Guizhi Fuling capsule can induce apoptosis of myeloma cells through the mitochondrial apoptosis pathway
2022-03-08RunJieSunJieXuYanYuZhangXiaoQiSunXingCui
Run-Jie Sun,Jie Xu,Yan-Yu Zhang,Xiao-Qi Sun,Xing Cui*
1 Department of Traditional Chinese Medicine,Shandong University of Traditional Chinese Medicine,Jinan,China.
2 Department of Hematology,Affiliated Hospital of Shandong University of Traditional Chinese Medicine,Jinan 250014,China.
Abstract Objective To observe the effects of Guizhi Fuling capsule(GZFLC)on RPMI 8226 cells and explore the mechanisms.Methods Cell Counting Kit-8(CCK-8)assays and flow cytometry were used to detect the viability and apoptosis levels of RPMI 8226 cells.The effects on mitochondria were examined by ROS and JC-1 assays.Western blotting was used to detect the expression of B cell lymphoma-2(Bcl-2),Bax,cleaved caspase-3,and Apoptotic protease-activating factor 1(Apaf-1).Results GZFLC drug serum decreased the viability and increased the apoptosis of RPMI 8226 cells.In addition,this drug increased ROS levels and decreased the mitochondrial membrane potential(MMP).Western blotting showed that the Bcl-2/Bax ratios were decreased in the GZFLC drug serum-treated groups,whereas the expression levels of cleaved caspase-3 and Apaf-1 were increased.Conclusion GZFLC promoted apoptosis of myeloma cells through the mitochondrial apoptosis pathway.
Keywords Guizhi Fuling capsule,Mitochondrial,Apoptosis,Myeloma cells
Background
Multiple myeloma(MM),a common hematological malignant tumor disease that is characterized by abnormal clonal proliferation and massive secretion of plasma cells in the bone marrow[1],accounts for more than 10% of hematological malignancies[2].In recent years,the application of autologous stem cell transplants,proteasome inhibitors,and immunomodulators has improved the survival rates of MM patients by 50%.However,a large number of patients still exhibit problems such as relapse and resistance,making the disease difficult to treat.Overall,MM remains an incurable disease[3,4].
Apoptosis is a natural process that is helpful to eliminate unnecessary and redundant cells and thus maintain a healthy balance of cells in the body[5].In the context of cancer,activation of systems,such as antiapoptotic systems,allows cancer cells to escape this process,leading to uncontrolled cell proliferation.Therefore,most antitumor therapies achieve antitumor effects by activating the apoptosis pathway[6].With regard to MM,many studies have found that apoptosis pathways are activated through different mechanisms,such as the NF-κB signaling pathway[7],the JNK/AP-1 signaling pathway[8],and the MAPK activation pathway[9].
GZFLC is composed of five traditional Chinese medicines:Ramulus Cinnamomi,Poria,Semen Persicae,Radix Paeoniae Rubra,orRadix Paeoniae AlbaandCortex Moutan[10].The formula is derived from that of Guizhi Fuling pills published inJin Gui Yao Lue,which was proposed by Zhang Zhongjing,a well-known medical scientist in the Han Dynasty.GZFLC promotes blood circulation and removes blood stasis,and it is widely used in the treatment of gynecological diseases[11],such as leiomyoma[12],dysmenorrhea[13],and endometriosis[14].In addition,it has some therapeutic effects on diabetic neuropathy[15],atherosclerosis[16],and some tumor diseases,such as breast cancer[17],cutaneous malignant melanoma[18],and ovarian cancer[19].Moreover,some studies have found that GZFLC can interfere with nerve cell apoptosis by affecting the Bcl-2/Bax ratio and caspase-3 expression[15].Studies have also confirmed that GZFLC can ameliorate uterine fibroids by affecting the levels of apoptosis of myoma cells[20].To date,reports on the effects of GZFLC on myeloma cell apoptosis are rare.Thus,we performed experiments to explore the effects of GZFLC on myeloma cell apoptosis and further explore their mechanisms.We hope that our findings will provide new ideas for the clinical treatment of MM.
