反硝化型甲烷厌氧氧化(DAMO)系统pH值耦合模型研究
2022-02-25楼菊青
吕 娇,楼菊青,徐 帆
反硝化型甲烷厌氧氧化(DAMO)系统pH值耦合模型研究
吕 娇,楼菊青*,徐 帆
(浙江工商大学环境科学与工程学院,浙江 杭州 310018)
对3个具有不同优势菌种反应器中厌氧氧化(DAMO)过程与pH值进行动力学耦合,结果表明,在25℃,Anammox-DAMO混培系统最大脱氮速率、硝酸盐初始抑制浓度和铵盐初始抑制浓度分别为3.95mg/(L·d),182.63,196.40mg/L;Nitrate-DAMO系统最大脱氮速率、硝酸盐初始抑制浓度分别为4.30mg/(L·d), 367.69mg/L;Nitrite-DAMO系统最大脱氮速率、亚硝酸盐初始抑制浓度分别为4.04mg/(L·d), 293.35mg/L.脱氮速率均随pH值增加先增大后减小,3个系统最佳脱氮pH值分别为7.5±0.2,7.2±0.2,7.8±0.2.脱氮动力学表明,3个不同系统DAMO反应过程均可用Haldane-pH值耦合模型描述. Nitrate-DAMO系统、Nitrite-DAMO系统脱氮过程还可用Monod-pH值耦合方程描述,Nitrate-DAMO系统细菌生长速率、硝酸盐亲和常数和抑制常数分别为1291.21cfu/(L·d), 295.23, 72.63mg/L;Nitrite-DAMO系统细菌生长速率、亚硝酸盐亲和常数和抑制常数分别为4040.42cfu/(L·d), 264.51, 5.02mg/L.
反硝化型甲烷厌氧氧化;脱氮性能;Hanldane方程;Monod方程;pH值;耦合模型
反硝化厌氧甲烷氧化(DAMO)过程,是以硝酸盐或亚硝酸盐为电子受体,以温室气体甲烷为唯一电子供体的甲烷厌氧氧化耦合反硝化过程[1-2].研究表明,该过程在湿地[3]、河流[4]、深水湖泊[5]等大多数淡水沉积物深层和水稻田中均存在,是自然界偶联碳氮循环的关键环节[6].主导该过程的功能微生物主要包括NC10门细菌的‘’()[1]和隶属于厌氧甲烷氧化古菌(ANME)中的一个簇ANME-2d的‘’()[2].DAMO过程在自然环境中也常见与厌氧氨氧化(Anammox)过程共存,两者可能会争夺NO2-,但也有可能会形成一种协同作用[7]: Anammox细菌消耗NO2-使其转化为NO3-,而后DAMO微生物还原NO3-[8].
数学模型是深入了解生物反应过程, 帮助和支持生物处理系统设计以及参数优化强有力的工具,现已被广泛应用于废水处理过程[9-10]. Ni等[11]对Anammox脱氮动力学模型的评价表明,格劳(Grau)二级基质去除模型和改进的斯托弗-金坎农(Stover-Kincannon)模型较一级基质去除模型、莫诺德(Monod)模型和康托斯(Contois)模型更适合描述Anammox脱氮过程;Rosenthal等[12]研究后发现Anammox细菌能够耐受亚硝酸盐浓度的升高,而亚硝酸盐敏感性系数并不适用于Anammox细菌的生长建模;Hu等[13]基于Monod模型和扩散反应模型开发了一个-DAMO模型用于探讨DAMO细菌的生长限制因素;Ni等[14]致力于模拟Anammox和DAMO微生物共培养系统模型,用以描述DAMO和Anammox微生物共培养系统生化过程.
目前未见有对硝酸盐型反硝化甲烷厌氧氧化(Nitrate-DAMO)过程的模型探讨.且已有的关于Nitrite-DAMO和Anammox的模型中也未考虑pH值这个对这些生化过程影响较大的因素.DAMO微生物与大多数传统异养反硝化类细菌类似,更偏好弱碱性环境[15].Zhu等[7]研究发现当pH值在5.9~7.4范围内,DAMO系统活性与pH值呈正相关.过往研究一般在pH值接近7.0的条件下培养DAMO微生物[2-3],过酸或过碱性环境可能会导致系统活性降低.然而目前未建立DAMO系统脱氮速率和系统内pH值的耦合模型,本文以稳定运行2000多天的3个具有不同优势菌种的DAMO反应器为研究对象,基于霍尔顿(Haldane)模型和Monod模型,建立pH值耦合模型,用以描述DAMO微生物和Anammox微生物混培系统、Nitrate-DAMO系统以及亚硝酸盐型反硝化甲烷厌氧氧化(Nitrite-DAMO)系统的反应动力学过程,探明电子受体与pH值对系统的协同影响,并确定该过程最佳脱氮速率和铵盐、硝酸盐、亚硝酸盐对DAMO系统初始抑制浓度,为完善DAMO过程理论和提升系统性能提供参考.
1 材料与方法
1.1 试验材料
1.1.1 试验系统 3个含DAMO微生物的系统分别是:Anammox-DAMO系统:以Nitrate-DAMO古菌(39.4%)和Anammox菌(45.8%)为优势菌种[16],以西溪河底泥、西湖底泥与农田水稻土壤的混合污泥为接种物,供给CH4、NH4+、NO3-,反应过程见图1A.
Nitrite-DAMO系统:以Nitrite-DAMO细菌(88.2%)为优势菌种[17],接种物与Anammox-DAMO系统相同,供给CH4、NO2-,反应过程见图1B.
