Alkali-metal(Li,Na,and K)-adsorbed MoSi2N4 monolayer: an investigation of its outstanding electronic, optical, and photocatalytic properties
2022-02-18ZhiyuanSunJingXuNsajigwaMwankemwaWenxingYangXianwenWuZaoYiShanjunChenandWeibinZhang
Zhiyuan Sun, Jing Xu, Nsajigwa Mwankemwa, Wenxing Yang,Xianwen Wu, Zao Yi, Shanjun Chen,∗and Weibin Zhang,∗
1 School of Physics and Optoelectronic Engineering, Yangtze University, Jingzhou 434023, China
2 School of Chemistry and Chemical Engineering, Jishou University, Jishou 416000, China
3 Joint Laboratory for Extreme Conditions Matter Properties, Southwest University of Science and Technology, Mianyang 621010, China
Abstract Single-layer MoSi2N4,a high-quality two-dimensional material,has recently been fabricated by chemical vapor deposition.Motivated by this latest experimental work, herein, we apply first principles calculations to investigate the electronic, optical, and photocatalytic properties of alkali-metal(Li, Na, and K)-adsorbed MoSi2N4 monolayer.The electronic structure analysis shows that pristine MoSi2N4 monolayer exhibits an indirect bandgap (Eg=1.89 eV).By contrast, the bandgaps of one Li-, Na-, and K-adsorbed MoSi2N4 monolayer are 1.73 eV,1.61 eV, and 1.75 eV, respectively.Moreover, the work function of MoSi2N4 monolayer(4.80 eV)is significantly reduced after the adsorption of alkali metal atoms.The work functions of one Li-, Na-, and K-adsorbed MoSi2N4 monolayer are 1.50 eV, 1.43 eV, and 2.03 eV,respectively.Then, optical investigations indicate that alkali metal adsorption processes substantially increase the visible light absorption range and coefficient of MoSi2N4 monolayer.Furthermore, based on redox potential variations after alkali metals are adsorbed, Li- and Naadsorbed MoSi2N4 monolayers are more suitable for the water splitting photocatalytic process,and the Li-adsorbed case shows the highest potential application for CO2 reduction.In conclusion,alkali-metal-adsorbed MoSi2N4 monolayer exhibits promising applications as novel optoelectronic devices and photocatalytic materials due to its unique physical and chemical properties.
Keywords: MoSi2N4, first-principles, alkali metal adsorbed, electronic structure, optical properties, photocatalysis
1.Introduction
In recent years, two-dimensional (2D) materials [1–4] have become of interest due to their unique properties and various applications that emerge in the single-layer limit.The most typical and earliest 2D material confirmed experimentally is graphene [5, 6], which possesses unique electronic, optical,and pyroelectric properties.In line with the continuous exploration and research of 2D materials, an increasing number of various 2D materials have been successfully synthesized through experiments [7, 8].MXenes (include 2D transition metal nitrides, carbides, and carbonitrides) are a rapidly developing family of 2D materials with nearly 30 members experimentally synthesized and dozens of them investigated theoretically [9, 10].They are widely applied in various applications because of their unique physiochemical properties, including outstanding electronic [11], photocatalytic [12], and mechanical [13] properties.Recently,Hong et al[14],during the chemical vapor deposition growth of nanolayered MoN2,introduced elemental Si to passivate its surface.As a consequence, centimeter-scale monolayer films of MoSi2N4can be grown.According to the report,MoSi2N4monolayer comprises the N–Si–N–Mo–N–Si–N atomic layer,and displays a non-magnetic nature, high strength, and outstanding stability.Meanwhile, its optical properties and bandgap were analyzed via the Tauc plot.It is found that MoSi2N4monolayer presents a high optical transmittance(average of 97.5%±0.2%) in the visible range, as well as exhibits an indirect bandgap semiconductor structure (bandgap ∼1.94 eV).
