Clinical practice guidelines for traditional Chinese medicine and integrated traditional Chinese and western medicine:a cross-sectional study of data analysis from 2010 to 2020
2022-03-08JieZhouJingGuoJiaYingWangQiaoHuangRongZhangZhengRongZhaoHongJieXiaXiangYingRenYiBeiSiJianPengLiaoYingHuiJinHongCaiShang
Jie Zhou ,Jing Guo ,Jia-Ying Wang ,Qiao Huang Rong Zhang ,Zheng-Rong Zhao ,Hong-Jie Xia ,Xiang-Ying Ren ,Yi-Bei Si ,Jian-Peng Liao ,Ying-Hui Jin ,Hong-Cai Shang 0,
1 Center for Evidence-Based and Translational Medicine,Zhongnan Hospital of Wuhan University,Wuhan 430071,Hubei Province,China.
2 School of Nursing,Wuhan University,Wuhan 430071,Hubei Province,China.
3 The Affiliated Hospital of Nanjing University of Chinese Medicine,Nanjing 210029,Jiangsu Province,China.
4 The Affiliated Wuxi People's Hospital of Nanjing Medical University,Wuxi 214000,Jiangsu Province,China.
5 Department of Neurotumor Disease Diagnosis and Treatment Center,Taihe Hospital,Hubei University of Medicine,Shiyan 442000,Hubei Province,China.
6 College of Acupuncture and Orthopedics,Hubei University of Chinese Medicine,Wuhan 430061,Hubei Province,China.
7 College of Nursing and Health,Henan University,Kaifeng 475001,Henan province,China.
8 The Second Clinical College,Wuhan University,Wuhan 430071,Hubei Province,China.
9 School of Public Health,Wuhan University,Wuhan 430071,Hubei Province,China.
10 Dongzhimen Hospital,Beijing University of Chinese Medicine,Key Laboratory of Chinese Internal Medicine of Ministry of Education,Beijing 100700,China.
Abstract Objective With the increasing publication of clinical practice guidelines(CPG)for Traditional Chinese Medicine(TCM)and Integrated Traditional Chinese and Western Medicine(IM),the standardization and scientifiction of its formulation have gradually attracted many people’s attention.To offer an overview of TCM and IM CPGs published over the past decade and analyze their general characteristics and methodological quality.Methods The China National Knowledge Infrastructure(CNKI)and WANFANG databases were searched for clinical practice guidelines and expert consensus papers from January 2010 to June 2021.Two researchers independently completed the literature screening and cross-checking according to the inclusion and exclusion criteria of CPGs and extracted information on general characteristics and methodological quality of CPGs.Results According to the selection criteria,231 CPGs(EB-CPGs=119,CBCPGs=112)were selected and the number of CPGs published in the 11 years showed an overall upward trend.The vast majority of CPGs used the Western naming system for the diseases,and only 11 CPGs were named of TCM diseases or symptoms.TCM treatments were recommended in 223 CPGs.There were 156 ancient Chinese Medicine literature sources cited in 231 CPGs and opinions and experiences of 62 TCM experts cited in 37 CPGs.The methodological quality of EB-CPGs for TCM and IM were significantly better than CB-CPGs in 11 items.Only 60 EB-CPGs and 7 CB-CPGs designated clear criteria for grading quality of evidence and strength of the recommendations and 74 CPGs presented both the level of evidence and the strength of recommendations.We classified all CPGs according to whether or not they used GRADE,and the results showed that the CPGs using GRADE had higher methodological quality and more standardized reports.Conclusion This research has shown that the quantity and quality of CPGs in both TCM and IM have improved over the time span,but the methodological quality,especially evidence citation,and the use of criteria for grading quality of evidence and strength of the recommendations,still needs to further improvement in the future.
Keywords Evidence-based CPG,Consensus-based CPG,Traditional Chinese medicine,Integrated traditional Chinese and western medicine,Methodological quality
Background
As a treasure of Chinese culture,Traditional Chinese Medicine(TCM)is widely used in medical practice.However,in the overall medical development and research field,Chinese medicine is still in a relatively weak position[1].China is the only country in the world that adopts Chinese and Western medicine in primary,secondary,and tertiary treatment systems[2].In recent years,the development of TCM has attracted the attention of the national government.The “Strategic Plan for the Development of Traditional Chinese Medicine(2016-2030)[3]” identifies the phased objectives of the development of TCM,emphasizing that not only does the TCM medical service system need to be improved,but also a standardized system for TCM needs to be established.Since the 1980s,the government has attached increasing importance to the development of TCM and the construction of a standardized system.The features and importance of TCM in health care have become increasingly prominent and the number of guidelines for TCM including integrated traditional Chinese and Western medicine(IM)has also increased rapidly[4].
According to the definition of the Institute of Medicine(IOM)in 2011[5],Clinical Practice Guidelines offer optimal guidance for patients with specific clinical problems based on evidence formed by systematic evaluation and comprehensive analysis of the strengths and weaknesses of various alternative interventions.TCM and IM have played a unique role in the prevention and treatment of diseases,such as SARS,influenza A,tumors,cardiovascular and cerebrovascular diseases.Standardized development,dissemination and implementation of TCM and IM clinical practice guidelines is a viable way to internationalize TCM[6].
With the increasing publication of clinical practice guideline(CPG)for TCM and IM,the standardization and scientifiction of its formulation have gradually attracted wide attention from researchers and medical practitioners.The low rigor and credibility of these CPGs leads to them having an unsatisfactory clinical utilization rate,so not allowing them to play a real guiding role in clinical practice[7-10].Domestic researchers have surveyed the application of TCM guidelines for 11 common diseases and found that 54.5% of the guidelines had never been cited[11].At present,there is no systematic in-depth study on the publication and quality of TCM and IM guidelines.Therefore,this paper has searched and analyzed the current TCM and IM guidelines published over the past decade to investigate their advantages and disadvantages,with the aim of promoting the standardization and normalization of guideline formulation.
Methods
Search strategy
The CPGs included in this study were based on previous retrieval work done by the research team.The key words for the searches included Chinese words for terms such as‘guidelines’,‘practice guideline’,‘clinical guideline’,‘clinical practice guideline’,‘consensus’,‘expert consensus’,‘expert consensus statements’,‘professional consensus’,‘recommendation’.We searched for these terms in title fields from China National Knowledge Infrastructure(CNKI)and WANFANG from January 2010 to June 2021.
Eligibility criteria
Inclusion criteria:(1)conforming to the definition of clinical practice guidelines proposed by IOM in 1990[12]or 2011[5].(2)Chinese version of original guidelines and consensus in the field of TCM and IM published in China and available as full text.We classified the guidelines into two types based on their title definition of CPGs,which were evidence-based CPG(EB-CPG)and consensusbased CPG(CB-CPG).Usually when evidence is only of low quality,guideline development groups label them as expert opinions and consensus statements.In this research we describe both expert opinions and consensus statements as CB-CPGs.The classification into EB-CPGs or CBCPGs and the differentiation between TCM or IM is based on the reports of their titles and verification from the text.Exclusion criteria:(1)Interpretation class,compilation,adaptation CPGs.(2)Translated versions of foreign guidelines.(3)Incomplete CPGs which omitted important information,such as brief versions that only include introductions,directories,abstracts and recommendations.
If several published versions of one CPG existed,only the version containing the greatest detail was included for research.If CPGs are updated,both previous and updated versions were included in the assessment.If CPGs are published in several parts,they were merged into one complete CPG for assessment.
Data extraction
The research team members formed the data extraction table for CPGs based on the general characteristics of CPGs and some items from the Appraisal of Guidelines for Research and Evaluation Ⅱ(AGREE-Ⅱ)instrument.The general characteristics and methodological quality of CPGs reflected the development of CPGs in China over the past 11 years.Two researchers independently completed the literature screening and cross-checking according to the inclusion and exclusion criteria of the guidelines.Any disagreement was resolved through discussion with a third author.Before data extraction,three evaluation members in the group were trained,and then two pre-tests were conducted.In order to reduce bias in understanding the extracted items,the extraction work was only commenced after a relatively consistent understanding of the data extraction content was reached.