Nitrate-DAMO系统:以Nitrite-DAMO细菌(62.2%)和Nitrate-DAMO古菌(26.5%)为优势菌种[18],以西溪河底泥、杭州七格污水处理厂二沉池活性污泥与储泥池厌氧消化污泥的混合污泥为接种物,供给CH4、NO3-,反应过程见图1C.
各试验系统内部始终保持厌氧状态,结合经高纯N2曝气30min的以去离子水为溶剂配制的新鲜营养液[19],KH2PO4、CaCl2×2H2O、MgSO4分别为0.05,0.30, 0.10g/L,微量元素液为1.25mL/L,利用0.1mol/L HCl或0.1mol/L NaOH使pH值维持在7.0±0.2,在25℃环境温度下运行稳定.

A. Anammox-DAMO系统;B. Nitrite-DAMO系统;C. Nitrate-DAMO系统
1.1.2 试验装置 如图2,反应器:直径16cm,高30cm,有效容积3.5L.取样口与排气口均设有阀门,所有阀门关闭时,反应器呈全封闭状态,内部环境厌氧.

图2 试验装置示意
a.排气口;b. 顶盖;c. 第一取样口;d. 第二取样口;e. 进样口;f. 搅拌子
1.2 试验方法
反应器已连续运行2547d,第1995~2367d的实验数据被用于校准和验证本文建立的DAMO反应过程动力学模型.第1995d开始作第1阶段,pH值控制在5.5±0.20,将Anammox- DAMO、Nitrate-DAMO系统内硝酸盐浓度控制为(10.0±0.50)mg N/L, Nitrite-DAMO系统内亚硝酸盐浓度控制为(10.0± 0.50)mg N/L,试验周期为7d,此后逐步将Anammox- DAMO系统硝酸盐浓度提升为(20.0±0.50), (30.0± 0.50), (50.0±0.50), (100.0±0.50), (150.0±0.50)mg N/L;Nitrate-DAMO系统硝酸盐浓度提升为(20.0± 0.50), (30.0±0.50), (50.0±0.50), (250.0±0.50) mg N/L; Nitrite-DAMO系统亚硝酸盐浓度提升为(20.0± 0.50), (30.0±0.50), (50.0±0.50)mg N/L.随着生物反应器性能的提高,在超过2054d的试验期间, Anammox-DAMO系统进水硝酸盐浓度从150.0mg N/L逐步提升至(250.0±0.50), (400.0±0.50), (500.0± 0.50), (800.0±0.50)mg N/L;Nitrate-DAMO系统进水硝酸盐浓度从250.0mg N/L逐步提升至(500.0±0.50), (750.0±0.50), (1000.0±0.50), (1250.0±0.50), (1500.0± 0.50)mg N/L;Nitrite-DAMO系统亚硝酸盐浓度从50.0mg N/L逐步提升至(100.0±0.50), (200.0±0.50), (350.0±0.50),(500.0±0.50),(650.0±0.50),(800.0±0.50)mg N/L.自第2085d开始,将Anammox- DAMO系统内铵盐浓度控制为(10.0±0.50)mg N/L,试验周期为7d,逐步将Anammox-DAMO系统进水铵盐浓度提升为(20.0±0.50), (30.0±0.50),(50.0±0.50), (100.0± 0.50),(150.0±0.50),(250.0±0.50),(400.0±0.50),(500.0±0.50),(800.0±0.50)mg N/L.此后2、3、4、5阶段控制pH值分别在6.0±0.20, 7.0±0.20, 8.0± 0.20, 8.5±0.20.
试验期间,每24h取水样3.0mL,经0.22μm微孔滤膜过滤后测定NH4+-N、NO3--N、NO2--N浓度以及pH值,同时监测系统中甲烷含量.利用普析TU-1901双光束紫外可见分光光度计测定NH4+- N、NO3--N、NO2--N的浓度[20].通过装配有FID的气相色谱仪(GC2030,岛津)测定顶空甲烷气体含量[21].利用梅特勒FG2便携式pH值计监测反应器在试验期间的pH值.
1.3 反应动力学模型
选用Haldane方程[式(1)][14]、Monod变型方程[式(2)、式(3)][13,22]为基础模型,利用Origin软件模拟氮素对DAMO过程的影响动力学.



式中:max为系统的最大脱氮速率, mg/(L·d);为硝酸盐、亚硝酸盐、铵盐浓度, mg/L;s为饱和系数, mg/L;K为硝酸盐、亚硝酸盐、铵盐对系统的初始抑制浓度,mg/L;max为微生物最大生长速率,cfu/(L·d);DA表示微生物产量系数;DA为微生物活性生物量,u/L;(NO2)为微生物对亚硝酸盐亲和常数, mg/L;'(NO2)为微生物对亚硝酸盐抑制常数,mg/L;(NO3)为微生物对硝酸盐亲和常数,mg/L;'(NO3)为微生物对硝酸盐抑制常数, mg/L.
N-DAMO系统脱氮速率在酸性环境随pH值升高而升高,在碱性环境随pH值的升高而降低[23],因此pH值对N-DAMO系统脱氮速率的影响可以看作一个近似一段的正弦函数方程[式(4)].

式中:为系统内pH值(取值范围0.0~14.0),、、、均为固定系数.
