Liver involvement in the course of thymoma-associated multiorgan autoimmunity: The first histological description
2022-03-02FrizioCitrellUmertoVespsiniGentiluiAnnCresenziAntonellBinhiVldimirVirzGiuseppeTonini
Frizio Citrell ,, Umerto Vespsini-Gentilui , Ann Cresenzi , Antonell Binhi ,Vldimir Virzì , Giuseppe Tonini
a Department of Medical Oncology, Policlinico Universitario Campus Bio-Medico, via Alvaro del Portillo, Rome 00128, Italy
b Area of Internal Medicine, Unit of Hepatology, Policlinico Universitario Campus Bio-Medico, via Alvaro del Portillo, Rome 00128, Italy
c Unit of Pathology, Policlinico Universitario Campus Bio-Medico, via Alvaro del Portillo, Rome 00128, Italy
TotheEditor:
Thymoma-associated multiorgan autoimmunity (TAMA) is a rare paraneoplastic syndrome described in patients with thymoma and characterized by multiorgan failure and graft-versus-host disease (GVHD) like pathology affecting the skin, the gastrointestinal tract, and the liver. To date, only 21 cases are reported in literature [1] , with some patients presenting gastrointestinal and hepatic manifestations, mainly colitis, diarrhea, and abnormal liver enzymes, but the hepatic involvement has not been histologically characterized yet. In the present study, we describe for the first time that liver involvement in a patient affected by TAMA resembles GVHD.
Herein, we report the case of a 60-year-old Caucasian male patient with a 6-year history of thymoma metastatic to mediastinal lymph nodes, bone and pleura. He underwent thymectomy on July 7, 2011, and due to disease recurrence 3 months after the surgery, he received 3 chemotherapy cycles according to CDDPTXL-EPI scheme (cisplatin 35 mg/mq + epirubicin 45 mg/mq + paclitaxel 100 mg/m2on day 1 and day 8 every 21 days). He received left pleural metastasectomy on March 21, 2012, started second-line therapy with octreotide since January 2016 (one administration every 28 days) [ 2 , 3 ], and had received a total of 29 administrations at the time of admission to our hospital. The patient was receiving periodic intravenous supplementation with 30 g immunoglobulins, with the last administration dated on March 22, 2018, to treat progressive hypogammaglobulinemia caused by concomitant Good’s syndrome.
He was referred to our hospital due to a 4-week lasting refractory diarrhea, disabling asthenia and refractory cough. Arterial blood gas test displayed chronic hypoxemic normocapnic respiratory failure: pH 7.45, pCO 2 35.9 mmHg, pO 2 52.9 mmHg, SO287.5%, HCO3-24.6 mmol/L. Cutaneous manifestations were absent. Blood tests showed severely increased cholestatic enzymes[gamma-glutamyl transferase 15 × upper limit of normal (ULN), alkaline phosphatase 2.5 × ULN, total bilirubin 3.5 × ULN with direct bilirubin 0.7 mg/dL], low total serum proteins (49 g/L) and serum albumin (28.1 g/L), low lymphocytic count [0.83 × lower limit of normal (LLN)], low hemoglobin (115 g/L), but normal aminotransferase levels and renal function.
The CT scan did not show progressive oncological disease and ruled out hepatic metastatic involvement. Diffuse and significant bronchial, inter- and intra-lobular septa thickening were detected,mainly affecting inferior lung lobes; multiple nodular and micronodular densification areas, some with the ground glass radiologic aspect, were present. Sputum culture was positive forE.coli, suggesting an infectious involvement likely favored by Good’s syndrome [4] .
C-reactive protein and procalcitonin were in the normal range and extensive laboratory screening for infectious diseases, including hepatitis A, B, C, E, Epstein-Barr virus, cytomegalovirus, herpes simplex virus 1/2, human immunodeficiency virus, parvovirus B19,rotavirus, intestinal bacteria,C.difficileand parasites,S.pneumonia,L.pneumophila,P.jiroveciiandM.tuberculosis, showed negative results.
