Terlipressin versus placebo in living donor liver transplantation
2022-03-02PschlisGvriilidisErnestHidlgoRoertSutcliffeKeithRoerts
Pschlis Gvriilidis , , Ernest Hidlgo , Roert P Sutcliffe , Keith J Roerts
a Department of Hepato-Pancreato-Biliary and Liver Transplant surgery, Queen Elizabeth University Hospitals Birmingham NHS Foundation Trust,Birmingham B15 2TH, UK
b Department of Hepato-Pancreatico-Biliary Surgery and Transplantation, Hospital Universitari Vall d’Hebron, Barcelona 08035, Spain
TotheEditor:
Terlipressin is a long-acting synthetic analogue of vasopressin,demonstrating several potential benefits in the context of living donor liver transplantation (LDLT). During the recipient hepatectomy, terlipressin reduces the portal flow. Consequently, it may mitigate the extent of bowel congestion following portal vein clamping. By decreasing portal hyperperfusion and hypertension,it protects the graft from further injury and improves renal blood flow. All the above described benefits result in decreased morbidity and improved surgical outcomes [1] .
A meta-analysis of the first three randomized controlled trials(RCTs) on the perioperative use of terlipressin demonstrated that infusion of terlipressin during LDLT significantly decreased heart rate during the anhepatic and neohepatic phases but had no effect on the levels of creatinine [1-4] . Since then, three more RCTs have been published [5-7] .
We aimed to track the accumulated evidence of terlipressin therapy over time. The primary outcome measure was the management of the portal vein pressure. Only RCTs that compared terlipressin to a placebo in LDLT were selected. Statistical analysis was conducted using Review Manager 5.3 software (Cochrane Collaboration, Oxford, UK).
Six studies [2-7] including 292 patients were selected from a pool of 110 studies. In these studies, 147 and 145 patients were included in the terlipressin and placebo treatment groups, respectively ( Table 1 ). The results were as follows:
Age, sex, body mass index (BMI), model for end-stage liver disease (MELD) score, and graft-to-recipient weight ratio (GRWR)were comparable between the two groups. Moreover, there were no significant differences in operative time, estimated blood loss,peak intraoperative lactate level, end of surgery lactate level, fresh frozen plasma, and colloids infusion between the two groups( Table 2 ).
Peak serum creatinine was significantly lower in the terlipressin group than that in the placebo cohort [mean difference (MD) = -0.27, 95% confidence interval: -0.52 to -0.02,P= 0.03,I2= 75%]( Fig. 1 ), while mean arterial pressures (MAP) during the anhepatic and neohepatic phases were significantly lower in the placebo group than those in the terlipressin cohort (MD = 4.78, 95% CI:1.64 to 7.92,P<0.01,I2= 47%; MD = 7.01, 95% CI: 3.47 to 10.55,P<0.01,I2= 69%, respectively).
There were significant differences in cardiac output in the anhepatic phase and heart rates in the anhepatic and neohepatic phases (MD = -0.56, 95% CI: -0.79 to -0.33,P<0.01,I2= 6%; MD = -6.48, 95% CI: -8.69 to -4.27,P<0.01,I2= 10%;MD = -6.82, 95% CI: -11.74 to -1.89,P<0.01,I2= 74%, respectively). Moreover, portal vein velocity was significantly slower in the terlipressin group than that in the placebo treatment group and the terlipressin group required less doses of inotropes compared to the placebo treatment group ( Table 2 ).
There were no significant differences in the baseline systemic vascular resistance (SVR), cardiac output in the neohepatic phase,hepatic artery resistive index (HARI) in the first postoperative day,urine output, intensive care unit stay, and length of hospital stay( Table 2 ).
No major discrepancies were detected between the results of the fixed- and random-effects models.
The overall quality of the included RCTs ranged from low to moderate. Achilles’ heel of the RCTs was performance and detection bias calculated. Only the study of Reddy et al. [6] was doubleblinded, while the others were not blinded to the outcome assessors.
The present study demonstrated that terlipressin significantly decreased peak serum creatinine, cardiac output in the anhepatic phase, portal venous velocity, and heart rate during the anhepatic and neohepatic phases, respectively. On the other hand, MAP during the anhepatic and neohepatic phases was significantly lower in the placebo treatment group, and consequently, the need for inotropes was significantly higher in the placebo treatment group than that in the terlipressin group. All the above findings support the evidence that the principal hemodynamic effects of terlipressin are increased peripheral vascular resistance, decreased cardiac output, improved renal blood flow, reduced portal hypertension, and selective splanchnic vasoconstriction [8] .