The advent of the MoSi2N4monolayer [15, 16] has inspired widespread attention in optoelectronics, valleytronics, spintronics, and so on.Yu et al [17] investigated the pristine lattice thermal conductivity of MoSi2N4monolayer based on the first principles phonon Boltzmann transport equation.The study showed that MoSi2N4monolayer displays high lattice thermal conductivity in 300 ∼800 K.Meanwhile, Bafekry et al [18] used a hybrid density functional theory to explore the thermal, mechanical, electronic,optical, and photocatalytic properties.The investigations revealed that pristine MoSi2N4monolayer has good thermoelectric properties, and is a promising photocatalyst for water splitting and CO2reduction.In addition, Yang et al [19]predicted that peak-to-valley conversion characteristics could be achieved in the MoSi2N4monolayer.
However, current research on the properties and performance of the modified MoSi2N4monolayer is still incomplete.It is well known that the modification of alkali metal adsorption on semiconductor materials will modify their electronic structure and optoelectronic properties because alkali metal atoms can easily lose electrons.In recent years,many research groups have reported the optoelectronic properties of alkali-metal-adsorbed 2D materials[20,21].For example,Jin et al[22]used density functional theory to study alkali-metal-adsorbed graphene and discovered that the alkali metal adsorption can the change optoelectronic properties of graphene.Cui et al[23]studied alkali-metal-adsorbed g-GaN using first principles calculations, and has shown that the optical absorption of g-GaN is extended due to the decoration of alkali metals.However, the fully electronic, optical, and photocatalytic properties of alkali-metal-adsorbed MoSi2N4monolayer are still unclear.
In this paper, the structural, electronic, optical, and photocatalytic properties of alkali-metal-adsorbed MoSi2N4monolayer were calculated via first principles calculations.Lithium(Li), sodium(Na), and potassium(K) atoms are adsorbed onto the 3×3×1 supercell of the MoSi2N4monolayer, respectively, to study the influence of the decoration of alkali metals.The most stable adsorption sites,work function, band structure (BS), density of state (DOS),optical constants of pristine and alkali-metal-doped MoSi2N4monolayers were studied in detail.Furthermore, the band alignments of alkali-metal-adsorbed MoSi2N4monolayer were analyzed to show the potential application in photocatalysis.Our findings suggest that the alkali-metal-adsorbed MoSi2N4monolayer is a potential 2D material for photocatalytic water splitting and CO2reduction.
2.Computational details
The VASP [24, 25] (Vienna ab initio Simulation Package)code was used to conduct first principles calculations in this work.The Perdew–Burke–Ernzerhof (PBE) [26–28] in a generalized gradient approximation (GGA) was used to optimize the geometric structure.The cutoff energy is 500 eV.Since the local density approximation (LDA) typically underestimates the equilibrium atomic distances while overestimating the model system’s adsorption energy, PBE/GGA can provide more precise estimations for geometric optimization.For the electronic and optical analysis, the convergence criterion for the self-consistent electronic loop was set to 10–6 eV.Meanwhile, a 3×3×1 k-point grid in the first Brillouin zone was applied.The maximum allowed force was 0.03 eV Å−1, and the maximum stress was 0.05 GPa.
The adsorption energy [29] for one Li, Na, or K atom adsorbed onto the MoSi2N4monolayer can be determined as

where Etotal, Epris, and ELi/Na/Kdenote the total energy of alkali-metal-adsorbed MoSi2N4,pristine MoSi2N4,and a free alkali metal atom, respectively.Negative (positive) values reveal that the adsorption is an exothermic (endothermic)reaction.