Gastroscopy and colonoscopy did not detect macroscopic lesion,but the former revealed diffuse inflammation of the gastric body and of the fundus, and the latter revealed inflammation of the rectum. Histologic examination for cytomegalovirus andH.pyloriwas negative. Lymphoplasmacytic cells in proximity to lamina propria, with focal epithelial apoptosis, and diffuse intraglandular mild granulocytic aggregates infiltrated the gastric mucosa. Lymphoplasmacytic aggregates, intraglandular granulocytes, focal or diffuse epithelial apoptosis, villous atrophy, and depletion of muciparous and Paneth cells were the pathological findings in the small and large intestinal mucosa. Ultrasonography and computed tomography (CT) scan did not show any hepatic macroscopic lesion. Similarly, magnetic resonance cholangiopancreatography (MRCP) did not reveal any defect or stenosis of the biliary tree.
We therefore proceeded to liver biopsy, which showed scattered neutrophils infiltrating the bile ducts. The limiting plate was generally preserved with minimal lymphocytic spill-over. Hepatocytes were characterized by sporadic evidence of chronic cholestasis with hepatocellular swelling and light immunostaining for cytokeratin 7 (cholate-stasis) ( Figs. 1 and 2 ) [5] .
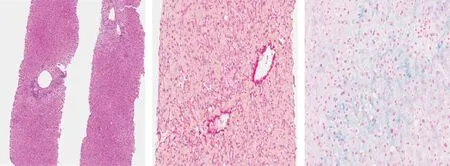
Fig 1. A: Two-thirds of the portal tracts presented a pathological process characterized by mild to moderate inflammatory infiltrate with lymphocytes and rare monocytes and plasma cells (Hematoxylin and Eosin staining, original magnification × 40). B: Central veins showed mild pericentral fibrosis (Syrius Red staining, original magnification ×200). C: Neither canalicular bile accumulation nor biliary plugs were observed (Perls staining, original magnification × 200). Steatosis was present in about 10% of hepatocytes.Perls staining for iron highlighted grade 1 hepatocellular siderosis without evidence of iron within the Kupffer cells.
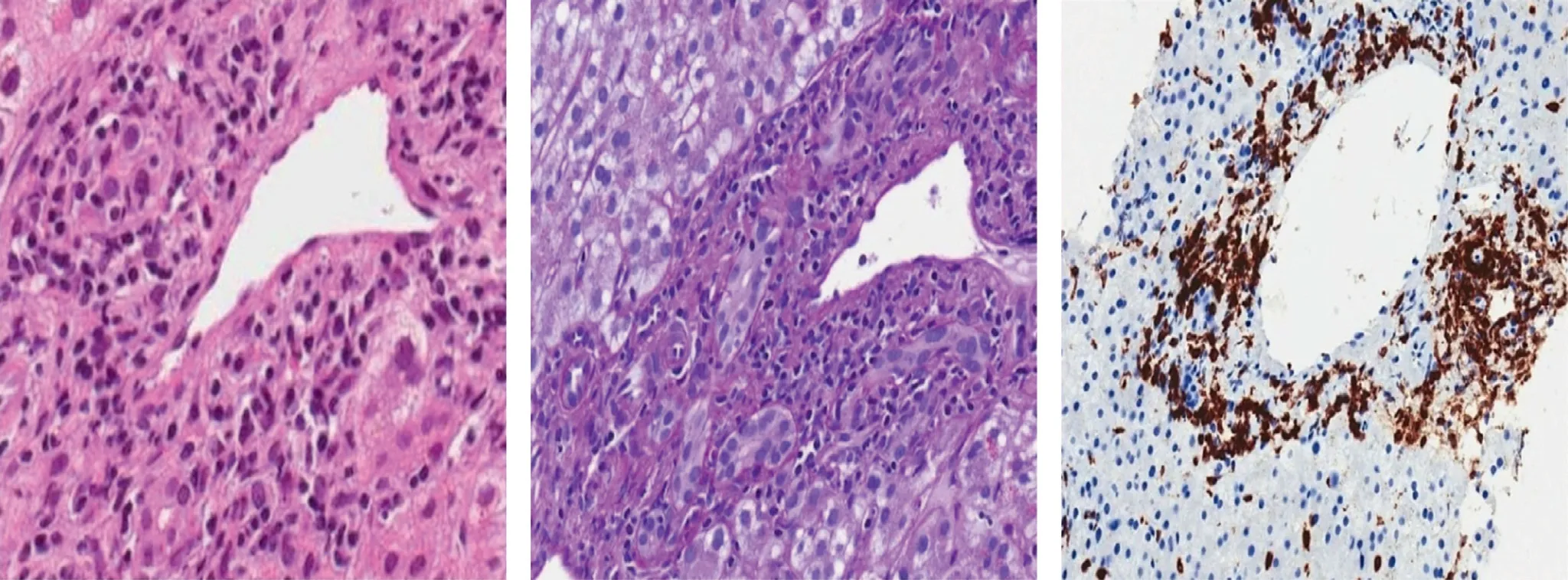
Fig 2. A: The main histological feature was the bile ducts damage: portal ducts were irregular in shape and surrounded and infiltrated by immune cells, mainly lymphocytes that resulted of T phenotype at immunohistochemistry (CD3 positive) (Hematoxylin and Eosin staining, original magnification × 200). B: Ductal epithelium showed degenerative vacuolization of the cytoplasm, variation in nuclear size with loss of polarity and sporadic presence of apoptotic nuclear debris (PAS-Diastase staining, original magnification × 200). C: Mild lobular inflammation predominantly composed of T lymphocytes was present (immunohistochemical staining for CD3, original magnification ×200). T lymphocytes were also involved in portal endothelitis and central perivenulitis.
Hepatic histology resembled GVHD-like patterns and the most likely diagnosis was hepatic involvement due to a systemic immune-mediated syndrome. Simultaneous pulmonary, hematological, gastrointestinal, and immunological damage were consistent with TAMA.