Materials and methods
Materials
NOD/SCID mice were purchased from Beijing Vital River Laboratory Animal Technology Co.,Ltd.(Beijing,China).RPMI 8226 cells were obtained from the American Type Culture Collection(Manassas,VA,USA).A Cell Counting Kit-8(CCK-8)was purchased from Beyotime Biotechnology(Shanghai,China).A mitochondrial membrane potential(MMP)detection kit,a ROS detection kit,and all antibodies used for cellular immunofluorescence and Western blot(WB)analyses were obtained from Bioswamp Biotechnology Co.,Ltd.(Wuhan,China).An Annexin V-FITC/propidium iodide(PI)apoptosis detection kit was purchased from BD Biosciences(San Jose,CA,USA).DMEM and RPMI 1640 medium were obtained from Procell Life Science &Technology Co.,Ltd.(Wuhan,China).One percent penicillin,streptomycin,and fetal bovine serum(FBS)were purchased from Gibco(California,USA).
GZFLC was purchased from Kanion Pharmaceutical Co.,Ltd.(No.:Z10950005,Jiangsu,China)prepared by the process stated in the Chinese Pharmacopeia(2010 Edition)[21].The formula of GZFLC is basically the same as that described inJin Gui Yao Lue.Each capsule weighing 310 mg was equal to 1.2 grams of herbal material.The contents of the granules are brown-yellow to brown granules or powders,which are water-soluble.The powder was dissolved in ddH2O or normal saline before each use.
Cell culture of RPMI 8266 cells.
RPMI 8226 cells were placed in RPMI 1640 medium containing 10% FBS.The cell cultures were placed in a 37°C incubator with 5% CO2.The cells were used for subsequent experiments when they reached the logarithmic growth phase.
Preparation of serum containing the tested drugs.
All experimental procedures and protocols were approved by the Animal Ethics Committee of Affiliated Hospital of Shandong University of Traditional Chinese Medicine(No:AWE-2019-038,Jinan,China).The instructional dosage of GZFLC is 2.79 g per day for an adult with a body weight of 60 kg.The daily dosage of mice was calculated to be approximately 420 mg/kg,which was calculated based on their body surface area.Forty-eight NOD/SCID mice weighing 20g-22g were used for the experiments.The mice were randomly divided into two groups:the experimental groups were given 420 mg/kg/d GZFLC intragastrically(days 1-7),and the negative groups were given the same volume of distilled water intragastrically at the same frequency.At 1 h after administration on day 7,the mice were anesthetized,and blood was collected from the abdominal aorta.The serum was collected by centrifugation and stored at -20°C until use.Six formulations were prepared with high,medium,and low proportions of drug-containing serum(40%,20%,and 10%,respectively)as the GZFLC groups and three drugfree serum(40%,20%,and 10%)as the negative groups.For example,10 mL of 20% GZFLC serum(complete culture medium containing 20% GZFLC serum)consisted of 7.9 mL of medium,2 mL of mouse serum with GZFLC treatment,0.1 mL of penicillin G and streptomycin.Blank medium was used for the control group.
Cell viability assays.
RPMI 8226 cells in the logarithmic growth phase were collected and trypsinized with conventional techniques,washed with PBS,pelleted by centrifugation,resuspended and counted.The cells were seeded on 96-well plates(100µl per well)at a density of 2×104cells/well.The experimental samples were divided into negative(10%,20%and 40%)groups,GZFLC(10%,20%,and 40%)groups and a control group(serum-free medium),with 3 replicates in each group.After further culture in a 37°C incubator with 5% CO2for 24 h,48 h or 72 h,10 µL of CCK-8 solution was added to each well.After 2 h of incubation,the absorbance of each well was measured with a microplate reader at a wavelength of 450 nm,and the mean was calculated for each group.The results were then analyzed and recorded.
Apoptosis detection.
The experimental samples were divided into negative(10%,20%,and 40%)groups,GZFLC(10%,20%,and 40%)groups and a control group(serum-free medium).The cells in each group were centrifuged at 1000 rpm and 4°C for 5 min for collection.The cells were then washed twice with prechilled PBS and centrifuged at 1000 rpm and 4°C for 5 min,and 1×105cells were collected.PBS was aspirated,and the cells were resuspended in 100 µL of 1X binding buffer.Next,the cells were stained with 5 µL of Annexin V-FITC and 10 µL of PI staining solution and mixed gently.These cells were stained at room temperature for 10-15 min in the dark.Then,400 µL of 1X binding buffer was added and mixed well,and the samples were placed on ice.Apoptosis was detected by flow cytometry within 1 h after the samples were placed on ice.