From the perspective of quantum mechanics, the interaction between photons and electrons in the system is described in terms of time-dependent perturbations of the ground electronic state,and the photon absorption or emission result in transitions between unoccupied and occupied states.The spectrum produced by excitation can be regarded as a joint DOS between the conduction bands (CB) and the valence bands(VB).The imaginary part ε2(ω)of the dielectric function [29, 30], which is a function of the photon frequency, can be described as
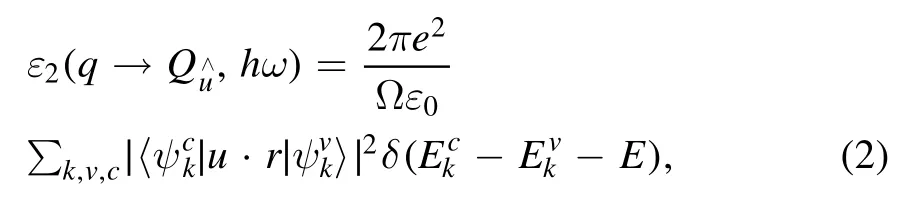
where k is the reciprocal lattice vector, ω is the incident photon frequency, u is the vector defining the polarization of the incident electric field, and the superscripts c and v denote the CB and VB, respectively.The real part ε1(ω) of the dielectric function, since the dielectric function shows a causal response,can be gained by the imaginary part of the Kramers−Kroning relations.Then, the optical absorption coefficient α(ω) can be obtained from ε1(ω) and ε2(ω) [31]:
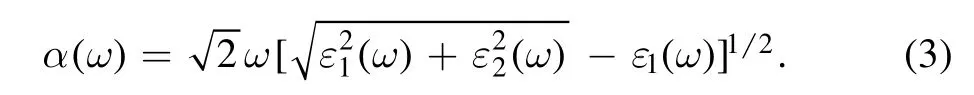
3.Results and discussion
The top and side views of the alkali-metal-adsorbed MoSi2N4monolayer structure and the three different adsorption sites are illustrated in figure 1.The top view illustrates that the pristine MoSi2N4monolayer,packing N,Si,and Mo atoms in a honeycomb lattice,is a 2D crystal with the optimized lattice constant a=2.897 Å.Li,Na,and K atoms were decorated on the surface of the MoSi2N4monolayer, which constitutes the alkali-metal-adsorbed MoSi2N4monolayer.From the side view, the MoSi2N4monolayer is composed of a N–Si–N–Mo–N–Si–N atomic layer,which can be regarded as a MoN2layer sandwiched between two slightly buckled honeycomb Si–N layers.We compared three different adsorption sites to determine the most stable sites of Li, Na, and K atoms adsorbed onto the MoSi2N4monolayer.The TN,TSi,and TMosites are directly above the N, Si, and Mo atoms.The adsorption energies of alkali-metal-decorated MoSi2N4, used to compare their stability,are calculated and shown in table 1.The data shows that all the adsorption energies of the three different sites are negative, indicating that the adsorption process of alkali metals onto the MoSi2N4monolayer is exothermic, which means all adsorption systems are stable.Furthermore,the TMois the most stable site due to the lowest adsorption energy.Therefore, the following discussion only refers to alkali metals decorated on TMoadsorption positions.
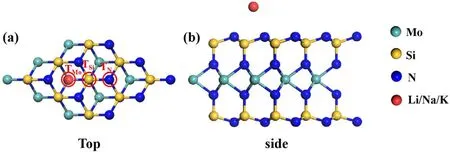
Figure 1.(a)Top view and the different adsorption sites of alkali metals decorated on MoSi2N4:the top of Mo(TMo),the top of Si(TSi),and the top of N(TN),(b)side view of the alkali metals decorated on MoSi2N4.The green,yellow,blue,and red spheres denote Mo,Si,N,and alkali metal atoms, respectively.
Table 2 displays the lattice parameters and bond lengths of alkali-metals-decorated MoSi2N4monolayer systems with four configurations.The lattice constant of the pristine MoSi2N4monolayer is 2.897 Å, which is consistent with previous reports [18].Furthermore, the lattice parameters of Li-,Na-and K-doped MoSi2N4are slightly bigger than that in pristine MoSi2N4monolayer.Still, the lattice parameters of alkali-metals-doped MoSi2N4do not increase with the tuning of the atomic number of alkali metal atoms.The bond lengthof Si-X (the bond length between Si and alkali metal atoms)or N-X (the bond length between N and alkali metal atoms)were shown in table 2.The results indicate that the bond lengths of Si-X or N-X increase as the atomic radius of alkali metal atoms increases.In addition, to study the bonding and interaction between the MoSi2N4monolayer, we calculated the average atomic charge populations of pristine and alkalimetal-decorated MoSi2N4, as shown in table 3.Due to the introduction of alkali metal atoms, the charge population of the MoSi2N4monolayer has changed.The results indicate that the charges of Mo and N vary slightly, but Si atoms gain electrons after the adsorption of alkali metals.There are∼0.99 |e|, 0.71 |e| and 0.84 |e| transfer from Li, Na and K atoms to Si atoms of MoSi2N4monolayer.