No definite therapeutic algorithm was clearly found in literature, thus the patient received symptomatic treatment:piperacillin-tazobactam was administered to treat pulmonary infection, oral budesonide and mesalazine and intrarectal mesalazine to partially relieve recurring diarrhea.
The patient was discharged after twelve days from hospitalization and prescribed 25 mg/d prednisone in association with oral budesonide to control systemic inflammation and to attenuate local gastrointestinal inflammation. Daily oxygen-therapy was also indicated, and diarrhea recurrence required oral rifaximin. Liver enzymes did not decrease during the follow-up.
The patient was reassessed twenty days after discharge: body weight decreased from 60 kg to 53 kg, the habitus was cachectic,diarrhea had almost disappeared, but cough was rising again. Because of low levels of lymphocytes, hypogammaglobulinemia and the need of a rapid and effective therapy, we preferred to increase the daily steroid dosage to almost 1.5-fold oral prednisone per day,corresponding to 75 mg/d, despite that GVHD treatment usually relies on immunosuppressive agents [6] .
The positron emission tomography (PET)/CT scan repeated 33 days after discharge showed a reduction in dimensional and glucose uptake in pleural and nodal metastatic sites, and evidenced ground glass areas affecting upper right and middle lung lobes. The patient died 44 days after discharge due to respiratory failure.
TAMA usually displays gastrointestinal, hepatic, and skin involvement, and diarrhea and rash are common at clinical onset. Pleiotropic manifestations may occur due to systemic inflammatory damage, such as myasthenia gravis and upper gastrointestinal erosions causing dysphagia; morbilliform eruption is the most common dermatological finding, while fissuring of palms and soles is also described. Paraneoplastic hematological disorders,such as hypogammaglobulinemia or pure red cell aplasia, are frequent. Anamnesis of thymoma and concomitant multiorgan damage, mainly gastrointestinal tract, liver and skin, with a GVHD-like disease histology, in absence of hematopoietic stem cell transplantation are consistent with the diagnosis of TAMA [7] .
To date, no histological hepatic description in the setting of TAMA is provided in literature and liver is not a frequent target of metastasization [8] .
Due to frequent skin involvement, cutaneous histology is the most commonly described symptom in the setting of TAMA and enlightens at least in part the pathogenesis. It mimics an acute GVHD with low concentration of regulatory T cells, substantial increase of CD8 + T lymphocytes infiltrating the epidermidis and high T helper 17 to CD4 + ratio. Regulatory T cell deficiency and selfdamaging T cell activity are the main actors of autoimmunity and inflammation [9] .