It is reported that acute kidney injury (AKI) after liver transplantation as defined by RIFLE (risk, injury, failure, loss of kidney function, and end-stage kidney disease) criteria is a risk factor associated with increased morbidity and mortality in the immediate postoperative period [9] . The reported incidence of AKI after deceased liver transplantation ranges widely from 11% to 94% [9] . Arecent study still reported an incidence as high as 63% [10] . The reported incidence of AKI after LDLT ranged from 21% to 68% [11] . A previous meta-analysis reported no significant differences in peak serum creatinine [1] . In contrast, the present study demonstrated that the difference was statistically significant. However, it should be interpreted with caution, because the definition of AKI varied widely among studies and none of the outcome assessors were blinded. Therefore, increased heterogeneity and detection bias may have influenced the results.

Table 1 Study characteristics.
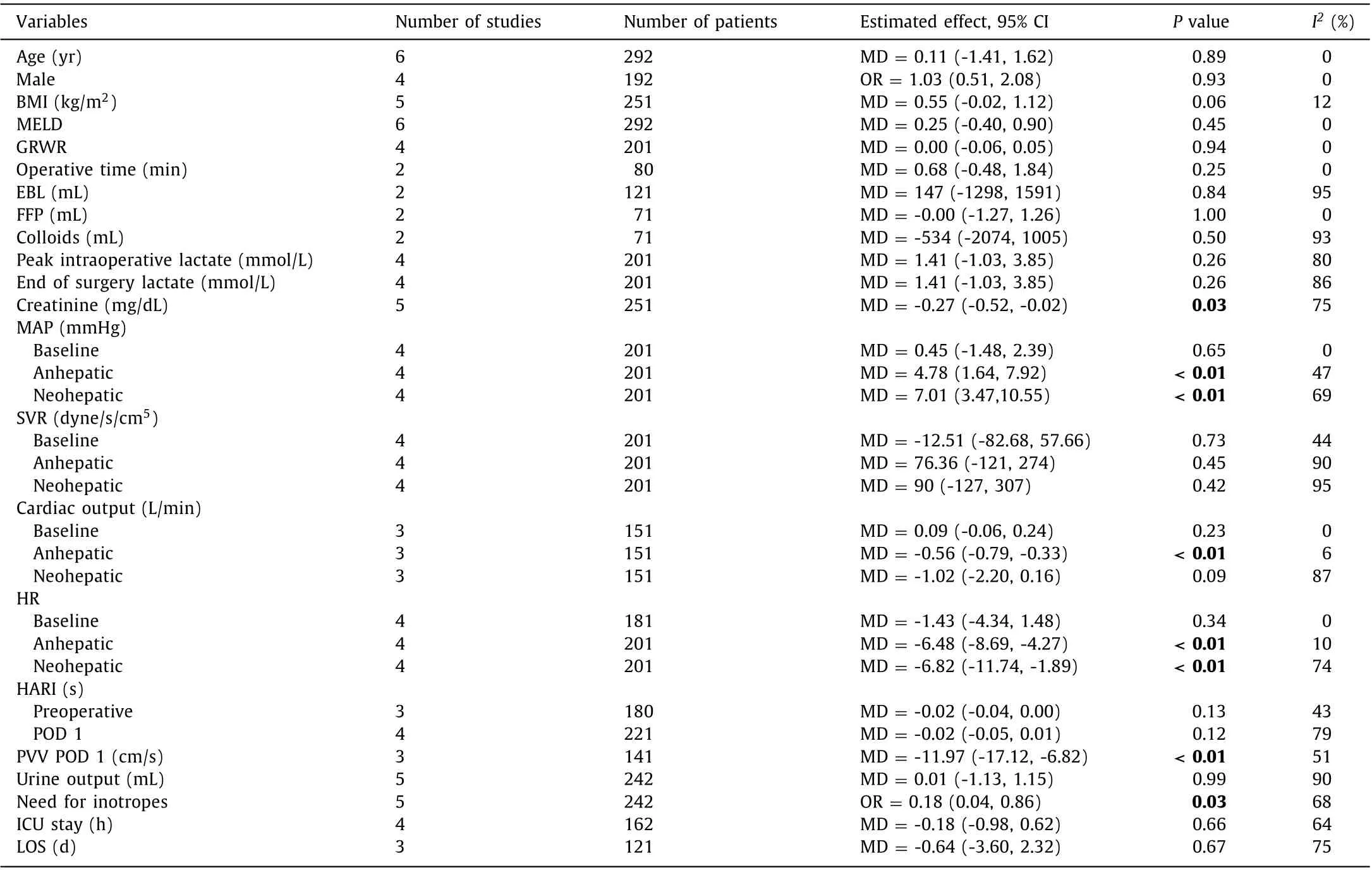
Table 2 Outcome of interests.