将式(1)、(2)、(3)与式(4)耦合建立反硝化型甲烷厌氧氧化系统脱氮速率-pH值耦合模型[式(5)、(6)、(7)]



2 结果与讨论
2.1 Anammox-DAMO系统脱氮拟合分析
由图3可知,在同等pH值条件下,0~800mg/L铵盐、硝酸盐影响Anammox-DAMO系统的脱氮速率均随盐浓度的增加呈先增加后下降趋势;在同等盐浓度条件下,5.5~8.5pH值影响下脱氮速率也随pH值的增加先增加后下降.Anammox-DAMO系统脱氮速率拟合参数见表1.
由图3a可知,Haldane-pH值耦合方程的拟合度(2)和残差平方和(SSE)分别为0.890和9.559, Anammox-DAMO系统的最佳脱氮速率为3.74mg/ (L·d),硝酸盐对系统的初始抑制浓度为182.63mg/L, 0~800mg/L NO3--N条件下系统脱氮速率符合Haldane-pH值方程.由图3b可知,Haldane-pH值耦合方程的2和SSE分别为0.867和12.330, Anammox-DAMO系统的最佳脱氮速率为3.95mg/ (L·d),铵盐对系统的初始抑制浓度为196.40mg/L,0~ 800mg/L NH4+-N影响下系统脱氮速率也符合Haldane-pH值方程.
由图3可知,硝酸盐浓度高于182.63mg/L,铵盐浓度高于196.40mg/L将限制DAMO古菌和Anammox细菌的生长,最佳盐浓度是图中拟合曲面的最高点.研究表明[24-25],随着盐度的增加,反硝化速率会受到抑制.因此当铵盐和硝酸盐浓度持续升高达到一定浓度后,Anammox-DAMO反应器反硝化速率下降.本文拟合结果中,硝酸盐对Anammox- DAMO系统脱氮抑制浓度为182.63mg/L,而Li等[26]发现在50~400mg/L,硝酸盐浓度增加对Anammox过程暂没有抑制作用,这可能是由于Li等[26]所使用的上流式污泥床-过滤器(UBF)反应器内微生物受到驯化,进水硝酸盐、铵盐浓度(300~400mg/L)较高,Anammox菌的耐受性相应提高;铵盐影响下的脱氮速率拟合结果低于Ni等[11]研究当铵浓度从210mg/L增加到380mg/L,氮负荷率(NLR)由0.43kg/ (m3×d)增加到0.72kg/(m3×d),反应器对基质浓度冲击具有良好的耐受性.这可能是由于Anammox细菌细胞产率低(0.08~0.11g/g,以VSS/NH4+-N计),生长缓慢且在高细胞浓度(1010~1011个/mL)时才具有活性[27],本试验所用反应系统内污泥生物量较Ni等[11]生物量低,导致Anammox细菌活性相对较低.

a. NO3--N-pH值影响;b. NH4+-N-pH值影响

表1 Anammox-DAMO系统动力学参数
图3中所示pH值对Anammox-DAMO系统脱氮速率的影响可以近似看作一段正弦曲线方程,在酸性范围内随pH值增大,脱氮速率逐步提升, 7.5± 0.2达到最佳脱氮速率,在碱性范围内随pH值增大,脱氮速率逐渐受到抑制.Achlesh等[28]通过构建二维等高线图和三维响应面图可视化温度和酸碱度对Anammox系统的交互影响结果表明, Anammox系统最佳pH值8.0~8.5,相较本文拟合结果更大,证明在Anammox-DAMO体系中,脱氮速率受DAMO古菌与Anammox菌共同作用,因此推测DAMO古菌更倾向于酸性环境,这与Lou等[17]推论相似.研究表明,游离氨(FA)对厌氧氨氧化系统有负面影响[29],且FA与pH值有关[21].本研究中碱性条件(7.0~8.5)下,随着pH值升高,FA浓度升高,脱氮速率下降.当pH=8.5,氨氮浓度为87.61mg/L时,FA浓度为13.34mg/L,此时脱氮速率不到最大脱氮速率的一半,而在酸性条件下(pH值5.5~7.0),对脱氮速率进行单因素方差分析发现并无显著性差异.说明在碱性条件下, Anammox- DAMO系统限制性抑制因子是FA.而在酸性条件下,抑制效果与FA浓度无关,只与离子化氨氮的浓度相关,离子化氨氮是限制性抑制因子.这与文献报道的最佳pH值范围(7.0~8.5)一致[30].
2.2 Nitrate-DAMO系统脱氮拟合分析
由图4可见,在相同pH值条件下,0~1500mg/L硝酸盐影响Nitrate-DAMO系统的脱氮速率随硝酸盐浓度的增加呈先增加后下降趋势;在相同硝酸盐条件下, pH值5.5~8.5范围内脱氮速率也随pH值的增加先增加后下降.Nitrate-DAMO系统脱氮速率拟合参数见表2.
由图4a可知,Haldane-pH值耦合方程的2和SSE分别为0.823和12.098, Nitrate-DAMO系统的最佳脱氮速率为4.30mg/(L·d),硝酸盐对系统的初始抑制浓度为367.69mg/L,0~1500mg/L NO3--N条件下系统脱氮速率符合Haldane-pH值方程.由图4b可知,Monod-pH值耦合方程2和SSE分别为0.822和12.098,Nitrate-DAMO系统的最佳脱氮速率为4.28mg/(L·d),细菌生长速率为1291.21cfu/(L·d),硝酸盐亲和常数和抑制常数分别为295.23,72.63mg/ L.0~1500mg/L NO3--N条件下系统脱氮速率也符合Monod-pH值方程.用这2个模型拟合后得到的结果吻合程度非常高.