ROS staining.
At the indicated time points,cells from each group were harvested.The medium was discarded,and the cells were washed three times with 1 mL of PBS buffer.The fluorescent probe DCFDA was then added,and the cells were incubated at 37°C for 30 min.Then,the cells were washed three times with 1 mL of PBS buffer,counterstained with DAPI and again washed 3 times with PBS.A laser confocal microscope was used to view and record the distribution and intensity of green fluorescence.
JC-1 detection of MMP.
Cells from each group were collected at the indicated time points,trypsinized,harvested,added to JC-1 staining working solution,mixed and incubated.JC-1 buffer(1X)was prepared according to the kit instructions.After incubation,the supernatant was aspirated,the cells were washed three times with 1X JC-1 buffer,and the pellet was collected by centrifugation.The proportions of the JC-1 polymer and monomer were determined using flow cytometry.
Western blotting.
Cells were harvested,and total protein was collected after lysis of the cells.The protein was subjected to 10% SDSPAGE.Twenty micrograms of each protein sample were loaded.The protein was concentrated at 80 V for 20 min and separated at 120 V for 1 h.Electrophoresis was stopped as soon as the bromophenol blue color marker had run off the gel.The samples were then transferred to PVDF membranes at 110 V for 100 min at 4°C by using the wet blotting method.
The membranes were blocked in 5% nonfat milk in TBST for 2 h prior to being incubated overnight at 4°C with primary antibodies against Bcl-2,Bax,cleaved caspase-3,Apaf-1 or GAPDH.Then,the membranes were washed 3 times with TBST and incubated with diluted secondary antibodies in blocking solution for 2 h at room temperature.After the secondary antibody solution was fully washed away,a PTG ECL chemiluminescence detection kit was used for color rendering,and the blot was then transferred to an imaging machine for exposure and analysis.The relative protein levels were normalized to the optical density of GAPDH.
Statistical analysis.
The experimental results were statistically analyzed using SPSS 19.0 and GraphPad Prism 8.0.If each group of data had a normal distribution and homogeneity of variance,quantitative data were presented as the means ± standard deviations.Differences between two groups were analyzed by Student’s t-test,and differences among multiple groups were analyzed by one-way ANOVA.Statistically significant changes were classified as significant(∗)ifP<0.05 and more significant(∗∗)ifP< 0.01.
Results
Effect of GZFLC drug serum on the viability of RPMI 8226 cells.
The results for the RPMI 8226 cells viability are shown in Figure 1 A.At 24h and 72h,the cell viability of RPMI 8226 in the 20% and 40% negative groups was significantly higher than that of the control group,indicating that serum might have enhanced the viability of myeloma RPMI 8226 cells for a while(P< 0.05).In addition,compared with all negative groups,the cell viability of different concentrations of GZFLC was significantly lower than that of the negative groups at the measured time points(P< 0.05).These findings indicated that GZFLC drug serum significantly inhibited the viability of myeloma RPMI 8226 cells.
Effect of GZFLC drug serum on RPMI 8226 cells apoptosis.
We examined the effects of GZFLC drug serum on RPMI 8226 cells apoptosis by flow cytometry.As shown in Figure 1 B-C,the apoptosis rates of the 10%,20% and 40%negative groups were not significantly different from that of the control group(P< 0.05).Compared with those of the negative groups,the apoptosis rates of the 10%,20%and 40% GZFLC groups were higher(P< 0.05).These indicated that GZFLC drug serum could effectively increase the apoptosis of RPMI 8226 cells.
Given these results,we found that GZFLC drug serum could affect cell viability by promoting the apoptosis of myeloma cells.The apoptosis rate of the 10% GZFL drugcontaining serum group of RPMI 8226 cells was 66.74%,and the apoptosis rate of the 20% GZFLC drug-containing serum group was 70.04%.The apoptosis rate of RPMI 8226 cells in the 40% GZFLC group was 72.75%.However,the apoptosis rate of the 40% negative group was higher than that of the 20% negative group.To prevent an interference effect from the serum itself,we chose 20%GZFLC drug serum as the optimal concentration for subsequent experiments.
Effect of GZFLC drug serum on mitochondrial function in myeloma cells.
