted 2-chloronicotinoyl chloride was dropwise added into ammonia water under an ice bath slowly. Then the Py-CONCO was synthesized easily and used without purification. Finally, the target compounds were gotten as white solid under mild condition.
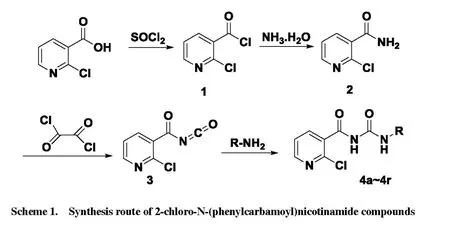
Scheme 1. Synthesis route of 2-chloro-N-(phenylcarbamoyl)nicotinamide compounds
These nicotinic acyl urea compounds have two amino groups, and two single peaks can be found around 9.5 and 10.5 ppm respectively. For the pyridine and benzene rings,these protons are in aromatic 6~8 ppm. For ESI-HR-MS, the measured value is according to the theoretical value within 0.003 errors.
3. 2 Crystal structure
The compound 2-chloro-N-((2,5-dichlorophenyl)carbamoyl)nicotinamide 4r was further characterized by X-ray diffraction single crystal analysis and is illustrated in Table 1 and Figs. 2 and 3. As shown in Fig. 2, the phenyl ring(C(8)~C(13)) is nearly in the same plane with pyridine ring(C(1), C(2), C(3), C(4), C(5), N(8)), in which the dihedral angle (θ) is 15.1º with plane equation 6.991x– 0.591y+7.169z= 3.9984 and 6.214x– 0.467y+ 13.226z= 8.0225 respectively, and the largest deviations from the leastsquares plane are 0.0062 and 0.0091 nm. The torsion angles of C(2)–C(6)–N(1)–N(2), C(6)–N(1)–C(7)–N(2) and N(1)–C(7)–N(2)–C(8) are 