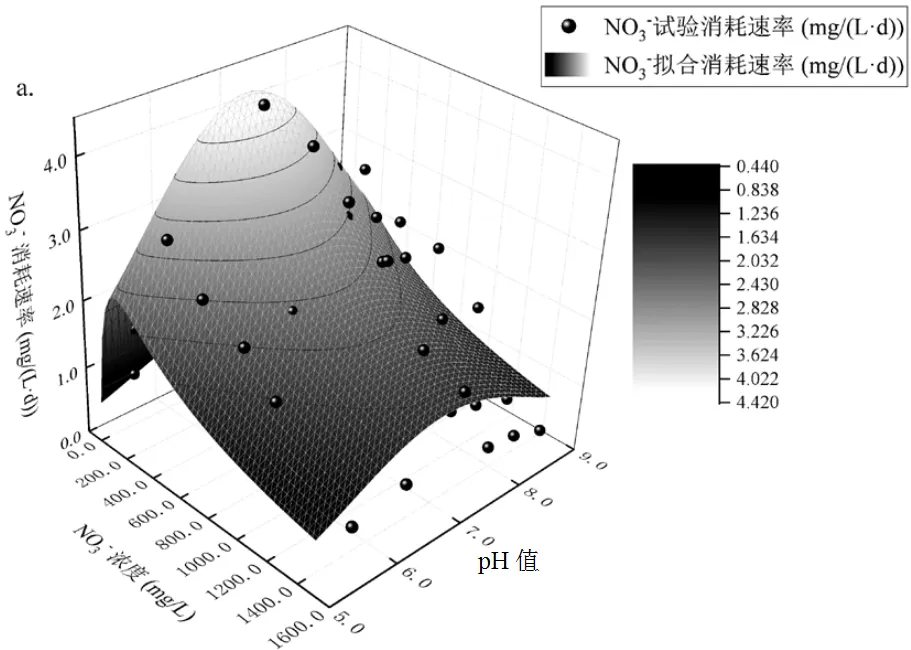
a. Haldane-pH值耦合模型;b. Monod-pH值耦合模型

表2 Nitrate-DAMO系统动力学参数
由图4可知,低浓度硝酸盐(0~350mg/L)影响下,Nitrate-DAMO系统脱氮能力与盐浓度呈正相关,这与Li等[31]发现的亚热带河口沉积物中DAMO速率与沉积物NO3--N呈正线性关系相类似.当硝酸盐浓度高于367.69mg/L时对系统产生抑制效应, Nitrate-DAMO系统脱氮性能随盐浓度升高而降低.盐度是决定DAMO细菌多样性和丰度的关键因素,高盐度对DAMO细菌和DAMO古菌的菌群结构、丰度以及活性产生抑制作用[21,32].Shen等[4]观察到盐度与杭州湾沉积物中DAMO细菌的丰度和活性呈负相关.这些结果证实了盐度会对DAMO过程产生一定的负面影响.随着盐度的增加,DAMO过程脱氮速率下降,也可能归因于盐度对反硝化作用的抑制[26].Li等[31]研究发现盐度也通过限制和抑制反硝化作用而间接影响DAMO系统反应速率.本研究也发现,当硝酸盐浓度达到一定浓度时,也对系统产生了负面作用,Nitrate-DAMO系统脱氮速率受到抑制.硝酸盐对Anammox-DAMO系统初始抑制浓度为182.63mg/L,对Nitrate-DAMO系统初始抑制浓度为367.69mg/L,这可能是由于DAMO细菌与DAMO古菌对高盐废水可以表现出更强的抗冲击负荷能力[33].
由图4可知,Nitrate-DAMO系统脱氮速率受pH值影响,先增高后降低,在pH7.2±0.2达到最佳. Nitrate-DAMO系统由62.2% Nitrite-DAMO细菌与26.5%Nitrate-DAMO古菌组成,脱氮速率受细菌与古菌协同影响,其中细菌起主导作用.与Nitrite-DAMO系统相比,由于偏弱酸性古菌影响, Nitrate- DAMO系统更适应中性环境,脱氮速率达到最佳,与Anammox-DAMO系统相比, Nitrate- DAMO系统内不含偏碱性的Anammox菌,最适pH值更低.
2.3 Nitrite-DAMO系统脱氮拟合分析
由图5可见,在相同pH值条件下,在0~800mg/L亚硝酸盐影响下,Nitrite-DAMO系统的脱氮速率随亚硝酸盐浓度的增加呈先增加后下降趋势;在同等亚硝酸盐条件下, pH值5.5~8.5时,脱氮速率也随pH值的增加先增加后下降.Nitrite-DAMO系统脱氮速率拟合参数见表3.