Fig. 1. Forest plot depicting serum creatinine and heart rate in anhepatic and neohepatic phases. A: Serum creatinine; B: heart rate in anhepatic phase; C: heart rate in neohepatic phase. CI: confidence interval; SD: standard deviation.
The included studies targeted MAP values between 65 and 70 mmHg and SVR values between 600 and 1300 dyne/s/cm5.It has been reported that maintaining MAP between 65 and 90 mmHg during liver transplantation reduces the risk of acute and chronic kidney injury [12] . Our study demonstrated no significant differences in SVR values during the anhepatic and neohepatic phases between the two groups. However, the placebo treatment group had significantly lower MAP values during the anhepatic and neohepatic phases and had a significantly higher need for inotropes compared to those of the terlipressin group.
It has been reported that the hallmarks of hyperdynamic circulatory syndrome in cirrhosis are increased cardiac output and heart rate and decreased SVR [13] , and that management of type I hepatorenal syndrome with terlipressin and albumin may reduce shortterm mortality and can be a bridge over transplantation [14] . The present study demonstrated the positive impact of terlipressin on heart rate reduction during anhepatic and neohepatic phases and the velocity of portal venous flow on the first postoperative day.
Reported complications of terlipressin are bradycardia, abdominal cramps, diarrhea, digital gangrene, and bowel ischemia [15] .From the included studies, only Reddy et al. focussed on this topic.They reported a significantly higher incidence of bradycardia and hypertension in patients with terlipressin than that in the placebo treatment group. Furthermore, three patients with severe bradycardia required special treatment and were withdrawn from the trial. They recommended careful and restraint use of terlipressin and close electrocardiographic monitoring during its use [6] .
The results of the present study should be interpreted in the context of its limitations. All the studies included were conducted in single centers. The total sample size was small. The definitions of outcomes varied among studies, and only one study investigated the complications of the terlipressin. Analysis of the risk bias demonstrated that only the study by Reddy et al. [6] was double-blinded and none of the rest blinded the assessors of the outcomes. Therefore, institutional, underpowered sample, performance, and detection bias may have influenced the results.
Perioperative use of terlipressin during LDLT demonstrated a significant and positive effect on MAP and heart rate during the anhepatic and neohepatic phases, which translated to significantly lower creatinine and less need for inotropes in the early postoperative period. A multicenter RCT adequately powered with predefined outcomes may shed further light on the topic.
Acknowledgments
None.
CRediT authorship contribution statement
Paschalis Gavriilidis : Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing - original draft. Ernest Hidalgo : Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing - original draft. Robert P Sutcliffe : Data curation, Formal analysis, Investigation, Validation, Visualization. Keith J Roberts : Data curation,Formal analysis, Investigation, Methodology, Project administration,Supervision, Validation, Writing - review & editing.
Funding
None.
Ethical approval
Not needed.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Targeting pancreatic ductal adenocarcinoma: New therapeutic options for the ongoing battle
- How open is the therapeutic horizon for pancreatic cancer patients?
- Fas -670 A/G polymorphism predicts prognosis of hepatocellular carcinoma after curative resection in Chinese Han population
- Meso-Rex bypass for the management of extrahepatic portal vein obstruction in adults (with video)
- The effect of SphK1/S1P signaling pathway on hepatic sinus microcirculation in rats with hepatic ischemia-reperfusion injury
- Liver involvement in the course of thymoma-associated multiorgan autoimmunity: The first histological description