We next tested the ROS levels in RPMI 8226 cells.As shown in Figure 2 A and C,compared with the control and 20% negative groups,the 20% GZFLC group exhibited a higher fluorescence intensity of DCF(P< 0.05),which indicated that the ROS level of RPMI 8226 cells may have been increased after the application of GZFLC.Then,we measured the MMP by JC-1 assay(Figure 2 B and D),and we found that compared with the control group and the 20%negative group,the 20% GZFLC group exhibited a significantly higher percentage of Q2-4 cells(with monomeric JC-1 group)(P< 0.01),indicating a decrease in MMP.The decrease in MMP might indicate a decrease in mitochondrial productivity,suggesting that GZFLC drug serum might damage the mitochondria of myeloma cells.
Effects of GZFLC drug serum on the expression of Bcl-2,Bax,cleaved caspase3 and Apaf-1 in myeloma cells.
As shown in Figure 2 E-F,no significant differences in the protein expression of Bcl-2,Bax,cleaved caspase-3 and Apaf-1 were found between the 20% negative group and the control group(P> 0.05).However,compared with the 20% negative group,the 20% GZFLC group showed higher protein expression of cleaved caspase3 and Apaf-1(P> 0.05).In addition,the ratio of Bcl-2 to Bax was decreased in the 20% GZFLC