176.43(15)°, –1.2(3)° and–175.91(15)° respectively, which showed the acyl urea bridge is nearly in the same plane. The torsion angles of O(1)–C(6)–N(1)–C(7) and O(2)–C(7)–N(1)–C(6) are–5.3(3)° and 3.5(3)° respectively, which indicated the two C=O groups are in the opposite orientations and nearly in the same plane. Compound 4r has four intermolecular hydrogen bonds and one intramolecular hydrogen bond. The parameters of five hydrogen bonds are listed in Table 2.They are linked together by N–H···N, C–H···Cl, C–H···O and N–H···O. From Fig. 1, the intramolecular N(2)–H(2)···O(1) hydrogen bond formed a six-membered ring in molecule 4r. The hydrogen bonding interactions strengthen the integration of the 3Dnetworks.
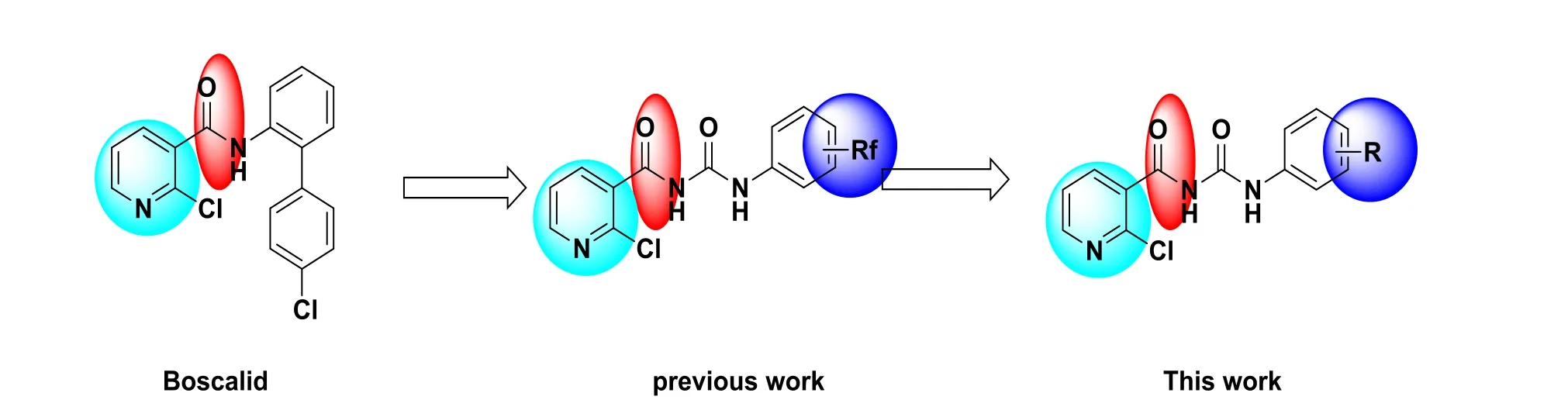
Fig. 1. Design method of nicotinic acyl urea derivatives
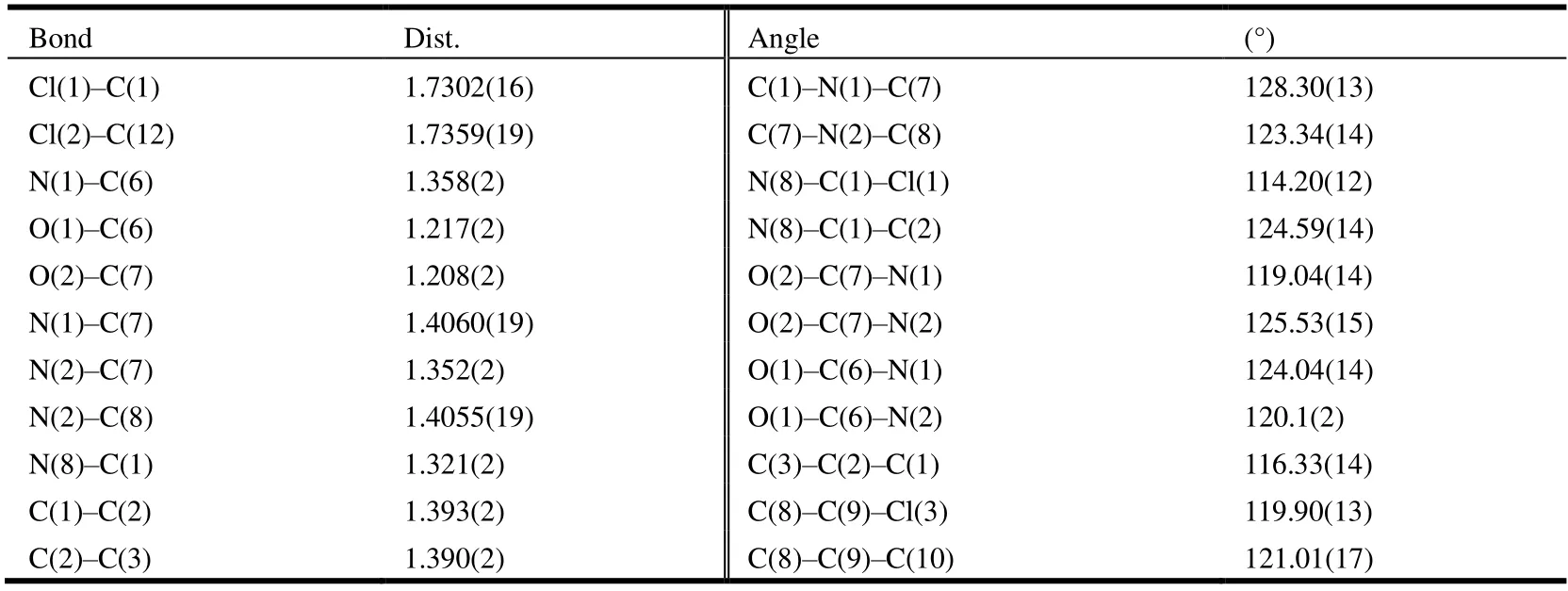
Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for the Title Compound 4r
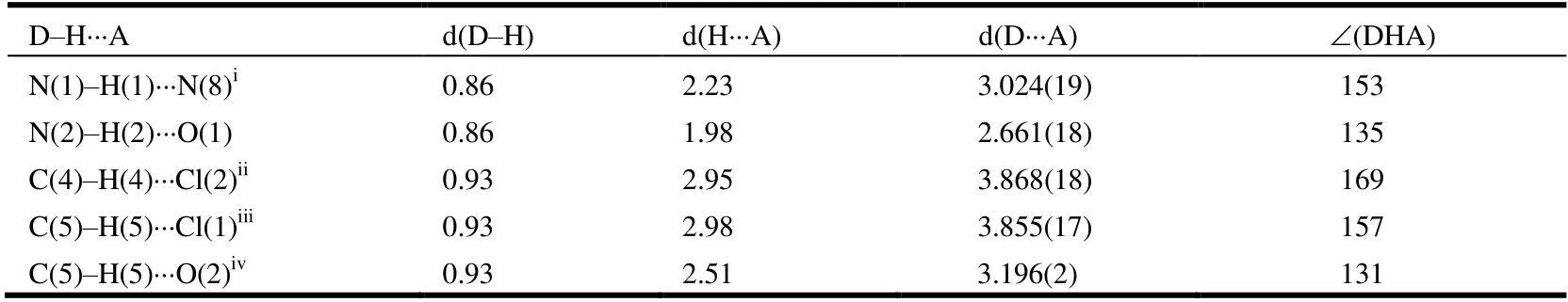
Table 2. Hydrogen-bond Parameters (Å) of the Title Compound 4r
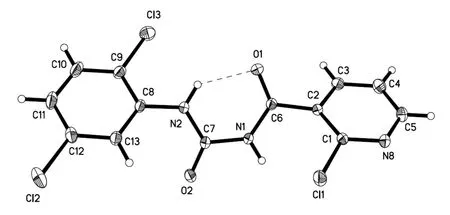
Fig. 2. Molecular structure of the title compound 4r
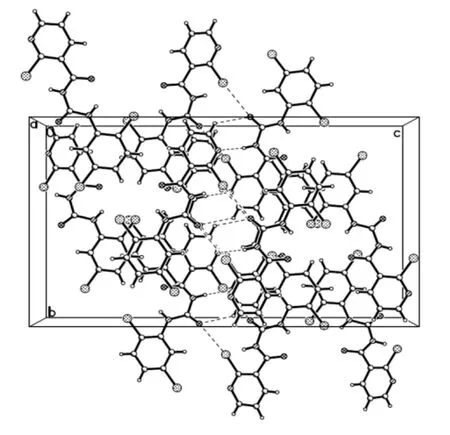
Fig. 3. Packing of the title compound 4r
3. 3 Fungicidal activity