Information on general characteristic included guideline title,year of publication,year of updating and interval between updates,development body and its classification(National Health Commission of the People's Republic of China,medical specialty societies including their branches,individuals including working groups and committees),number of pages of CPGs document,number of references,guideline type(EB-CPG or CB-CPG),classification of TCM or IM,theme(diagnosis,treatment,prevention,prevention and treatment,diagnosis and treatment,nursing,rehabilitation,infectious disease prevention and control),CPGs users target population(under or over 18 years),Grading quality of evidence and strength of recommendations,TCM recommendation(decoction,Chinese patent medicine,TCM injection,traditional exercises,acupuncture,external TCM therapies including Tuina(therapeutic massage),enema,application by patching or mounting,cupping,fumigation,herb bath,etc.).Diseases were classified according to the International Classification of Disease revision 11(ICD-11).
Through the consensus process,we extracted the following 11 information categories from the 23 items of AGREE II to reflect the current methodological status of CPGs for TCM or IM.(1)Multidisciplinary development teams:these were described as diverse groups including more than two of the following representatives:relevant technical experts or health professionals,end-users,representatives of groups most affected by the recommendations,methodologists(assessing evidence and developing guidelines informed by evidence,or health economist or technical experts in equity and human rights).(2)Systematic literature searching:the article clearly points out accessing and rigorously searching at least 4 databases in English and Chinese,(e.g.,PubMed,Cochrane library,CNKI).(3)Identifies the characteristics of TCM evidence in retrieval and selection of evidence,such as the search or use of ancient books and literature on TCM,opinions and experience of TCM experts.(4)Recommendations based on evidence of systematic reviews of the scientific literature:at least one piece of the evidence supporting a recommendation came from a systematic review or meta-analysis.Systematic review was described as “a review of a clearly formulated question that uses explicit and systematic methods to identify,select,and critically assess relevant research,and to extract and analyze data from the studies that are included in the review”.(5)Quality evaluation of included literature.(6)Clear criteria for grading quality of evidence and strength of recommendations used,and whether they are based on or combined with TCM evidence.(7)The designation of level of evidence.(8)The presentation of strength of recommendations.(9)The declaration of conflicts of interest.(10)The identification of sources of funds:divided into national,provincial and municipal governmental funds which come from governmental organizations;hospitals and universities;medical specialty societies and no funding(including not funded and no report).(11)The consideration of factors such as feasibility,economy,security,equity,acceptability,values,and patient preferences in the formulation of each recommendation.
Data analysis
This paper reports only descriptive statistics,using Microsoft Excel software entry and collation of data to give a frequency and percentage summary.Inter-rater reliability was assessed by Kappa statistics.Comparisons of EB-CPGs and CB-CPGs in methodological characteristics were conducted using chi-square test or Fisher’s exact test.Mann Kendall Trend Test(M-K test),a non-parametric method,was adopted to identify monotonically increasing or decreasing trends of methodological characteristics over years,a positive z value indicated a monotonic upward trend and a negative one indicated downward trend.The statistical software SPSS 25.0 was used for data analysis and a two-sidedPvalue of < 0.05 was considered as statistically significant.
Results
Flow of included studies
A total of 29,186 articles were identified,of which 18,078 were considered potentially relevant;after selection,a total of 450 guidelines were eligible.231 guidelines(EBCPGs=119,CB-CPGs=112)were selected according to the selection criteria(See Figure 1).There was high agreement between the authors extracting data(Kappa= 0.835;95%CI 0.675~0.883;P< 0.001).Two authors discussed the differences in data extraction with the third author,and reached a consensus by re-examining the CPGs.
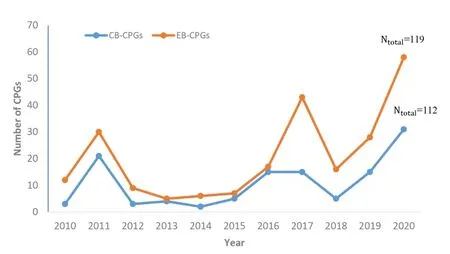
Figure 2.Trends in the number of CPGs for TCM and IM from 2020 to 2021
General characteristics of guidelines
Number and themes of CPGsFrom 2010 to 2020,the numbers of CPGs published for TCM and IM have shown an overall upward trend,and the number of CB-CPGs publications in the past four years was significantly higher than that of EB-CPGs.The total number of CPG publications was the lowest in 2013,and the number of publications increased rapidly from 2018 to 2020,reaching a maximum of 58 in 2020,accounting for 25.11%(58/231)of the total publications,including 31 EB-CPGs and 27 CB-CPGs(See Figure 2).
Diagnosis and treatment were the main themes of CPGs,accounting for 69.70%(161/231).In addition,the remaining CPGs themes were treatment(15.15%),diagnosis(0.87%),prevention and control of infectious diseases(6.06%),prevention and treatment(4.33%),nursing(0.43%),rehabilitation(3.46%).According to the classification of CPGs in the field of TCM and IM,there were 154 CPGs for TCM and 77 CPGs for IM,accounting for 66.67%(154/231)and 33.33%(77/231),respectively.
Classification of TCM therapies in recommendationsAccording to the recommendations given in the article,the most widely used TCM treatment method is decoction,accounting for 90.91%(210/231)of the total number of publications,including 108 EB-CPGs and 102 CB-CPGs.The second most widely used TCM treatment method was Chinese patent medicine treatment,which was recommended by 98 EB-CPGs and 79 CB-CPGs,accounting for 76.62%(177/231)of the total number of publications.A total of 87 EB-CPGs and 78 CB-CPGs recommended acupuncture therapy,accounting for 71.43%(165/231).68 EB-CPGs and 51 CB-CPGs recommended external TCM treatment,accounting for 51.52%(119/231),of which 21.21%(49/231)recommended Tuina.In addition,13.42%(31/231)of CPGs recommended traditional exercises,and 9.96%(23/231)recommended treatment using TCM injections The remaining 8 CPGs did not recommend TCM-related diagnosis and treatment methods,which were(1)Guideline for Western Medicine Diagnosis and TCM Syndrome Differentiation of IgA Nephropathy,(2)Guideline for TCM pediatric clinicaldiagnosis and treatment of children with insufficiency of the spleen using medicated cuisine(formulation),(3)Traditional Chinese Medicine Treatment Guidelines for Coronary Heart Disease Before and After Percutaneous Coronary Intervention,(4)Standardization Guidelines for Chinese Medicine Rehabilitation,(5)Expert consensus on phase I cardiac rehabilitation after coronary artery bypass grafting in the integrative medicine,(6)Traditional Chinese medicine core nursing knowledge and practical ability training standards:an expert consensus,(7)Expert Consensus on Selection Criteria for Ancient Medical Cases of Sepsis in Traditional Chinese Medicine,(8)Expert Consensus on study and application of Traditional Chinese Medicine Knowledge by Western Pharmacists in General Hospitals(Beijing,2020).
Development organizations and diseases addressed by CPGsEighty EB-CPGs and 95 CB-CPGs were formulated by the medical specialty societies,accounting for 75.76%(175/231)of the total number of publications,followed by 47.62%(110/231)Chinese Association of Traditional Chinese Medicine,21.21%(49/231)Chinese Society of Integrated Traditional Chinese and Western Medicine,5.19%(12/231)World Federation of Chinese Medicine Associations.There were 23.81%(55/231)of CPGs developed by individuals(only describing the establishment of a working group or committee),and the remaining 1 EB-CPG did not report the development organizations.Details are shown in Supplementary Table 1.
CPGs covered a broad range of diseases.The most addressed diseases were digestive system diseases(25.54%),followed by circulatory system diseases(9.96%),certain infectious or parasitic diseases(9.96%),respiratory system diseases(6.93%),symptoms,signs or clinical findings,not elsewhere classified(6.94%),diseases of the skin(6.49%),diseases of the musculoskeletal system or connective tissue(6.06%),diseases of the genitourinary system(4.33%).The classification of diseases is shown in Table 1.