Table 1.The adsorption energy for alkali-metal-adsorbed MoSi2N4 monolayer with different sites.

Table 2.The lattice parameters and bond length of pristine and alkali-metal-adsorbed MoSi2N4 monolayers.The dSi−X (dN−X)means the bond length between Si(N) atom and alkali metal atoms.

Table 3.The average atomic charge populations of pristine and alkali-metal-adsorbed MoSi2N4 monolayers.
To explore the effect of alkali metal adsorption on the electronic structure of the MoSi2N4monolayer, we investigated the BS and DOS of pristine and alkali-metal-adsorbed MoSi2N4monolayers, as shown in figures 2 and 3, respectively.Figure 2(a)shows that the pristine MoSi2N4monolayer exhibits an indirect bandgap semiconductor structure since the maximum valence band(VBM)and minimum conduction band (CBM) are at two different k points.The calculated bandgap was 1.89 eV, which agrees with the values of experimental measurement(1.94 eV)in previous reports[14].

Figure 2.Band structures of pristine and alkali-metal-adsorbed MoSi2N4 monolayers.
The band structures of the alkali-metal-adsorbed MoSi2N4monolayer are shown in figures 2(b)–(d).The Li-,Na-,and K-adsorbed MoSi2N4monolayers exhibit an indirect bandgap,which is similar to the pristine MoSi2N4monolayer.Compared to the pristine system,the number of energy bands in the CB of the adsorbed systems increases.Besides,the CB of the doped systems shifts toward the low-energy region.Hence, the bandgaps of the doped systems are reduced.The corresponding bandgaps are 1.73 eV, 1.61 eV, and 1.75 eV for the Li-, Na-, and K-adsorbed MoSi2N4monolayers.The bandgap reduction demonstrates that the BS of the MoSi2N4monolayer can be regulated and modified by the adsorption of alkali metals.From the viewpoint of optical properties, less energy is required to complete the transition of electrons from the VB to the CB in an alkali-metal-adsorbed MoSi2N4monolayer, which means that the photocatalytic properties of the MoSi2N4monolayer will be improved after the introduction of alkali metals.Meanwhile, the optical absorption edge of the alkali-metal-adsorbed MoSi2N4monolayer will shift to the low-energy region compared with the pristine MoSi2N4monolayer.Comparing four different configurations, the smallest bandgap of Na-adsorbed MoSi2N4monolayer (1.61 eV) may imply the highest photocatalyst.
Furthermore, the influence of alkali metal dopants in higher concentrations on the BS of MoSi2N4monolayer,especially the effect on the bandgap, has been studied using the same method.The variation for the bandgaps as a function of the adsorbed alkali metal atoms is shown in figure 3(a).The results indicate that the bandgap significantly decreases as the number of doped alkali metal atoms increases.When an alkali metal atom is adsorbed onto the MoSi2N4monolayer,the Na atom adsorbed case shows a more obvious modulation effect.However,when three alkali metal atoms are doped,the corresponding bandgaps are 1.42(3Li), 1.46(3Na), and 1.47 eV(3K), respectively.The bandgap values of the 3Li-,3Na-, and 3K-adsorbed MoSi2N4monolayers are close,implying that the bandgap does not depend on dopant types when three alkali metal atoms are adsorbed onto the MoSi2N4monolayer.Interestingly, the bandgaps of the MoSi2N4monolayer decorated with Li dopants decreased almost linearly as the number of Li atoms increased.