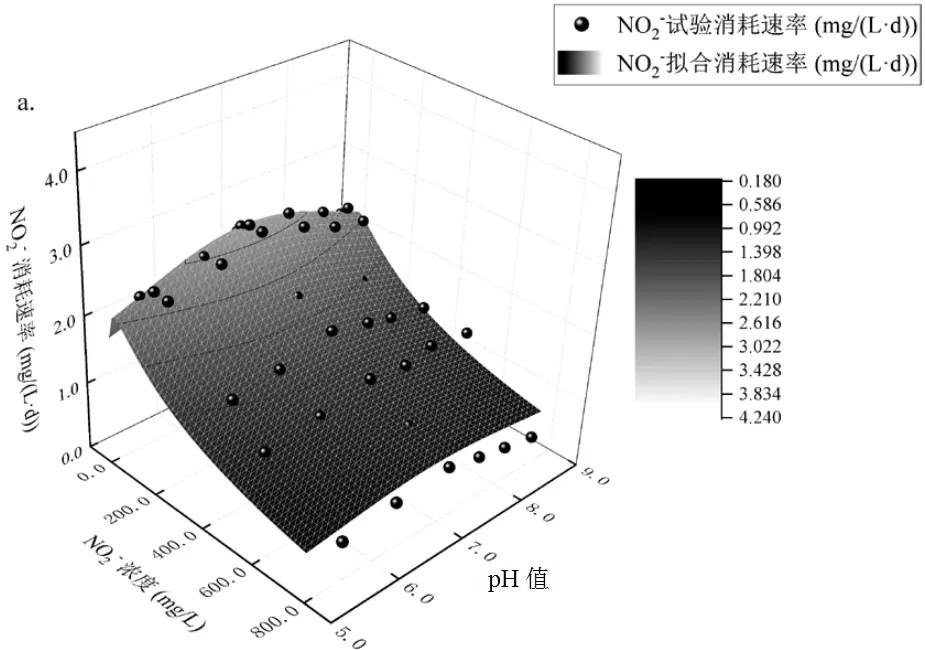
a. Haldane-pH值耦合模型;b. Monod-pH值耦合模型
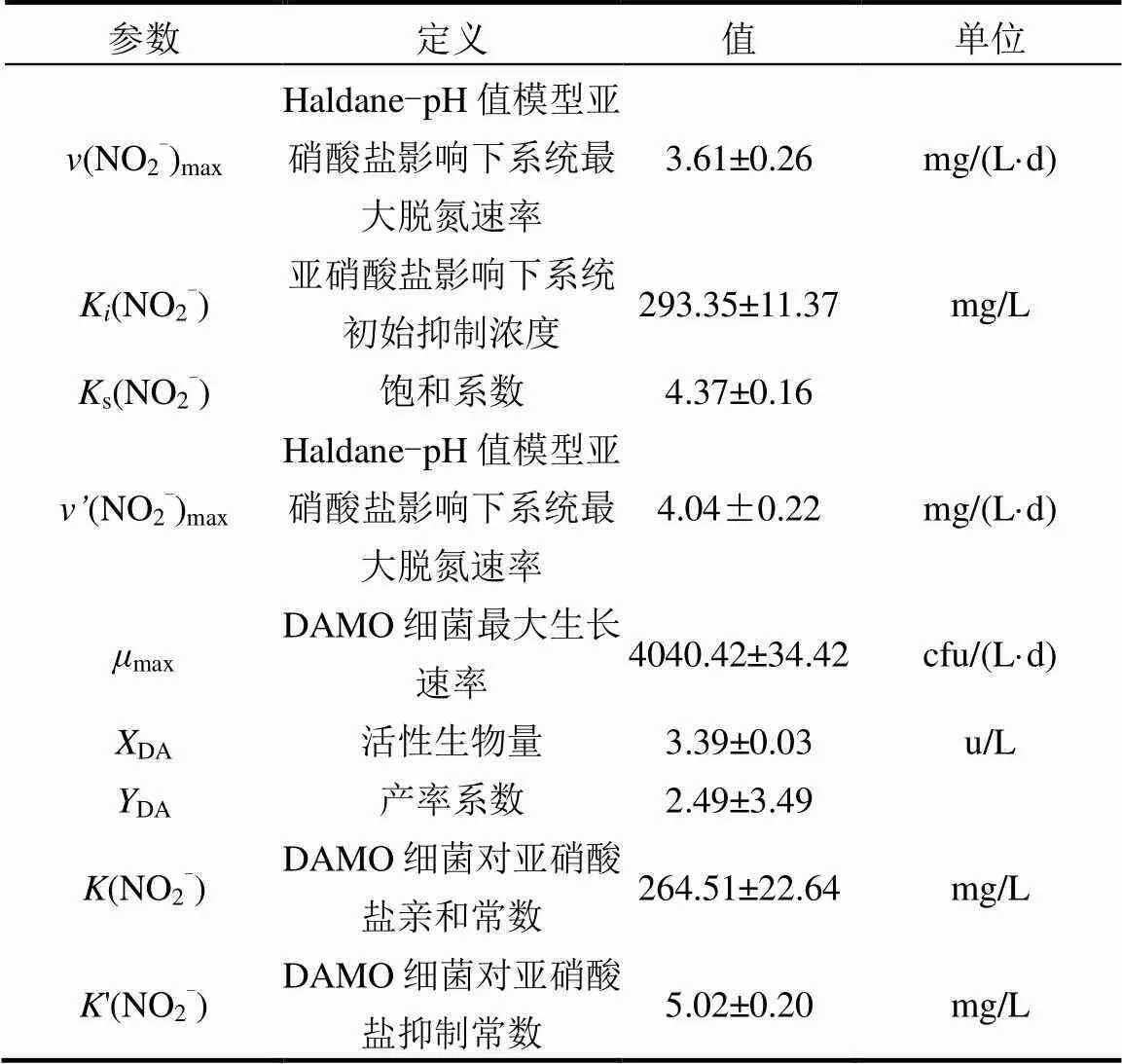
表3 Nitrite-DAMO系统动力学参数
由图5a可知,Haldane-pH值耦合方程的2和SSE分别为0.954和1.629, Nitrite-DAMO系统的最佳脱氮速率为3.61mg/(L·d),亚硝酸盐对系统的初始抑制浓度为293.35mg/L, 0~800mg/L NO2--N条件下系统脱氮速率符合Haldane-pH值方程.由图5b可知,Monod-pH值耦合方程的2和SSE分别为0.955和1.591,Nitrite-DAMO系统的最佳脱氮速率为4.04mg/(L·d),细菌生长速率为4040.42cfu/(L·d),亚硝酸盐亲和常数和抑制常数分别为264.51, 5.02mg/ L.0~800mg/L NO2--N条件下系统脱氮速率也符合Monod-pH值方程.