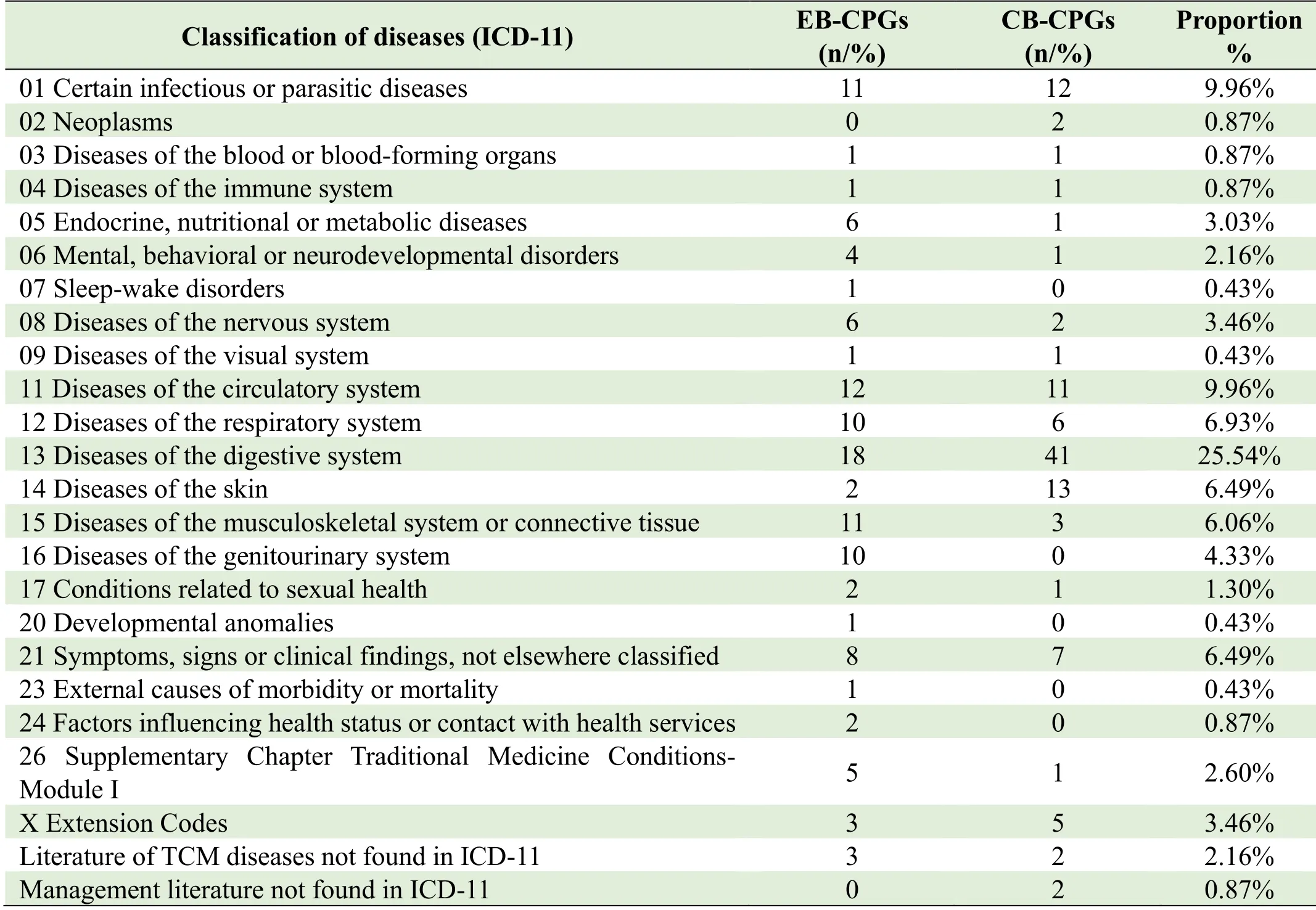
Table 1.The classification of diseases in CPGs from 2020 to 2021
Among the 231 CPGs,there were 11 CPGs using the TCM disease or syndrome naming system,including 7 using the TCM disease names(chest obstruction with pain,snake strand sore,nasal obstruction,syndrome of malnutrition,sweating syndrome,snoring,bloody semen)and 3 TCM syndrome names(syndrome of heat and stasis,constitution of spleen deficiency,stagnation of blood),accounting for 4.76%(11/231)of the total number of publications.There were two management consensuses in CB-CPGs,includingTraditional Chinese medicine core nursing knowledge and practical ability training standard:an expert consensus;Expert Consensus on study and application of Traditional Chinese Medicine Knowledge by Western Pharmacists in General Hospitals(Beijing,2020).
Target population and CPGs usersThirty-four(14.72%)CPGs targeted patients under 18 years old of which only 1 CPG was for infants,and 57(24.68%)CPGs targeted patients over 18 years old.The remaining 140 CPGs did not specify the target population,accounting for 60.61%(140/231)of the total number of publications.
CPGs users included 6.49%(15/231)TCM doctors,8.23%(19/231)TCM and Western Medicine doctors,1.73%(4/231)TCM and IM doctors,TCM,2.16%(5/231)Western Medicine and IM doctors.There were 188 CPGs which did not report CPGs users,accounting for 81.39%(188/231)of the total number of publications.
Methodological characteristics of guidelines
The results of methodological information assessment of CPGs are shown in Figure 3.
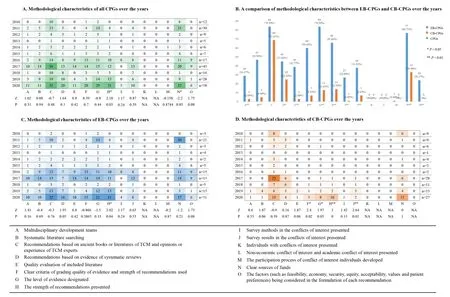
Figure 3.Methodology characteristics of all CPGs from 2020 to 2021
Citation of ancient literature of TCM and experience and opinion of TCM expertsA total of 153(66.23%)of the 231 CPGs cited ancient books and literatures of TCM and experience and opinions of TCM experts including 70.59%(84/119)EB-CPGs and 61.61%(69/112)CPCPGs.
A total of 156 ancient Chinese medicine literature sources were cited in 231 CPGs,of whichTreatise on ColdDamagewas cited most frequently,which was cited by 36 EB-CPGs and 35 CP-CPGs,accounting for 30.74%(71/231),followed by 29.00%(67/231)Beneficial Formulas from the Taiping Imperial Pharmacy,25.97%(60/231)Essentials from the Golden Cabinet,23.38%(54/231)Correction of Errors in Medical Works,22.51%(55/231)The Complete Works of[Zhang]Jing-yue.The top 20 citations of ancient books and literatures of TCM are presented in Table 2.
Thirty-seven CPGs cited opinions and experiences of 62 TCM experts,19 CPGs cited National Famous Chinese Medicine Practitioner;17 CPGs cited National physician master;10 CPGs cited Provincial Famous Chinese Medicine Practitioner;5 CPGs cited Municipal Famous Chinese Medicine Practitioner;4 CPGs cited Qi Huang scholars' treatment experience and opinions;1 CB-CPGs quoted the opinions and experience of ancient Chinese medicine expert Wang Bing in traditional exercises for heart disease and 19 CPGs cited other TCM experts.Among the cited TCM experts,3 EB-CPGs cited the experience of Professor Xu Jingfan,a master of TCM,in the treatment of digestive system diseases.The three CBCPGs cited the experience and methods of Professor Li Junxiang,a scholar of Qi and Huang,in the treatment of liver cirrhosis and ascites diseases.Specific references to TCM expert opinions and experiences are contained in Table 3.