Antifungal activities of compounds 4a~4r and positive control fluxapyroxad againstSclerotinia sclerotiorum(SS),Phytophthora infestans(PI),Fusarium oxysporum(FO),Phytophthora capsici(PC),Gibberella zeae(GZ),Rhizoctonia solani(RS),Cercospora arachidicola(CA),Alternaria solani (AS),Physalospora piricola(PP) andBotrytis cinerea(BC)were tested at 50 ppm according to published method[42], with the results shown in Table 3. The antifungal activity results showed some compounds exhibited good inhibitory againstB. Cinerea,G. zeae,P.capsici,andP. piricola.Compounds 4e, 4f and 4i still possessed good inhibitory (> 63%) againstB. cinerea,which is the same as fluxapyroxad. Most of the title compounds show good inhibitory againstP. piricola, such as compounds 4b, 4d, 4h, 4q, 4r and 4s. For theG. zeae,four compounds(4f, 4g, 4i, and 4q) exhibited good activity (57.1%). But the control displayed weak activity (28.6%) againstG. zeae.There is no significant fungicidal activity againstA. solani,F.oxysporum,P. infestans,R. solani,andC. arachidicola. ForF. oxysporum, only compounds 4g (40%) and 4r (40%)exhibited moderate activity, which is the same as the positive control (29.4%). As the same asC. arachidicola, most of these compounds exhibited low activity (< 40%), which are weaker than the positive control (100%). For theP. capsici,compound 4b (70%) exhibited good activity, while the others exhibited low activity.