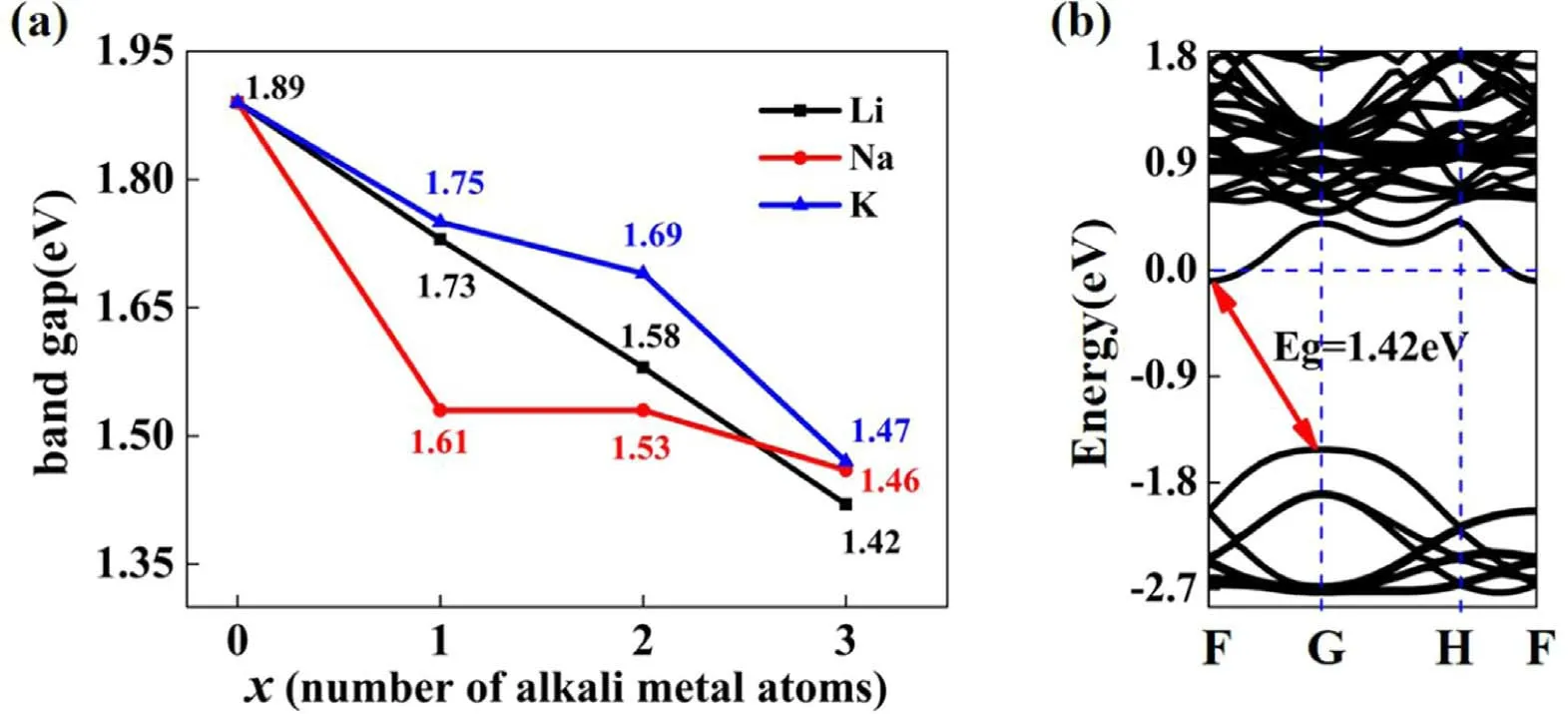
Figure 3.(a)Shows the bandgap variation as a function of the number of adsorbed alkali metal atoms.(b)Band structures of 3Li-adsorbed MoSi2N4 monolayer.
In addition, the BS of the 3Li-adsorbed MoSi2N4monolayer is calculated and shown in figure 3(b).The result demonstrates that the CBM moves down below the Fermi level, converting these 2D materials into an n-type semiconductor.Meanwhile, the upper VB of the 3Li-adsorbed MoSi2N4monolayer shifts toward the high energy range as a whole.The CBM and the VBM approach each other, resulting in a reduction in the bandgap.The reduction in the bandgap means that the photocatalytic activity of the MoSi2N4monolayer will improve as the alkali metal concentration increases.Because the band structures of 3Na(K)-adsorbed MoSi2N4monolayer are similar to that of 3Liadsorbed MoSi2N4monolayer,we just show the 3Li-adsorbed case as representative.