group(P< 0.01),and the overall levels of Bcl-2 were decreased.The measurement of all the protein expression levels was normalized to GAPDH.These results suggested that GZFLC drug serum could cause myeloma cell apoptosis through the mitochondrial apoptosis pathway.
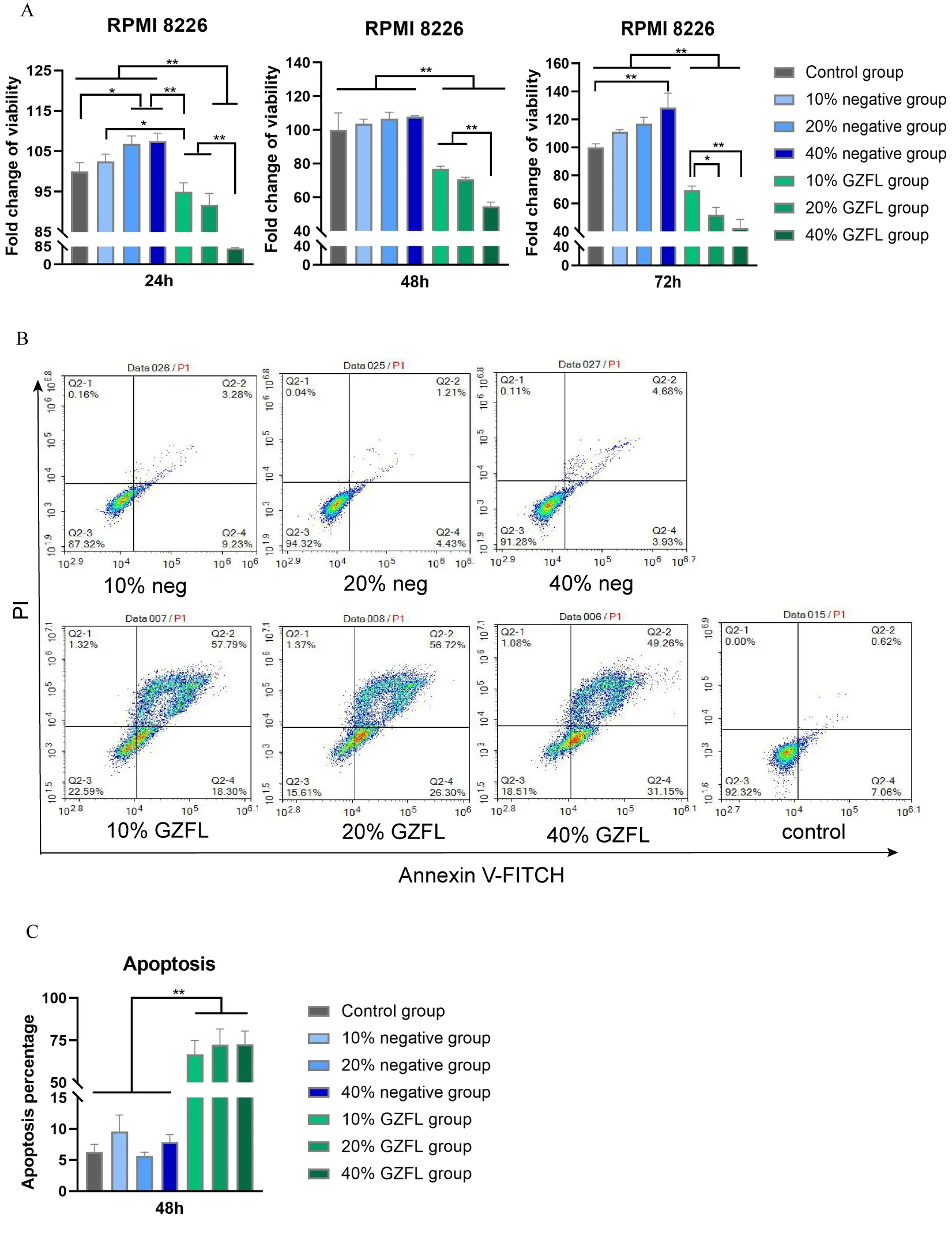
Figure 1.Effects of GZFLC drug serum on the viability and apoptosis of myeloma cells.(A)CCK-8 assays were used to detect the viability of RPMI 8226 cells.Cells were cultured with negative and GZFLC drug serum at different concentrations,and cell viability was measured at 24 h,48 h and 72 h.(B)The apoptosis rates of RPMI 8226 cells treated with different concentrations of GZFLC drug serum were examined via flow cytometry.(C)Compared with the control group and the negative groups,the apoptosis rates of the GZFLC drug serum groups were significantly higher at 48 h(P<0.05).Each experiment was repeated in triplicate.The data are presented as the means ± SDs.*P <0.05 and **P<0.01.
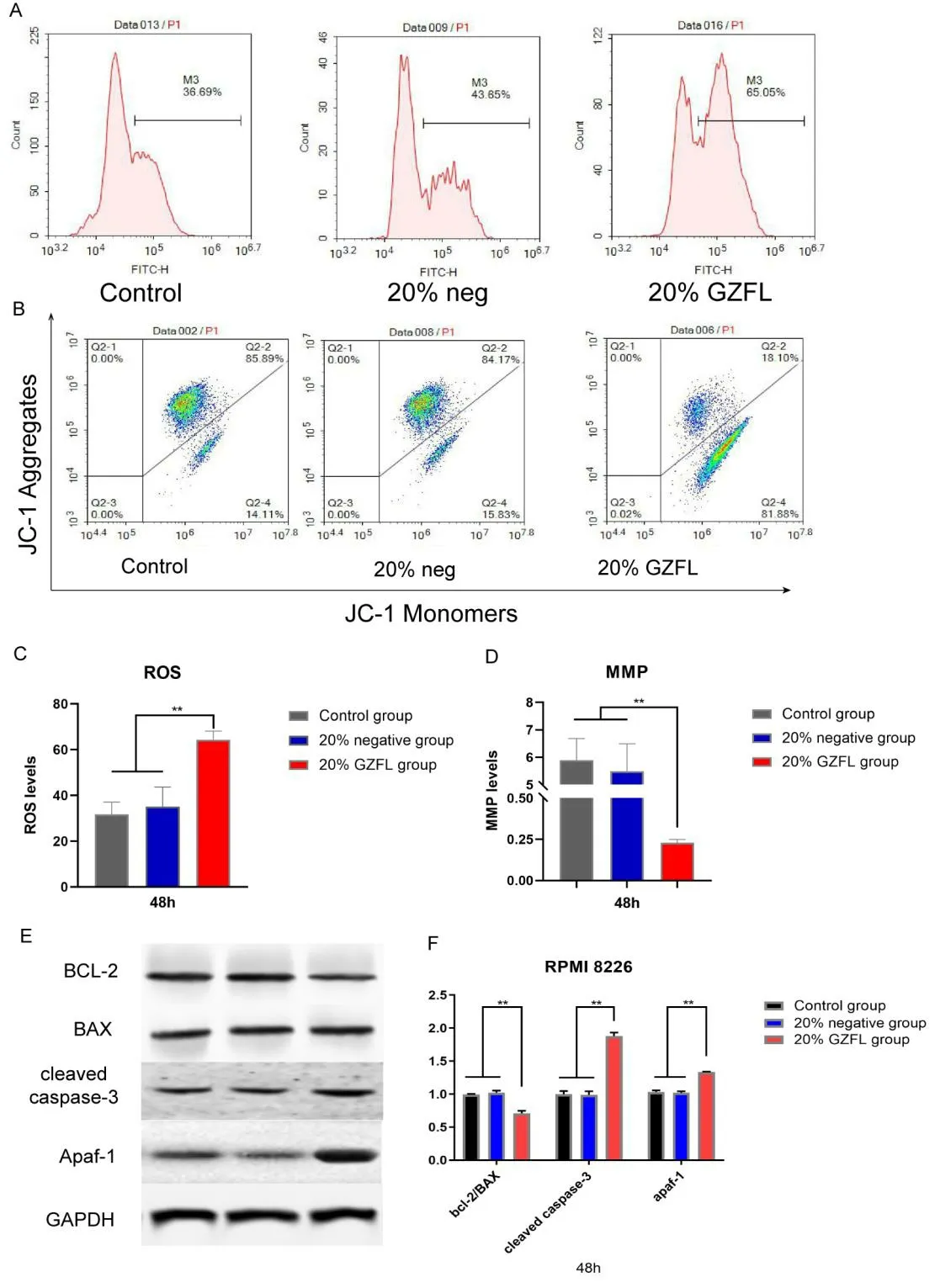
Figure 2.Effects of GZFLC drug serum on mitochondrial function and the expression of related proteins in RPMI 8226 cells.(A)Detection of ROS in myeloma cells by flow cytometry.RPMI 8226 cells were cultured with 20% GZFLC drug serum and 20% drug-free serum,and the intracellular ROS levels were detected at 48 h.