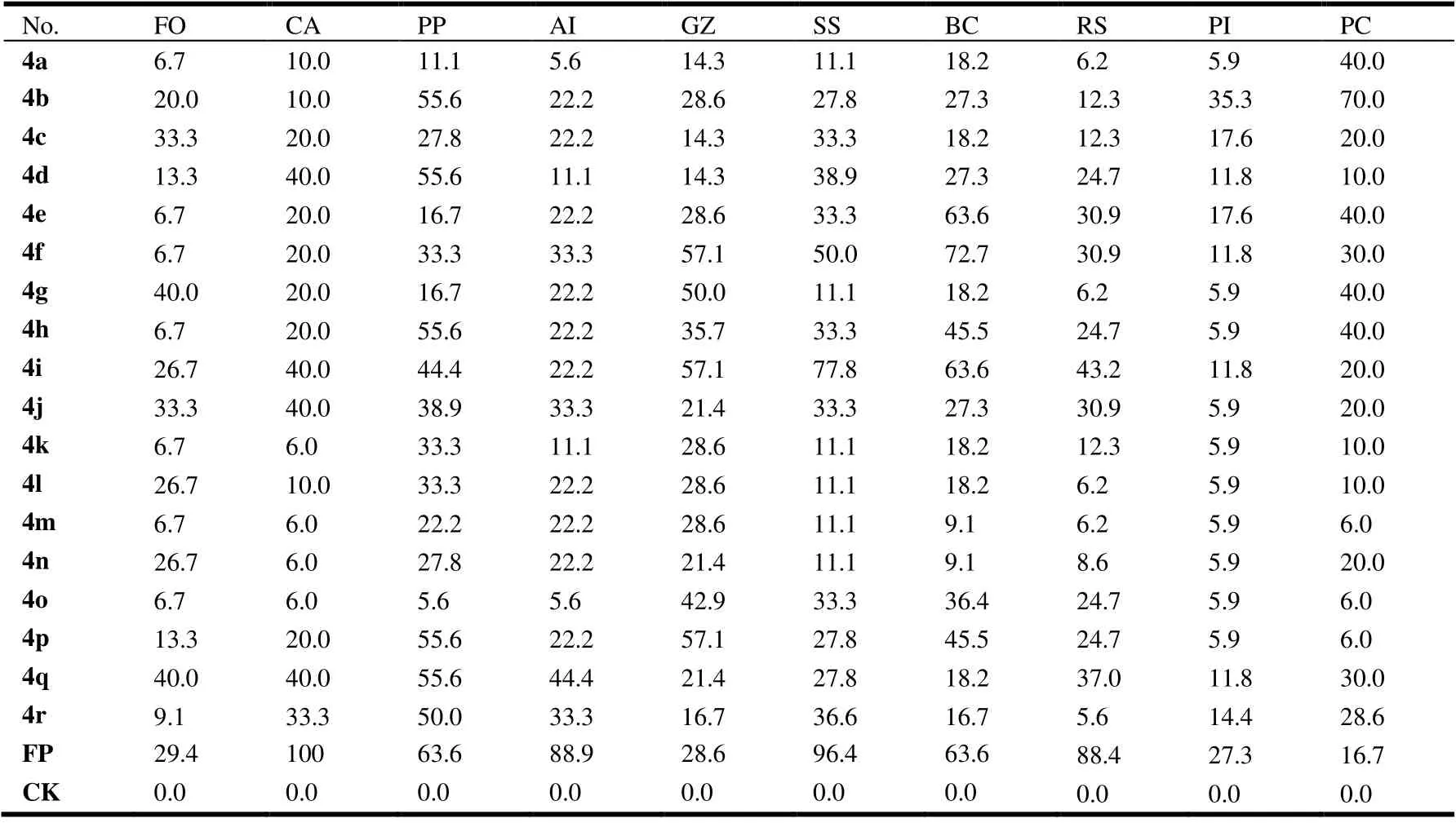
Table 3. Fungicidal Activity of the Title Compounds 4a~4r at 50 μg/mL
3. 4 Docking study
In order to study the mode of action of these compounds,the molecular docking was carried out between compound 4i and the enzyme SDH (PDB:2FBW) using DS 2.5. The docking results indicated that compound 4i can well occupy active site of SDH (Fig. 4). From Fig. 2, aπ-cation interaction exists between compound 4i and Arg 43 amino acid residue of SDH with the distance of 3.9 Å. There is another hydrogen bond (distance 2.0 Å) between the C=O of compound 4i and Trp 173 amino acid residue of SDH. From the docking, the pyridine ring and amide group are key active group in this fungicide, which is the same as the lead compound boscalid.