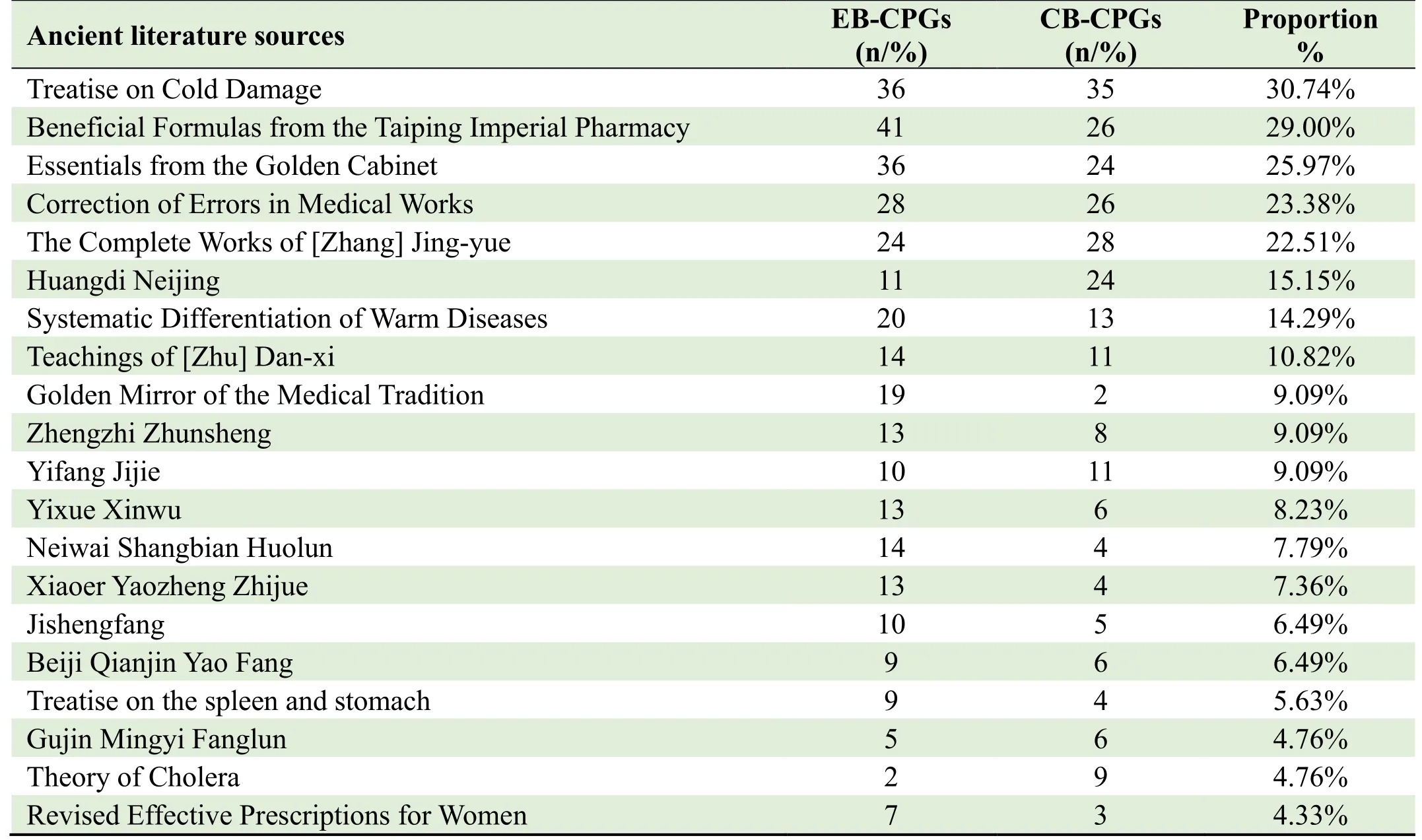
Table 2.Top 20 citations of ancient books and literatures of TCM cited in CPGs from 2020 to 2021
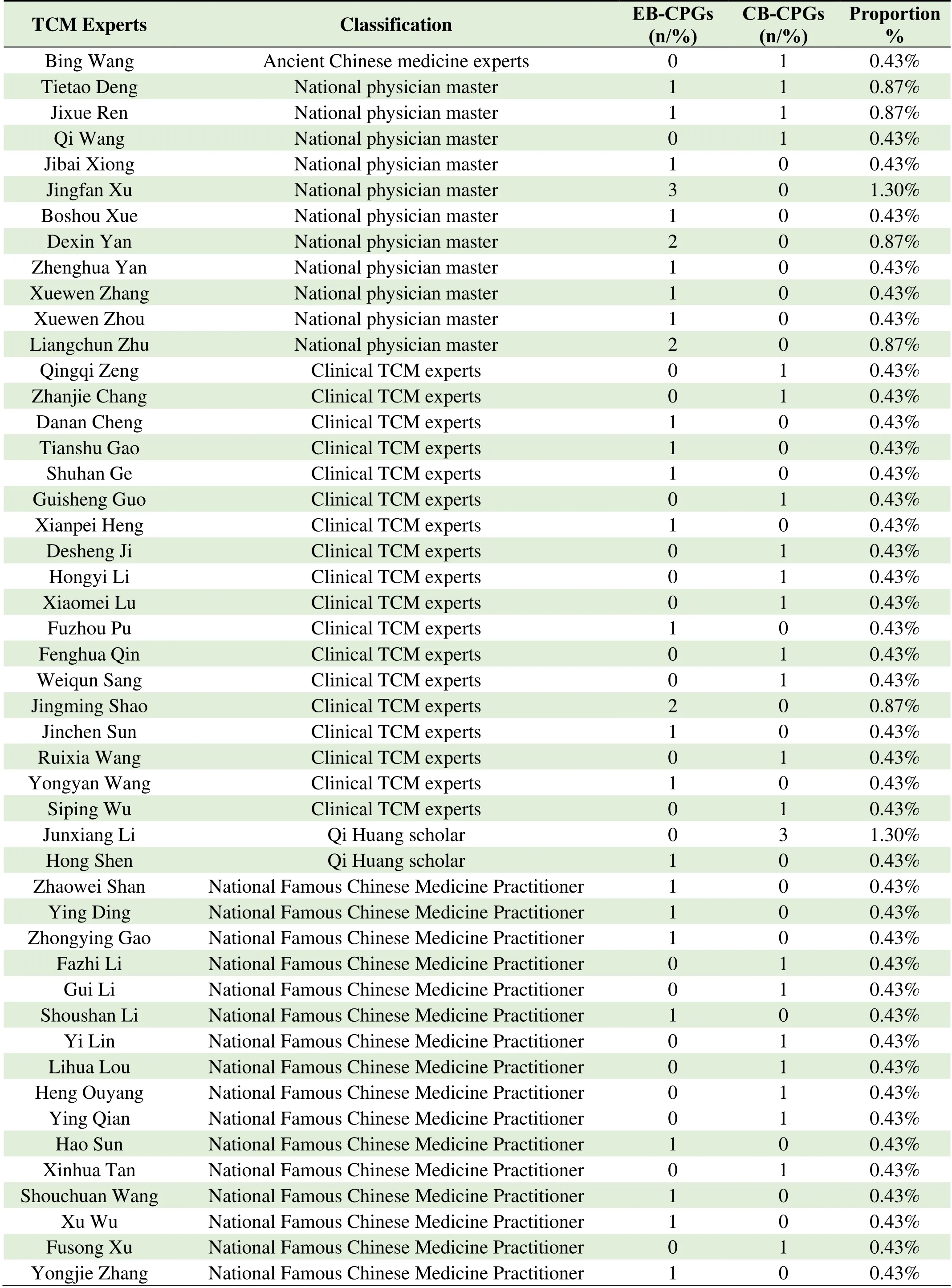
Table 3.TCM expert opinions and experiences cited in CPGs from 2020 to 2021
Wenxia Zhao National Famous Chinese Medicine Practitioner 0 2 0.87%Fusheng Zhou National Famous Chinese Medicine Practitioner 0 1 0.43%0.43%Yun Cui Provincial Famous Chinese Medicine Practitioner 0 1 0.43%Ruli Ai Provincial Famous Chinese Medicine Practitioner 0 1 0.43%Maoliang Qiu Provincial Famous Chinese Medicine Practitioner 2 0 0.87%Ruiqiang Fan Provincial Famous Chinese Medicine Practitioner 0 1 Lining Wang 0.87%Jingri Xie Provincial Famous Chinese Medicine Practitioner 1 0 0.43%Provincial Famous Chinese Medicine Practitioner 1 1 Baochun Zhang 0.43%Heping Zhao Provincial Famous Chinese Medicine Practitioner 0 1 0.43%Provincial Famous Chinese Medicine Practitioner 1 0 0.87%Lingtai Wang Municipal Famous Chinese Medicine Practitioner 1 0 0.43%Ping Liu Municipal Famous Chinese Medicine Practitioner 1 1 0.43%Peiting Zhu Municipal Famous Chinese Medicine Practitioner 1 0 0.43%Peifen Yue Municipal Famous Chinese Medicine Practitioner 1 0
Grading quality of evidence and strength of recommendationsA total of 84 EB-CPGs and 13 CBCPGs designated the level of evidence,66 EB-CPGs and 14 CB-CPGs presented the strength of recommendations,and 74 of 231(32.03%)CPGs presented both the level of evidence and the strength of recommendations.Significant differences between EB-CPGs and CB-CPGs were observed in this methodological characteristic(Item G and H,P< 0.01),which is shown in Figure 3B.Compared with EB-CPGs,CB-CPGs have improved significantly in the item of presenting the level of evidence over the time-span(Item G,P < 0.05)(See Figure 3D).CPGs,(P< 0.05)EBCPGs(P < 0.05)and CB-CPGs(P< 0.01)all have improved significantly in the item of presenting the strength of recommendations over the time-span(Item H,See Figure 3A,3C and 3D).