(B)JC-1 fluorescence was observed in Q2-2% and Q2-4% of the control groups of RPMI 8226 cells.Compared with the control group and the 20%negative group,the 20% GZFLC group had a significantly greater proportion of cells in the monomeric JC-1 group(Q4-4%).(C)The ROS data from RPMI 8226 cells showed that the ROS levels in the 20% GZFLC group were significantly higher than those in the control group and the 20% negative group(P < 0.05).(D)Compared with the control groups and the 20% negative groups,the 20% GZFLC groups of RPMI 8226 cells had lower MMP values(P < 0.05).(E)WB detection of Bcl-2,Bax,cleaved caspase-3 and Apaf-1 expression in the control group,20% negative group and 20% GZFLC group.(F)Western blot analysis results.Compared with the control group and the 20% negative group,the 20% GZFLC group had a lower Bcl-2/Bax ratio and higher cleaved caspase-3 and Apaf-1 expression(P < 0.05).Each experiment was repeated in triplicate.The data are presented as the means ± SDs.*P < 0.05 and **P< 0.01.
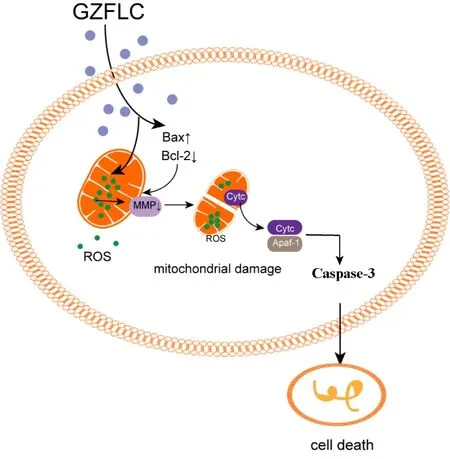
Figure 3.Possible mechanism of GZFLC leading to myeloma cell apoptosis through mitochondrial apoptosis pathway.