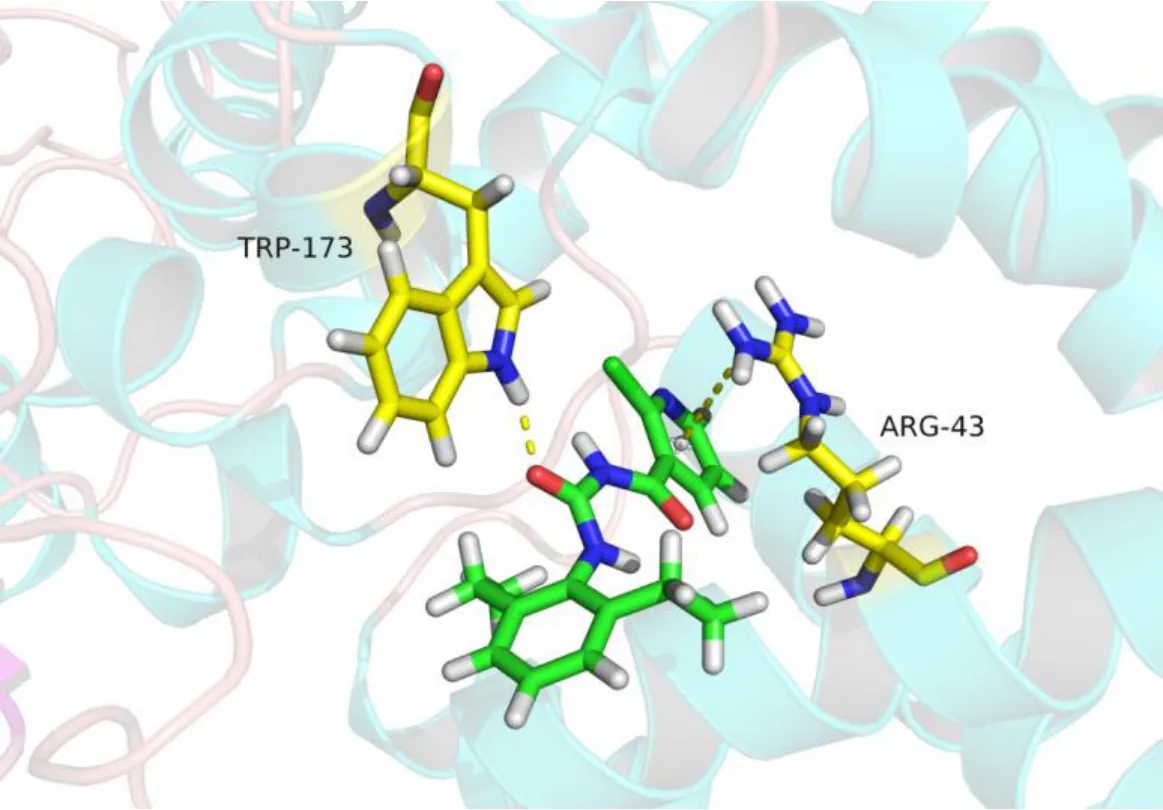
Fig. 4. Docking mode of compound 4i and SDH
杂志排行
结构化学的其它文章
- Synthesis, Crystal Structure and Fungicidal Activity of 3,4-Dichloro-5-(6-chloro-9-(4-fluorobenzyl)-9H-purin-8-yl)isothiazole①
- Synthesis, Crystal Structure and Antifungal Activity of New Furan-1,3,4-oxadiazole Carboxamide Derivatives①
- Syntheses, Crystal Structures, Anticancer Activities and DNA-Binding Properties of the Dibutyltin Complexes Based on Benzoin Aroyl Hydrazone①
- A New Borate-phosphate Compound CsNa2Lu2(BO3)(PO4)2:Crystal Structure and Tb3+ Doped Luminescence①
- Drug Design, Molecular Docking, and ADMET Prediction of CCR5 Inhibitors Based on QSAR Study①
- Self-organized TiO2 Nanotube Arrays with Controllable Geometric Parameters for Highly Efficient PEC Water Splitting①