Nitrite-DAMO系统脱氮趋势与其他研究的亚硝酸盐对活性污泥中多种微生物的抑制范围相比,表现出对高浓度亚硝酸盐更强的抗冲击负荷的能力.Dapena-Mora等[33]发现,当亚硝酸盐浓度为5~40mg/L时,会严重抑制厌氧氨氧化反应.亚硝酸盐积累浓度达到140~700mg/L时,会对反硝化细菌的活性产生抑制作用[34].由图4b可知,Monod-pH值耦合模型能够较好地描述Nitrite- DAMO系统脱氮过程,这与Hu等[13]拟合结果相似,且该模型可以在一定时间内很好地预测废水中亚硝酸盐的浓度.亚硝酸盐消耗率在达到最大值后下降[35-36]也与本文拟合结果相符.
如图5,Nitrite-DAMO系统脱氮速率在各浓度下均随pH值增大先提升后降低,在7.8±0.2达到最佳.这与He等[21]的拟合结果类似:pH7.6左右修正方程的最佳拟合值.与大多数异养反硝化菌类似, Nitrite-DAMO细菌更倾向于pH7.0~9.0的弱碱性环境[37].过往研究对于N-DAMO的培养大多保持在6.8~7.6[38-39],7.6左右的pH值脱氮速率优于7.0,以上结论均与本文拟合结果相符.He等[40]认为,游离亚硝酸盐氮(FNA)是反硝化过程的抑制因子.Chen等[41]发现,当pH8.0~8.9时, FNA浓度较低,即使亚硝酸盐氮浓度高达2000mg/L,也未对系统产生抑制作用.Puyol等[42]发现,膨胀颗粒污泥床(EGSB)厌氧氨氧化工艺对pH值冲击非常敏感,亚硝酸盐在反应器中积累产生的高FNA浓度的抑制作用在酸性pH值冲击后恶化了反应器性能.本实验中当温度为25℃, pH6.0,亚硝酸盐氮浓度74.72mg/L时,FNA浓度74.72mg N/L,Nitrite- DAMO系统脱氮率不到最佳脱氮速率的1/2.单因素方差分析表明,在酸性条件下,亚硝酸盐氮对体系的影响与FNA值有关,FNA是限制性抑制因子.在碱性条件下,亚硝酸盐对体系的抑制作用与FNA值无关,此时离子化的亚硝酸盐是限制性抑制因子.该结果与Puyol等[42]的研究结果一致.
3 结论
3.1 Anammox-DAMO、Nitrate-DAMO、Nitrite- DAMO系统耦合pH值反应动力学皆符合Haldane- pH值方程.其中,Anammox-DAMO系统最大脱氮速率为3.95mg/(L·d),硝酸盐初始抑制浓度为182.63mg/L,铵盐初始抑制浓度为196.40mg/L; Nitrate-DAMO系统最大脱氮速率为4.30mg/(L·d),硝酸盐初始抑制浓度为367.69mg/L;Nitrite-DAMO系统最大脱氮速率为4.04mg/(L·d),亚硝酸盐初始抑制浓度为293.35mg/L.
3.2 Nitrate-DAMO系统和Nitrite-DAMO系统也符合Monod-pH值耦合模型,且拟合结果与Haldane-pH值方程拟合结果具有很高的吻合程度. Nitrate-DAMO细菌生长速率为1291.21cfu/(L·d),硝酸盐亲和常数为295.23mg/L,硝酸盐抑制常数为72.63mg/L;Nitrite-DAMO细菌生长速率为4040.42cfu/(L·d),亚硝酸盐亲和常数为264.51mg/L,亚硝酸盐抑制常数为5.02mg/L.
3.3 各系统脱氮速率受pH值影响皆呈一段正弦曲线分布,其中Anammox菌、Nitrite-DAMO细菌适应弱碱环境,Nitrate-DAMO古菌更倾向于弱酸环境,在3类微生物的协同作用下Anammox-DAMO系统最佳脱氮速率pH值为7.5±0.2, Nitrate-DAMO系统为7.2±0.2,Nitrite-DAMO系统为7.8±0.2.
[1] Ettwig K F, Butler M K, Le Paslier D, et al. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria [J]. Nature, 2010,464(7288): 543-548.
[2] Haroon M F, Hu S, Shi Y et al. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage [J]. Nature, 2013,500(7464):567-570.
[3] Hu B L, He Z F, Geng S, et al. Cultivation of nitrite-dependent anaerobic methane-oxidizing bacteria: impact of reactor confgurafion [J]. Applied Microbiology and Biotechnology, 2014,98(18):7983-7991.
[4] Shen L D, Liu S, He Z F, et al. Depth-specific distribution and importance of nitrite-dependent anaerobic ammonium and methane- oxidising bacteria in an urban wetland [J]. Soil Biology and Biochemistry, 2015,83:43-51.
[5] Deutzmann J S, Stief P, Brandes J, et al. Anaerobic methane oxidation coupled to denitrification is the dominant methane sink in a deep lake [J]. Proceedings of the National Academy of Sciences of the United States of America, 2014,111(51):18273-18278.
[6] 翟 俊,马宏璞,陈忠礼,等.湿地甲烷厌氧氧化的重要性和机制综述 [J]. 中国环境科学, 2017,37(9):3506-3514.