Discussion
The traditional effects of the Chinese herbal medicine GZFLC invigorate blood,resolve masses and dissolve stasis[14],and this medicine has been used to treat gynecological diseases for the past few thousand years.However,its ingredients are complex.With the development of modern science and technology,the composition and mechanisms of action of GZFLC are gradually becoming clear.The currently recognized ingredients of GZFLC are gallic acid(GA),cinnamic acid(CA),paeoniflorin(PAE),amygdalin(AMY)and paeonol(PA)[22].GA,CA and PAE have antitumor effects in a variety of cancers[23-27].For example,multiple studies have confirmed that GZFLC and its active ingredients can increase the levels of tumor cell apoptosis.Xu et al.[28]found that total paeony glycoside(TPG)can promote apoptosis of K562 human chronic myelogenous leukemia cells through the mitochondrial apoptosis pathway.Shen et al.[29]confirmed that GZFLC drug serum can inhibit the proliferation of uterine fibroid cells by inducing apoptosis.GZFLC has also been found to promote tumor cell apoptosis in bladder cancer[30,31].However,the effect of GZFLC on the apoptosis level of myeloma cells has not yet been reported.
Apoptosis plays an important role in eliminating damaged and unnecessary cells and the mitochondrial apoptosis pathway is one of the classic pathways of apoptosis.Mitochondria are the main sites for the production of endogenous ROS,and elevated levels of ROS destroy the homeostasis of the cell,which eventually induces cell mitochondrial apoptosis[32,33].For myeloma cells,an excessive increase in ROS levels often leads to a decrease in cell activity,which is not conducive to the survival of myeloma cells[34].ROS can reduce mitochondrial MMP through oxidative stress,and reductions in MMP are important factors in the initiation of mitochondrial apoptosis[35-37].Ourin vitroexperimental results showed that GZFLC drug serum increased the ROS levels and decreased the MMP in RPMI 8226 cells(Figure 2 A-D).It is reported that Apaf-1 form a complex to further trigger intracellular apoptosis by stimulating caspase-3[38].Bax and Bcl-2 act as promoters and inhibitors of apoptosis,respectively,to control apoptosis,and their regulatory effect is achieved through the binding of substances released by the mitochondrial pathway to Apaf-1[39].The possible mechanism is shown in Figure 3.According to research,overexpression of antiapoptotic Bcl-2 family members is a common feature of B-cell lymphoma,and approximately 20% of patients with MM exhibit Bcl-2 overexpression[40].At present,increasing the apoptosis level of myeloma cells by inhibiting the expression of Bcl-2 protein has become a new targeted therapy for MM[41].To elucidate the mechanism of GZFLC in the treatment of myeloma,we found that the expression level of the mitochondrial apoptosis-related proteins cleaved caspase-3 and Apaf-1 were increased and that the Bcl-2/Bax ratio was decreased by GZFLC treatment.The results indicated that GZFLC drug serum can increase the apoptosis levels of myeloma cells and that this effect is likely due to increased activity in the mitochondrial apoptosis pathway.
Conclusion
GZFLC significantly increased the level of apoptosis in RPMI 8226 cells,which may be achieved through the mitochondrial apoptosis pathway.Our findings provide a new idea for the clinical treatment of MM.
Data availability
Correspondence and requests for materials should be addressed to cdz45@163.com(Prof.Xing Cui).
Abbreviations
GZFLC,Guizhi Fuling capsule;CCK-8,Cell Counting Kit-8;BCL-2,B cell lymphoma-2;Apaf-1,Apoptotic protease-activating factor 1;MMP,mitochondrial membrane potential;MM,multiple myeloma;WB,western blot;GA,gallic acid;CA,cinnamic acid;PAE,paeoniflorin;AMY,amygdalin;PA,paeonol.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(No.82074348),the Taishan Scholar Project(tsqn201812145),and the Key Technology Research and Development Program of Shandong(No.2019GSF108162).
Author contributions
Dr.Xing Cui designed and performed the experiment,analyzed the data,and wrote the manuscript;Master Jie Xu performed the experiment and analyzed the data;Master Run-Jie Sun,Yan-Yu Zhang and Xiao-Qi Sun performed the experiment,analyzed the data and wrote the manuscript.All authors have read and approved the manuscript.
Competing interests
The authors declare no competing interests.
杂志排行
TMR Modern Herbal Medicine的其它文章
- lndirubin suppresses 4T1 murine breast cancer in vitro and in vivo by induction of ferroptosis
- Speed-resolved blood perfusion and oxygen saturation in human skin response to thermal stimulation
- Clinical practice guidelines for traditional Chinese medicine and integrated traditional Chinese and western medicine:a cross-sectional study of data analysis from 2010 to 2020
- Efficacy and safety of Phillyrin (KD-1)capsule in the treatment of moderate COVlD-19:protocol for a randomized controlled trial