50.42%(60/119)EB-CPGs and 6.25%(7/112)CBCPGs designated clear criteria for grading quality of evidence and strength of the recommendations.EB-CPGs were better developed than CB-CPGs in this item,and there was a significant statistical difference between the two(Item F,P<0.01)(See Figure 3B).Compared with EB-CPGs,CB-CPGs have improved significantly in this item over the time-span(Item F,P<0.05)(See Figure 3D).
From 2010 to 2020,there are 6 criteria for grading quality of evidence and strength of the recommendations:12.12%(28/231)the grading standards for TCM literature by Professor Wang Shouchuan[13];9.09%(21/231)the Grading of Recommendations Assessment,Development and Evaluation(GRADE)criteria by the International Evidence Classification Working Group[14];7.79%(18/231)Evidence Classification of Clinical Research Based on Evidence Body by Professor Liu Jianping[15];3.46%(8/231)Delphi classification standard proposed by International Infection Forum(ISF)in 2001;0.87%(2/231)National Clinical Guidelines Database Evidence Rating System;0.43%(1/231)Oxford Centre For Evidence Based Medicine,OCEBM.
Among the 21 CPGs using GRADE,4 EB-CPGs used modified GRADE criteria named from 1 to 4.Among them,Modified GRADE 4 was combined with the evidence characteristics of the guidelines for TCM and IM,so as to make it more suitable for the guidelines in this field.The grading standards for TCM literature by Professor Wang Shouchuan;Evidence Classification of Clinical Research Based on Evidence Body by Professor Liu Jianping and self-defined criteria for grading quality of evidence and strength of the recommendations also reflect the TCM features in criteria for grading quality of evidence.A total of 34.45%(41/119)of EB-CPGs and 5.36%(6/112)of CB-CPGs use above mentioned TCM related criteria.Details are shown in Table 4.
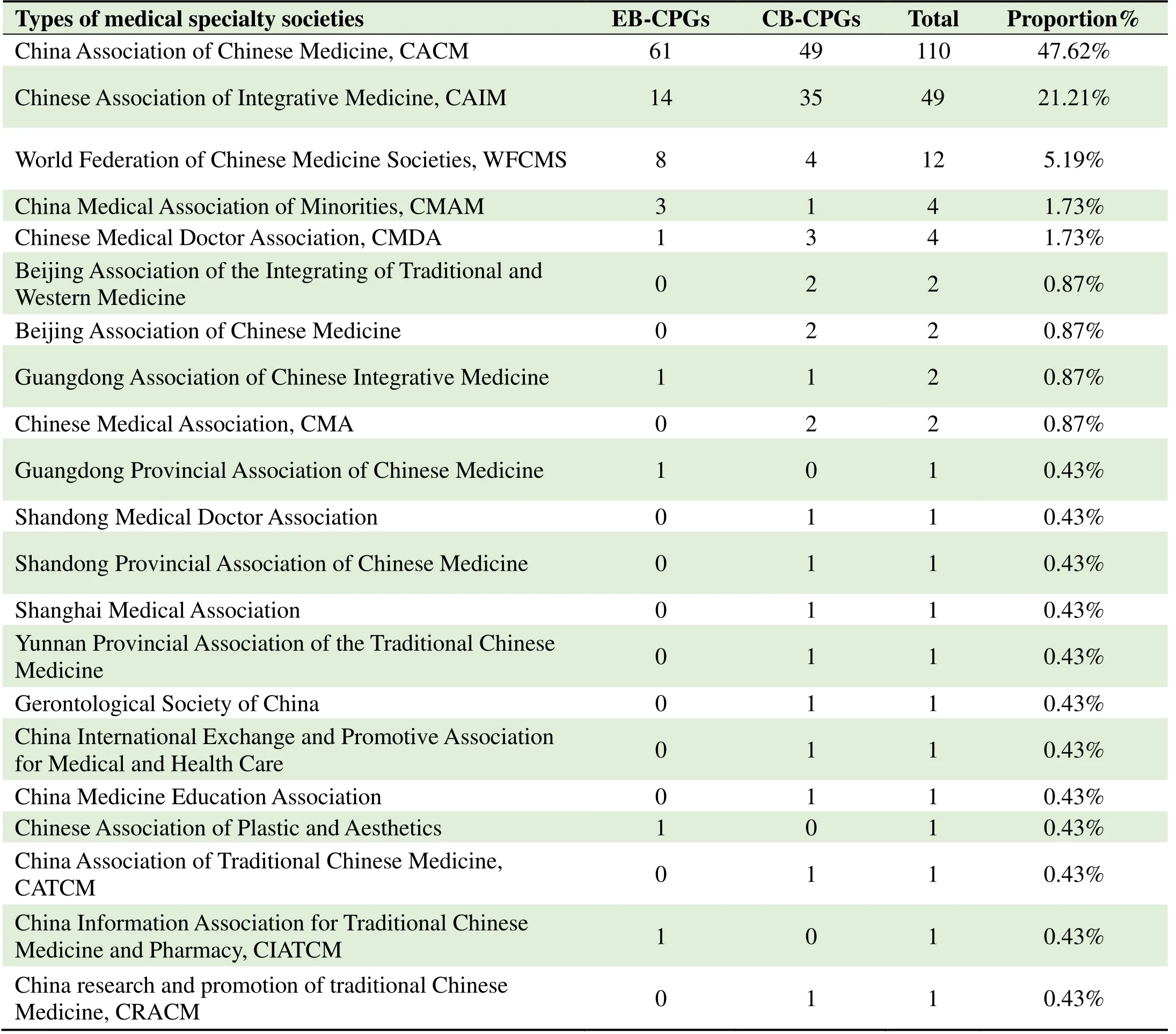
Table 1.Number of medical specialty societies in development of CPGs from 2020 to 2021
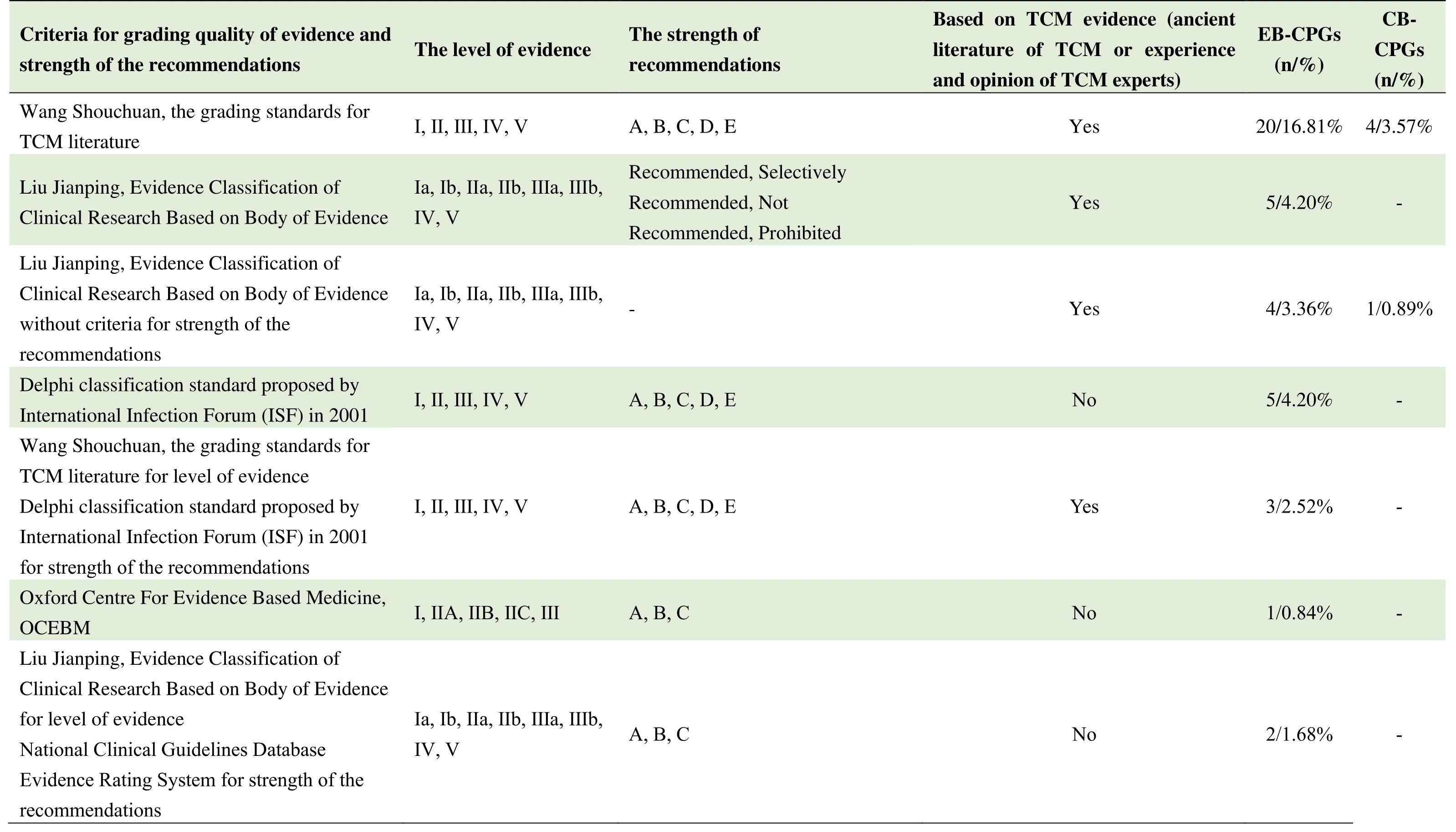
Table 4 Criteria for grading quality of evidence and strength of the recommendations used in the published guidelines from 2010-2020
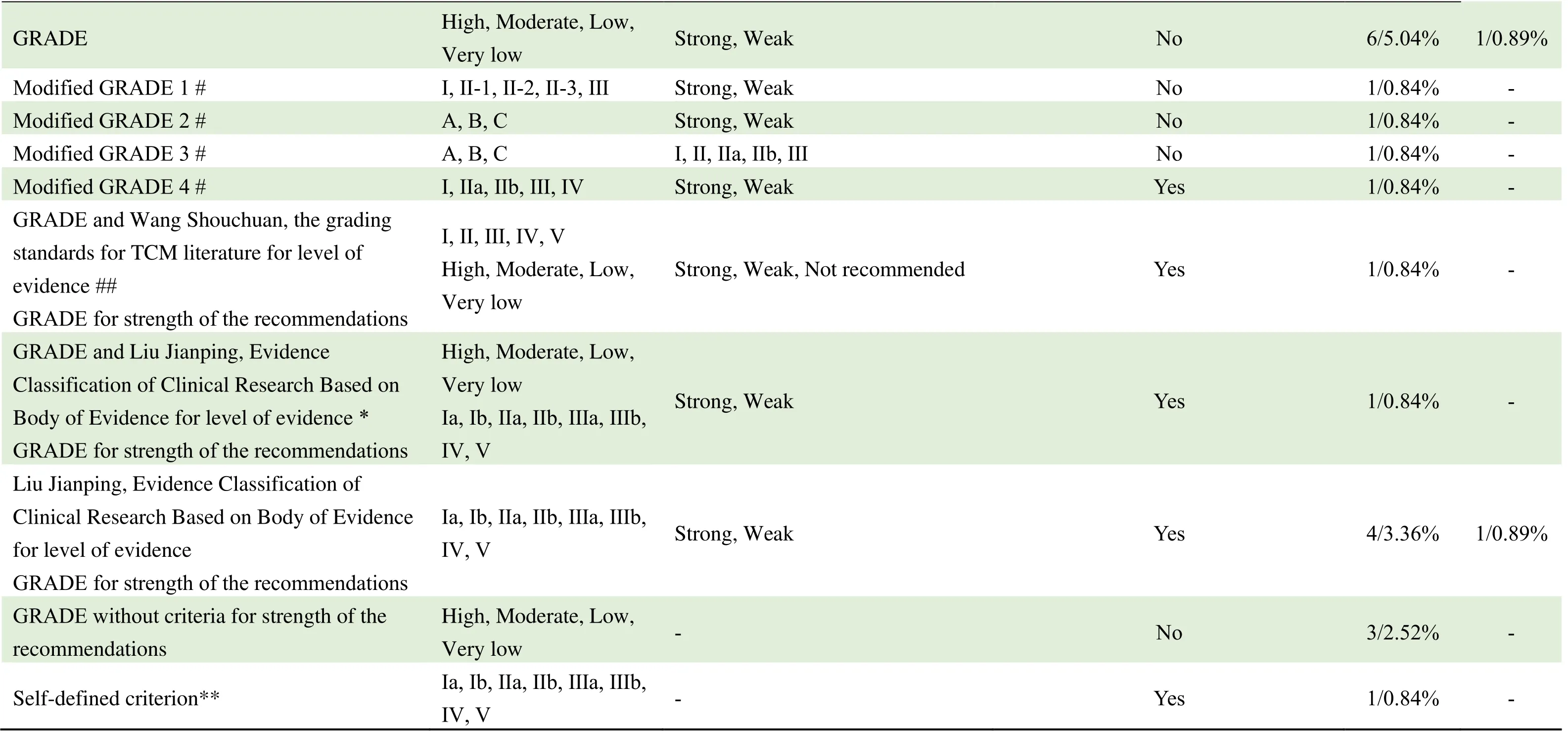
#:4 EB-CPGs used modified GRADE criteria named from 1 to 4.##:The reported literature was based on GRADE and the literature from TCM books was based on the grading standards for TCM literature by Professor Wang Shouchuan to grade the quality of evidence.*:GRADE was used to appraisal the bodies of evidence and recommendations;the body of evidence and recommendation of TCM prescriptions were graded by Evidence Classification of Clinical Research Based on the Body of Evidence by Professor Liu Jianping.**:the self-defined criterion was formulated with reference to GRADE,Liu Jianping 's Evidence Classification of Clinical Research Based on the Body of Evidence,and evidencebased clinical practice guidelines of TCM,combined with the clinical characteristics of the disease.