Zhai J, Ma H P, Chen Z L, et al. Review on the importance and mechanisms of anaerobic oxidation of methane in wetlands [J]. China Environmental Science, 2017,37(9):3506-3514.
[7] Zhu G, Jetten M S M, Kuschk P, et al. Potenial roles of anaerobic ammonium and methane oxidation in the nitrogen cycle of wetland ecosystems Interactions between denitrifying anaerobic methane oxidizing bacteria and anammox bacteria [J]. Applied Microbiology and Biotechnology, 2010,86(4):1043-1055.
[8] Strous M, Heijnen J J, Kuenen J G et al. The sequencing batch reactor as a powerful tool for the study of slowly gr owing anaerobic ammonium-oxidizing microorganisms [J]. Applied Microbiology and Biotechnology, 1998,50(5):589-596.
[9] Amand L, Carlsson, B. Optimal aeration control in a nitrifying activated sludge process [J]. Water Research, 2012,46(7):2101-2110.
[10] Young M N, Marcus A K, Rittmann B E. A Combined Activated Sludge Anaerobic Digestion Model (CASADM) to understand the role of anaerobic sludge recycling in wastewater treatment plant performance [J]. Bioresource Technology, 2013,136:196-204.
[11] Ni S Q, Sung S W, Yue Q Y, et al. Substrate removal evaluation of granular anammox process in a pilot-scale upflow anaerobic sludge blanket reactor [J]. Ecological Engineering, 2012,38(1):30-36.
[12] Rosenthal A, Ramalingam K, Beckmann K, et al. Experimental evaluation of the nitrite sensitivity coefficient in granular anammox biomass [J]. Water Science and Technology, 2013,68(9):2103-2110.
[13] Hu B L, He Z F, Cai C, et al. Mdodeling a nitrite-dependent anaerobic methane oxidation process: Parameters identification and model evaluation [J]. Bioresource Technology, 2013,147(2013):315-320.
[14] Ni B J, Yuan Z G, Hu S H, et al. Modeling of simultaneous anaerobic methane and ammonium oxidation in a membrane biofilm reactor [J]. Environmental Science & Technology, 2014,48(16):9540-9547.
[15] Shen L D, Huang Q, He Z F, et al. Vertical distribution of nitrite-dependent anaerobic methane-oxidising bacteria in natural freshwater wetland soils [J]. Applied Microbiology and Biotechnology, 2015,99(1):349-357.
[16] Lou J Q, Wang X L, Li J P, et al. The short- and long-term effects of nitrite on denitrifying anaerobic methane oxidation (DAMO) organisms [J]. Environmental Science and Pollution Research, 2018, 26(5):4777-4790.
[17] Lou J Q, Li J P, Wang X L, et al. Response mechanism of denitrifying anaerobic methane oxidation microorganisms to ammonia [J]. Environmental Chemistry, 2019,17(1):17-27.
[18] Li J P, Lou J Q, Lv J. The effect of sulfate on nitrite-denitrifying anaerobic methane oxidation (nitrite-DAMO) process [J]. Science of the Total Environment, 2020,731(3/4):139-160.
[19] Raghoebarsing A A, Pol A, Pas-Schoonen K T, et al. A microbial consortium couples anaerobic methane oxidation to denitrification [J]. Nature, 2006,440(7086):918-921.
[20] 魏复盛.水和废水监测分析方法(第4版) [M]. 北京:中国环境科学出版社, 2002.
Wei F S. Analytical methods for water and wastewater monitoring (4th Edition) [M]. Beijing: China Environmental Science Press, 2002.
[21] He Z F, Geng S, Shen L D, et al. The short- and long-term effects of environmental conditions on anaerobic methane oxidation coupled to nitrite reduction [J]. Water Research, 2015,68:554-562.
[22] 徐 富,缪恒锋,任洪艳,等.低浓度废水厌氧处理中不同动力学模型对比研究 [J]. 中国环境科学, 2013,33(12):2184-2190.
Xu F, Miao H F, Ren H Y, et al. Comparative study of different kinetic models in anaerobic treatment low-strength wastewater [J]. China Environmental Science, 2013,33(12):2184-2190.
[23] Lou J Q, Lv J, Yang D Y, et al. Effects of environmental factors on nitrate-DAMO activity [J]. Water Air and Soil Pollution, 2020,231(6): 263-270.
[24] Rysgaard S, Sloth N P. Effects of salinity on NH4þadsorption capacity, nitrification, and denitrification in Danish estuarine sediments [J]. Estuaries and Coasts, 1999,22(1):21-30.
[25] Liu C, Hou L J, Liu M, et al. Coupling of denitrification and anaerobic ammonium oxidation with nitrification in sediments of the Yangtze Estuary: importance and controlling factors [J]. Estuar. Estuarine Coastal and Shelf Science, 2019,220:64-72.
[26] Li Z X, Peng Y Z. Biphasic effect of nitrate on anaerobic ammonium oxidation (anammox) and related kinetic modeling [J]. Chemosphere, 2020,238:124654.
[27] Isaka K, Date Y, Sumino T, et al. Growth characteristic of anaerobic ammonium-oxidizing bacteria in an anaerobic biological filtrated reactor [J]. Applied Microbiology and Biotechnology, 2006,70(1):47-52.
[28] Achlesh D, Pang C C, Kasturi D, et al. Statistical analysis to evaluate the effects of temperature and pH on anammox activity [J]. International Biodeterioration & Biodegradation, 2015,102:89-93.