The total density of states(TDOS)and partial density of states(PDOS)of the pristine MoSi2N4monolayer are shown in figure 4(a).The lower VB (from −3.5 to −6 eV) was mainly contributed by the N 2p, while the upper VB (from−3.5 to 0 eV)was contributed primarily by the Mo 4d state.Besides,the CB originates from the hybridization of the Mo 4d and the Si 3p states.Figures 4(b)–(d)present the DOS of the adsorbed systems.The impurity bands of alkali metal s states locate above the Fermi energy, which is partially occupied with a bandwidth of ∼1.7 eV.Meanwhile, the s states of the alkali metals are hybridized with the N 2p, Si 3p,and Mo 4d states at 0.2 ∼1.9 eV,indicating the impurity state generated by the alkali metal mainly contributes to the CB.The impurity state is the reason for the increased number of energy bands in the CB.In addition, the alkali metal atoms interact with other atoms and resonate at ∼1 eV,which also proves that the alkali-metal-adsorbed MoSi2N4systems are stable.
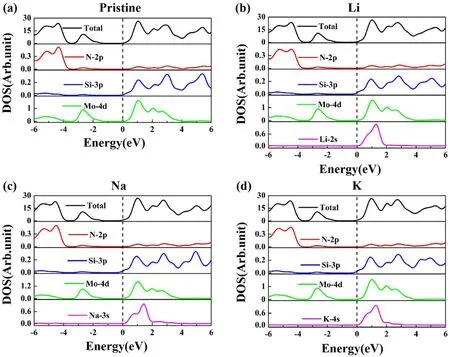
Figure 4.DOS of pristine and alkali-metal-adsorbed MoSi2N4 monolayers.
Furthermore, some occupied levels are introduced into the Si 3p states below the Fermi energy.In this case,the electronic in-band transition from the occupied bands to the unoccupied bands under irradiation may cause intense absorption in the long-wavelength visible region.These occupied levels introduced into the Si 3p states reduce the bandgap of alkali-metal-adsorbed MoSi2N4monolayer.Specifically, the alkali metal s state is above the Fermi level,so the introduction of the alkali metal s state does not directly cause the CB to move down.But the alkali metals lose electrons after adsorption, and the Si 3p state shifts to the low-energy region due to the electrons obtained by Si.In figures 3(a)–(d), it can be seen that the CBM of Si 3p states is from 0.15 eV (pristine) to −0.28(Li), −0.34(Na),and −0.32 eV(K), which causes the CB to move down and reduces the bandgap.
The work function [32, 33] is a critical factor to counterpoise the optoelectronic properties of materials.We investigate the work function variations of the surface in the following section.The work function (Φ) of materials can be defined as the minimum energy required to remove an electron from the surface.The variation in Φ is closely related to the change of surface conductivity,especially the single layer.Accurate calculation of Φ is also helpful in determining the direction of charge flow on the surface.Traditionally, the work function is defined as Φ [33]:

where EFand D were the Fermi level and the electronic potential at a vacuum region away from the surface,respectively.The decrease (increase) of work function can be induced by either a reduced(enhanced)surface dipole or a rising (lowering) of its intrinsic volume of Fermi energy.To investigate the influence of alkali metal adsorption on work function, we calculated the work function of pristine and adsorbed MoSi2N4monolayer systems.Figure 5(a)presents the work function of pristine MoSi2N4monolayer with the calculated work function was 4.80 eV.Figure 5(b)shows the variation of work function in the alkali-metaladsorbed MoSi2N4monolayer.The calculated work functions are 2.03 eV, 1.50 eV, and 1.43 eV for Na-, Li-, and K-adsorbed MoSi2N4monolayers.As can be seen, the work function values were significantly reduced following the decoration of the Li, Na, and K atoms.As a result, the work function of the MoSi2N4monolayer can be adjusted with different alkali metal absorption.Furthermore, the decreased work function reveals that the alkali-metaladsorbed MoSi2N4monolayer can be applied to fieldemission devices.