Thymoma is believed to be an “immunological source” of neoantigens potentially responsible for paraneoplastic disorders [10] .No etiological link is reported between thymoma and paraneoplastic hepatic injury with the exception of an autoimmune hepatitis report in which thymic neoplastic cells were identified as a likely antigenic reservoir [11] .
The most recent and complete review describing TAMA includes liver immune-mediated involvement in the clinical course,but liver biopsy has never been fully described in literature [7] .
Since liver biopsy of our patient showed GVHD-like patterns that were similar to other gastrointestinal examined tissues, we hypothesize that the underlying pathogenesis is different from auto-immune hepatitis and auto-immune cholangitis, and more frequently associated with thymoma. Steroids, milestones for autoimmune hepatitis and auto-immune cholangitis treatment, were in fact only partially effective.
Hepatic GVHD is not a frequent and easy diagnosis; liver biopsy is sometimes not specific and clinical manifestations require proper evaluation. Main confounder factors are previous administration of pre-transplant chemotherapy or immunotherapy, GVHD prophylaxis and infections. Hepatic GVHD is characterized by biliary epithelium damage: nuclear pleomorphism, loss of nuclear polarity, nuclear overlap, cytoplasmic vacuolization, eosinophilic change of the cytoplasm, and rarely apoptosis. Interlobular bile ducts are more often involved but ductular proliferation is generally poor. Lymphocytes are the most frequent white cells and endothelitis is sometimes evident. No specific histology currently defines chronic hepatic GVHD [12] .
Liver biopsy should be considered in presence of plausible clinical diagnosis of GVHD when gastrointestinal biopsy results are negative. Biopsy is not “performed” at the clinical onset of GVHD when inflammatory damage is not definitely arisen. There is no direct correlation between entity of biliary damage and response to therapy, thus grading of hepatic GVHD is not recommended [12] .
A histologic diagnostic algorithm and scoring system for hepatic GVHD have been proposed by Stueck et al. [13] . The authors demonstrated that GVHD, compared to the drug-induced control group, presents more frequently bile duct loss or damage, cholestasis, acidophilic bodies and lower ductular reaction.
A complete clinical assessment led to the diagnosis of TAMA in our case. We describe for the first time that not only intestinal and cutaneous lesions but also hepatic involvement during TAMA resembles GVHD.
Our daily experience suggests to first rule out hepatic metastasis if progressive increase in cholestatic enzymes occurs in a patient diagnosed with thymoma. Once malignancy and potential hepatotoxic agents are excluded, physicians should consider thymoma-associated hepatic autoimmune involvement if the biopsy mirrors a GVHD histology in the absence of malignant cells and the prevalent symptoms are gastrointestinal and cutaneous.
To our knowledge, this is the first description of immunemediated hepatic damage during TAMA, and a GVHD-like pattern was found.
TAMA requires a multidisciplinary management since it carries poor prognosis and needs tailored therapy due to the frequent multiorgan damage and co-morbidities; further studies are necessary to clarify mechanisms of underlying immune deregulation.
Acknowledgments
None.
CRediT authorship contribution statement
Fabrizio Citarella: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing -original draft,Writing - review & editing. Umberto Vespasiani-Gentilucci : Data curation, Methodology, Supervision, Writing - review & editing.Anna Crescenzi: Data curation, Methodology. Antonella Bianchi:Data curation, Methodology. Vladimir Virzì: Conceptualization,Data curation. Giuseppe Tonini: Data curation, Methodology,Project administration, Validation, Writing - original draft, Writing - review & editing.
Funding
None.
Ethical approval
Not needed.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Targeting pancreatic ductal adenocarcinoma: New therapeutic options for the ongoing battle
- How open is the therapeutic horizon for pancreatic cancer patients?
- Terlipressin versus placebo in living donor liver transplantation
- Fas -670 A/G polymorphism predicts prognosis of hepatocellular carcinoma after curative resection in Chinese Han population
- Meso-Rex bypass for the management of extrahepatic portal vein obstruction in adults (with video)
- The effect of SphK1/S1P signaling pathway on hepatic sinus microcirculation in rats with hepatic ischemia-reperfusion injury