[29] Coma M, Verawaty M, Pijuan M, et al. Enhancing aerobic granulation for biological nutrient removal from domestic wastewater [J]. Bioresource Technology, 2012,103(1):101-108.
[30] Strous M, Van Gerven E. Effects of aerobic and microaerobic conditions on anaerobic ammonium-oxidizing (Anammox) sludge [J]. Applied & Environmental Microbiology, 1997,63(6):2446-2448.
[31] Li X F, Laic D Y F, Gao D Z. Anaerobic oxidation of methane with denitrification in sediments of a subtropical estuary: Rates, controlling factors and environmental implications [J]. Journal of Environmental Management, 2020,273:111151.
[32] Chen J, Zhou Z C, Gu J D. Occurrence and diversity of nitrite dependent anaerobic methane oxidation bacteria in the sediments of the South China Sea revealed by amplification of both 16SrRNA and pmoA genes [J]. Applied Microbiology and Biotechnology, 2014, 98(12):5685-5696.
[33] Dapena-Mora A, Fernández I, Campos J L, et al. Evaluation of activity and inhibition effects on Anammox process by batch tests based on the nitrogen gas production [J]. Enzyme and Microbial Technology, 2007,40(4):859-865.
[34] Valenzuela E I, Prieto-Davó A, López-Lozano N E, et al. Anaerobic methane oxidation driven by microbial reduction of natural organic matter in a tropical wetland [J]. Applied Environment Microbiology, 2017,83(11):1-15.
[35] Ettwig K F, Shima S, Pas-Schoonen K T, et al. Denitrifying bacteria anaerobically oxidize methane in the absence of Archaea [J]. Environmental Microbiology, 2008,10(11):3164-3173.
[36] Vadivelu V M, Yuan Z G, Fux C, et al. The inhibitory effects of free nitrous acid on the energy generation and growth processes of an enriched nitrobacter culture [J]. Environmental Science and Technology, 2006,40(14):4442-4448.
[37] Kampman C, Hendrickx T L, Luesken F A, et al. Enrichment of denitrifying methanotrophic bacteria for application after direct low-temperature anaerobic sewage treatment [J]. Journal of Hazard Materials, 2012,227:164–171.
[38] Tang Y, Zhou C, Ziv-El M, et al. A pH-control model for heterotrophic and hydrogen-based autotrophic denitrification [J]. Water Research, 2011,45(1):232-240.
[39] Ettwig K F, van Alen T, van de Pas-Schoonen K T, et al. Enrichment and molecular detection of denitrifying methanotrophic bacteria of the NC10phylum [J]. Applied Environment Microbiology, 2009,75(11): 3656-3662.
[40] He S L, Zhang Y L, Niu Q G, et al. Operation stability and recovery performance in an Anammox EGSB reactor after pH shock [J]. Ecological Engineering, 2016,90:50-56.
[41] Chen S K, Juaw C K, Cheng S S. Nitrification anddenitrification of high-strength ammonium and nitritewastewater with biofilm reactors [J]. Water Science and Technology, 1991,23(79):1417-1425.
[42] Puyol J M, Carvajal-Arroyo R, Sierra-Alvarez J A. Nitrite (not free nitrous acid) is the main inhibitor of the anammox process at common pH conditions [J]. Biotechnology Letters, 2014,36(3):547-551.
pH coupling model of denitrifying anaerobic methane oxidation (DAMO) system.
LU Jiao, LOU Ju-qing*, XU Fan
(College of Environmental Science and Engineering, Zhejiang Gongshang University, Hangzhou 310018, China)., 2022,42(2):612~619
This study tried to couple the pH value with the reaction kinetics of the denitrification-type methane anaerobic oxidation process. The Denitrifying Anaerobic Methane Oxidation (DAMO) reaction rate and pH value were coupled and evaluated in three reactors with different dominant bacteria. The results show that the Anammox-DAMO maximum denitrification rate and the initial inhibition concentration of nitrate ammonium at 25℃ were 3.95mg/(L×d), 182.63mg/L and 196.40mg/L, respectively. The maximum denitrification rate and the initial inhibition concentration of nitrate in Nitrate-DAMO system were 4.30mg/(L×d) and 367.69mg/L, respectively. The maximum denitrification rate and the initial inhibition concentration of nitrite in Nitrite-DAMO system were 4.04mg/(L×d) and 293.35mg/L, respectively. The denitrification rate of the systems increased first and then decreased with the increase of pH. The optimum pH was 7.5±0.2, 7.2±0.2, 7.8±0.2, respectively. The bacterial growth rate, nitrate affinity constant and inhibition constant of Nitrate-DAMO system were 1291.21cfu/(L×d), 295.23mg/L and 72.63mg/L, respectively; The bacterial growth rate, nitrite affinity constant and inhibition constant of Nitrite-DAMO system were 4040.42cfu/(L×d), 264.51mg/L and 5.02mg/L, respectively. All the three DAMO systems could be described by the coupled Haldane pH model; the denitrification process of Nitrate-DAMO system and Nitrite-DAMO system could also be described by Monod-pH coupling equation.
denitrifying anaerobic methane oxidation;denitrification performance;Hanldane equation;Monod equation;pH;coupling model
X703
A
1000-6923(2022)02-0612-08
吕 娇(1996-),女,浙江湖州人,浙江工商大学硕士研究生,主要研究方向为废水生物处理及资源化.发表论文1篇.
2021-05-21
浙江省自然科学基金资助项目(LY21D030003);浙江工商大学研究生科研创新基金一般项目(19020160029)
* 责任作者, 副教授, ljq7393@163.com