Sources of funding for guidelinesA total of 110(47.62%)CPGs indicated where there were clear sources of funding,including 64.71%(77/119)EB-CPGs and 29.46%(33/112)CB-CPGs.Although CPGs have improved in this area of the methodological quality over the time-span(P< 0.05,Item N)(See Figure 3A),a significant statistical difference still remains between EB-CPGs and CB-CPGs(P< 0.01,Item N)(See Figure 3B).
Most funding(26.41%,61/231)came from the National Administration of Traditional Chinese Medicine,including 46.22%(55/119)EB-CPGs and 5.36%(6/112)CB-CPGs.In addition,12.99%(30/231)were supported by national projects,2.60%(6/231)by provincial projects,8.66%(20/231)by municipal projects and 0.87%(2/231)by associations.51.52%(119/231)CPGs did not report a clear source of funding.
Multidisciplinary development teams13.85%(32/231)of the CPGs established multidisciplinary development groups during the formulation process and the expert group consisted of professionals from at least two different disciplines,this included 29 EB-CPGs and 3 CB-CPGs.EB-CPGs scored significantly higher for this item(Item A,P< 0.01)(See Figure 3B).The remaining 199 CPGs did not report multidisciplinary cooperation,accounting for 86.15%(199/231)of the total number of publications.
The expert groups of 27 CPGs include clinical experts and methodological experts(experts in evidence-based medicine,statistics,health economics,philology,clinical epidemiology,and guidelines development methodology).Among 154 CPGs for TCM,25 have developed multidisciplinary cooperation.Among them,9 CPGs inviting Western medicine clinical experts accounted for 5.84%(9/154),and 6 CPGs inviting IM clinical experts accounted for 3.90%(6/154).
Retrieval and Appraisal of evidence22.51%(52/231)CPGs were based on a complete literature search,including 39.50%(47/119)of EB-CPGs and 4.46%(5/112)of CB-CPGs.It was clear that there was a significant difference between EB-CPGs and CB-CPGs(Item B,P<0.01)(See Figure 3B).3.03%(7/231)of CPGs had more than 100 references,all of which are EB-CPGs.
From 2010 to 2020,there were 30.30%(70/231)of the EB-CPGs whose recommendations were based on evidence of systematic reviews,which consisted of 37.82%(45/119)EB-CPGs and 22.32%(25/112)CB-CPGs.There was a statistically significant difference between EB-CPGs and CB-CPGs(Item D,P<0.05)(see Figure 3B).
There were 42 CPGs citing quality evaluation,accounting for 18.18%(42/231)of the total number of publications.Among them,there were 39 EB-CPGs and 3 CB-CPGs,accounting for 32.77%(39/119)and 2.68%(3/112)of the total number of publications,respectively.There were significant differences between the EB-CPGs and CB-CPGs(Item E,P<0.01)(see Figure 3B).
Developing recommendationsAmong the 231 CPGs,only 24 EB-CPGs took into account the feasibility,economy,security,equity,acceptability,values,and patient preferences in the formation of recommendations,accounting for 20.17%(24/119).There were significant differences between EB-CPGs and CB-CPGs(Item O,P<0.01)(see Figure 3B).
Conflicts of interestA total of 45(19.48%)CPGs published from 2010 to 2020 stated the conflicts of interest survey results of the drafters,including 31.93%(38/119)in the EB-CPGs and 6.25%(7/112)in the CB-CPGs.The difference between the two was statistically significant(Item J,P< 0.01)(see Figure 3B).CB-CPGs have improved significantly in the item of reporting the survey results of conflicts of interest over the time-span(Item J,P< 0.01)(See Figure 3D).
Among the 231 CPGs,12.12%(28/231)described the conflicts of interest survey method,of which EB-CPGs accounted for 22.69%(27/119)and CB-CPGs accounted for 0.89%(1/112).The difference between the two was statistically significant(Item I,P<0.01)(see Figure 3B).
Only 0.84%(1/119)of EB-CPGs presents the participation process of individual conflicts of interest guidelines.
Effect of GRADE on the methodological quality of CPGs
In 2010-2020,there were 9.09%(21/231)CPGs that used GRADE methodology of which 10.65%(19/119)were EB-CPGs and 4.72%(2/112)were CB-CPGs.We statistically analyzed 15 methodological quality evaluation items of those using GRADE and those not using it.From the following statistical analysis,it can be concluded that the methodological quality of CPGs using GRADE classification system is relatively high in the formulation process.There were significant differences in the methodological quality in 9 items of CPGs using GRADE and those not using it(P< 0.01).Supplementary Figure 1 shows the details.
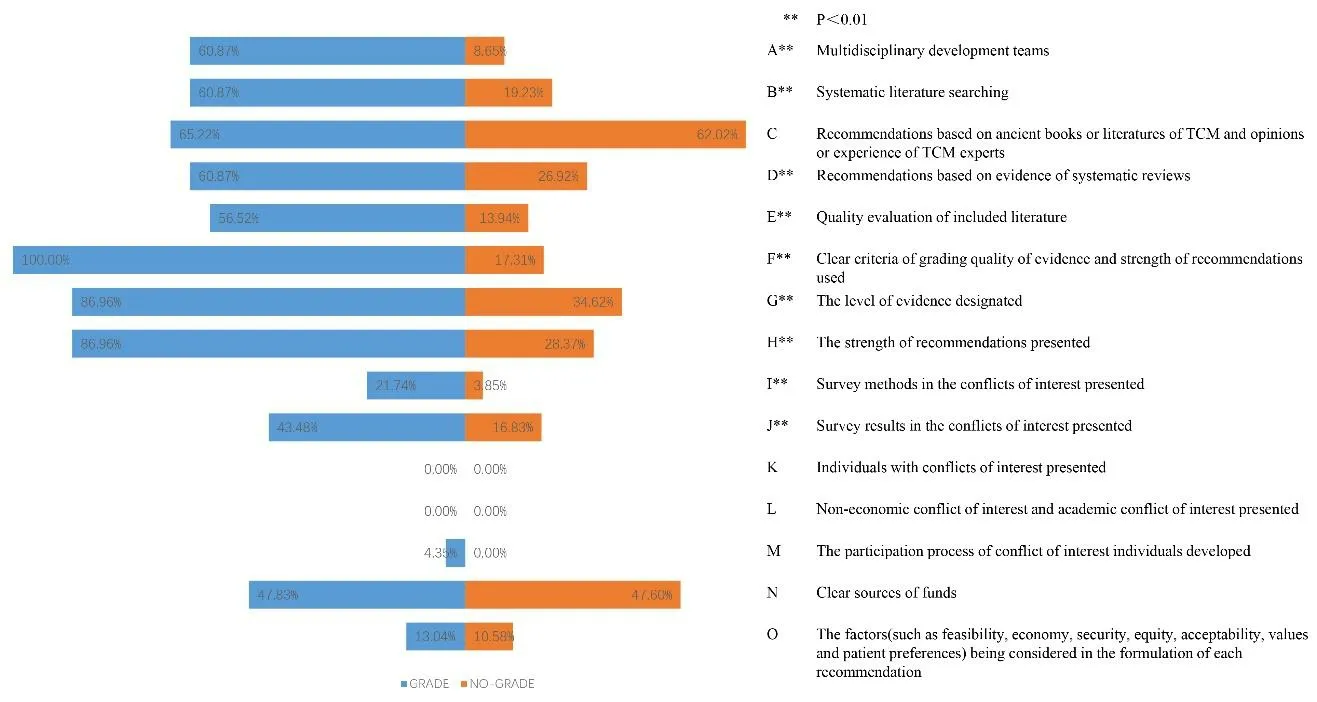
Figure 1.Methodological characteristics in CPGs using GRADE versus those not from 2020 to 2021
Discussion
After descriptive and analytical data analysis of the general information characteristics and methodological characteristics of 119 EB-CPGs and 112 CB-CPGs on TCM and IM from 2010 to 2020,it was found that the number of CPGs published in the 11 years showed an overall upward trend,which was consistent with the current focus on the development of CPGs standards and guidelines for TCM and IM.The vast majority of CPGs used the Western diseases naming system,and only 11 CPGs were found to be named using the TCM diseases or symptoms naming system.A total of 223 CPGs recommended TCM treatment,including decoction,Chinese patent medicine,acupuncture,external TCM therapies,Tuina,traditional exercises and TCM injection.As the most widely used and the most equipped TCM treatment,decoction was recommended in most CPGs.In our study,over the eleven-year time span,CPGs were of poor quality in the item of clearly identifying the target population and guideline users,with 60.61% and 81.39%of CPGs not reporting the target population and guideline users,respectively,and no patient version of the guidelines were found.In additional,only a few CPGs have been updated in this study.The methodological quality of the TCM and IM CPGs included in this study was generally poor.Only one-seventh of the CPGs established a multidisciplinary expert panel in the development of guidelines and there was low participation of methodological experts in the development process.Less than a quarter of CPGs conducted sufficient evidence retrieval and literature quality appraisal.Less than one third of the CPGs clearly pointed out the criteria for grading quality of evidence and strength of the recommendations.41.99% and 34.63% CPGs of the CPGs presented evidence level and recommendations strength,respectively.Only one-fifth EB-CPGs considered the feasibility,economy,security,equity,acceptability,values,and patient preferences in the formation of their recommendations.Of CPGs that reported conflicts of interest surveys the methods and results of the drafters were unsatisfactory.
Both EB-CPGs and CB-CPGs were included in this study,and they differed significantly in quality.From the evaluation results of this study,EB-CPGs were superior to CB-CPGs in several methodological quality evaluation items.Particularly,EB-CPGs showed higher quality in the evaluation and citation of evidence.However,in terms of quality improvement over time,the EB-CPGs included in this study showed significant quality improvement only in presenting the strength of recommendations;whereas the methodological quality of the CB-CPGs showed more significant improvement over time,mainly in specifying criteria for grading quality of evidence and strength of the recommendations,designating the level of evidence and the strength of recommendations,and reporting the conflicts of interest survey results.EB-CPGs are optimal guidelines for patient-specific clinical problems,based on evidence from systematic review and a comprehensive balance of pros and cons of various alternative interventions[5].In contrast to EB-CPGs,CB-CPGs usually lack a uniform definition and are defined differently by different international guideline development organizations.At present,it is generally accepted that CB-CPG is an industry guidance document that is less rigorously produced than EB-CPG.A CB-CPG is a recommendation in a particular medical field that is formulated by medical specialty societies or experts having a certain influence in a certain field based on consensus approach(e.g.,Delphi method)and limited evidence[2,16].In our study,the CB-CPGs were not highly cited for evidence,and only a small proportion of them were developed based on systematic evaluation,quality assessment of the included literature,and clear indications of criteria for grading quality of evidence and strength of the recommendations;mostly recommendations were reached through the expert consensus process only.It is important to clarify that CBCPGs are not only experts’ opinions,but also need to be supported by relevant evidence,otherwise biased recommendations may be obtained,which may result in poor clinical guidance[17-18].A more rigorous recommendation based on higher-level evidence and a standardized development process may be an important direction for future guidelines and consensus development in the field of TCM and IM[19].
About two-thirds of the CPGs in this study cited ancient literature of TCM and TCM experts experience or opinions as sources of evidence.TCM has a long history and inherits the wisdom of the Chinese people for about five thousand years,and ancient literature and experts experience or opinions in Chinese medicine are the characteristic evidence of TCM.Ancient literature has significant advantages in serving as a basis for decision making in TCM[20].Firstly,the large number of ancient literature sources in TCM,covering a wide range of disease areas,can provide supporting evidence for most diseases;secondly,citing ancient literature as evidential support is more in accordance with the characteristics of clinical decision-making in TCM.However,there are still obstacles to using the citation of ancient literature as an evidence source for clinical evidence-based TCM as evidence formulated in the guidelines often has an incomplete evaluation system[20-21].The methodology of guideline development requires classification and grading of evidence,but the existing evidence classification methods,especially the international recognized evidence classification and grading methods,are based on modern medical evidence,so this presents a problem when using ancient literature as evidence and how to complete the classification and grading in the guideline development process is a question yet to be solved.In our study,a certain number of CPGs were unable to be graded using the internationally unified grading criteria for evidence quality and recommendations.Although some scholars[21-27]in China have developed grading standards for ancient literature they are only dealing with this one issue,they are unable to make a comprehensive evaluation with the evidence of modern medical research and still cannot promote and disseminate ancient literature internationally.At the same time,more and more highquality TCM studies have been published in international authoritative medical journals[28-31],laying the foundation for the modernization and internationalization of TCM.Therefore,based on previous research conclusions[32-35]and our considerations,we recommend that real-world studies based on ancient literature should be conducted and applied as a source of evidence for CPGs.Modern medical evidence in TCM not only reflects the important methods and interventions in ancient literature,but also translates ancient literature into modern medical studies,which will facilitate its dissemination internationally and its standardization of criteria for evidence quality and grading of recommendations in the guideline development process.
The experience and opinion of TCM experts mainly refers to the experienced prescriptions or opinions of ancient and modern famous TCM practitioners.The classification of expert opinion is not consistent among the existing TCM evidence quality standards[13,22,24,36].Liu Jianping[37]has put forward the path from“experience” to “evidence” for TCM diagnosis and treatment characteristics:which is,to obtain information through observation of TCM medical experience,integrate the information and refine theories to build a knowledge system,and then develop evidence through research.In all,expert experience can also be proved through real world studies by future generations,and effective dialectical ideas and treatments strategies can be passed down.Modern medical evidence based on expert experience and opinion to support guideline development may be a future trend.
Over the past century,the clinical TCM has been facing competition and challenges from Western medicine.It is a new trend in the development of TCM to realize the complementarity between Chinese and Western medicine by giving full play to the advantages of TCM.For example,the examination and diagnosis of diseases and the understanding of diseases through modern medicine provide TCM with more accurate and reliable objects for evidence-based treatment,and the combination of evidence-based and disease-based diagnosis further improves the diagnosis and evidence-based level of TCM.There are significant differences between the theoretical and practical systems of TCM and Western Medicine[38].In some CPGs[39-41],there was a mixed use of multiple criteria for grading the quality of evidence or strength of the recommendations.For example,the reported literature was based on the international evidence grading system,while the TCM-related evidence quality grading standards were mainly used for the grading of TCM ancient literature and TCM expert opinions.We do not advocate the using of multiple evidence quality or recommendation criteria in the development of a guideline,mainly because the systemic and holistic nature of classification and grading criteria in the development and formulation process can be interrupted with the fragmentation or combination of different criteria.For instance,GRADE is based on the body of evidence of different study types and different initial evidence levels before applying rating down and rating up factors[42].For different clinical problems,confidence in best estimates of magnitude of effects,feasibility,as well as the cost of transformation of evidence to decision making are taken into account in the GRADE model,which are not fully available in other criteria.
In this study,we classified all CPGs according to whether they used the GRADE or not,and the results showed that the CPGs using GRADE had higher methodological quality and showed more standardized reporting.There are still many challenges in the application of the international evidence classification and grading system to TCM and IM CPGs,but it is undeniable that they are the scientific standard and so the way forward.Some scholars[43-45]believed that GRADE is still one of the most effective methods for the construction of the TCM clinical system,and recommend the application of the GRADE to TCM/IM clinical practice guidelines.As the methodology of CPGs in TCM and TCM continued to mature and the quality of the included studies gradually improves,the application of GRADE in CPGs of TCM and IM will gradually mature and increase[45].In addition,we noticed that four EB-CPGs[46-49]attempted to use GRADE in the development process.However,due to the unavailability of GRADE for TCM evidence,they adapted GRADE without detailing the adaptation method of the modified GRADE criteria.These challenges are mainly caused by the lack of classification and grading of TCMrelated evidence included in internationally accepted common standards.As mentioned above,we do not advocate the using of modified GRADE criteria and transforming TCM evidence into modern medical evidence before being cited by CPGs can solve these problems.Similarly,real-world studies based on TCM evidence can be classified and graded using GRADE.
This paper has some limitations:(1)We only searched the CNKI and Wanfang without searching for English guidelines and consensus statements developed by Chinese researchers.When screening the literature,we only classify TCM and IM from the literature titles,so some literature may have been missed.(2)In this study,the complete form of AGREE II was not used for methodological quality evaluation in the included literature,so it does not provide a systematic understanding of the literature quality and the research perspective is relatively limited.
Conclusion
High-quality,clinically relevant CPGs for TCM and IM are needed to guide practitioners to make more rational clinical decisions,standardize medical practices,direct the active development of TCM,and promote the standardization and internationalization of TCM[50].This research has shown that the quantity and quality of CPGs in TCM and IM have improved over the time span.With the increasing development of CPGs in TCM and IM,it is hoped that the methodological quality,especially evidence citation,and the use of criteria for grading quality of evidence and strength of the recommendations,will become more standardized and scientific so promoting standardization and internationalization of CPGs in TCM and IM.
Data availability
Supplementary data are available online at TMR Modern Herbal Medicine.Correspondence and requests for materials should be addressed to jinyinghuiebm@163.com(Prof.Ying-Hui Jin).
Abbreviations
CPG,Clinical Practice Guidelines;TCM,Traditional Chinese Medicine;IM,integrated traditional Chinese and Western medicine;CNKI,China National Knowledge Infrastructure;IOM,Institute of Medicine;EB-CPG,CBCPG,evidence-based CPG;consensus-based CPG;AGREE-Ⅱ,Appraisal of Guidelines for Research and Evaluation Ⅱ.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(No.82174230 and No.81904055).We express our gratitude to Jean Glover from Tianjin Golden Framework Consulting for English editing.
Author contributions
Jie Zhou and Jing Guo were the main writers of the article;they completed the collection and analysis of relevant literatures and written the initial draft(including substantive translation and revision).Jie Zhou,Zheng-Rong Zhao,Hong-Jie Xia,Xiang-Ying Ren,Yi-Bei Si and Jian-Peng Liao participated in the collection and check of literatures and materials.Jie Zhou,Qiao Huang and Rong Zhang completed data analysis and visualization.Ying-Hui Jin,Hong-Cai Shang and Jia-Ying Wang were the designers in charge of the project and directed the article writing.All authors read and agreed on the final text.
Competing interests
The authors declare no competing interests.

Table 1.Number of medical specialty societies in development of CPGs from 2020 to 2021

Figure 1.Methodological characteristics in CPGs using GRADE versus those not from 2020 to 2021
杂志排行
TMR Modern Herbal Medicine的其它文章
- Blood stasis syndrome:clinical multi-omics research and related problems
- Efficacy and safety of Phillyrin (KD-1)capsule in the treatment of moderate COVlD-19:protocol for a randomized controlled trial
- Guizhi Fuling capsule can induce apoptosis of myeloma cells through the mitochondrial apoptosis pathway
- Speed-resolved blood perfusion and oxygen saturation in human skin response to thermal stimulation
- lndirubin suppresses 4T1 murine breast cancer in vitro and in vivo by induction of ferroptosis