Figure 5.(a) Work function for pristine MoSi2N4, (b) the change of Φ for alkali-metal-adsorbed MoSi2N4 systems.
To verify our prediction for the influence of the modified electronic structure on the enhanced photocatalytic behavior,we computed the optical constants of alkali-metal-decorated MoSi2N4monolayer.Figure 6(a) displays the real part ε1(ω)of pristine and Li-,Na-,and K-decorated MoSi2N4monolayer systems.The pristine MoSi2N4monolayer exhibits a static dielectric constant ε1(0) of 3.8.For alkali-metal-adsorbed MoSi2N4monolayer, the ε1(0) are 13.9 (Li), 14.9 (Na) and 13.7(K)respectively.The result indicates that the ε1(0)of the doped systems are markedly larger than that of the pristine system.Hence, the optical properties of MoSi2N4monolayer are tunable and can be modified by decorated alkali metals.It is well known that the optical properties of the material are highly dependent on its electronic BS.It has also been discovered in the previous article [34, 35] that the dielectric constant increases as the energy gap of the material decreases.Therefore, the decoration of alkali metals reduced the bandgap, resulting in the increase of the dielectric constant.The high dielectric constant value will reduce the charge carrier recombination rate, thereby improving the photovoltaic performance of the solar cell.The imaginary part ε2(ω) for pristine and Li-, Na-, and K-decorated MoSi2N4monolayers was demonstrated in figure 6(b).For pristine MoSi2N4monolayer, three main peaks are located at 5.2 eV, 7.1 eV,and 13.2 eV.The peak at 5.2 eV originates from the electronic transition between the N 2p states in the lower VB and the Si 3p states in the CBM (figures 2 and 3).The electronic transition between Si 3p and Mo 4d states in the VB results in a peak at 6.50 eV.The weak peak at 13.2 eV is due to the electronic transition between N 2p and Si 3p states(not drawn in the electronic structure).For the alkali-metal-adsorbed MoSi2N4monolayer, the peak at 5.2 eV found in the pristine MoSi2N4monolayer disappeared.However, a new peak,which originates from the electronic transition between the Si 3p and s states of the alkali metal cations, arises at 0.72 eV after the introduction of Li,Na,and K atoms.In addition,in a higher energy range than around 7 eV, the spectral shapes of all the MoSi2N4monolayer systems were almost same.Thus,the different alkali metal dopants mainly influence the optical properties in the low-energy range.

Figure 6.(a) Shows the real part ε1(ω) and (b) imaginary part ε2(ω) of dielectric function for pristine and alkali-metal-adsorbed MoSi2N4 monolayers.
The absorption coefficients α(ω) for the pristine and Li-,Na-, and K-decorated MoSi2N4monolayers were calculated from the obtained ε1(ω) and ε2(ω).Figure 7(a) shows the absorption spectra of the MoSi2N4monolayer systems with four different configurations.For the pristine MoSi2N4monolayer, the absorption coefficient gradually decreases in the visible range (380 ∼780 nm).When the wavelength is greater than 780 nm, the α(ω) of the pristine MoSi2N4monolayer is almost 0, which means that the pristine MoSi2N4monolayer can hardly absorb infrared light.In contrast, the absorption coefficient numbers increase remarkably with the wavelength increase after alkali metals are adsorbed.The α(ω)at 380 nm are 0.34(pristine),0.57(Li),1.09(Na), and 0.89(K) (×105cm−1), while the α(ω) at 780 nm are 0.02(pristine), 0.92(Li), 1.34(Na), and 1.14(K)(×105cm−1), respectively.Compared to the three configurations of MoSi2N4monolayer systems, the Na-decorated MoSi2N4monolayer shows a stronger visible light absorption ability due to the largest absorption coefficient.The above results reveal that alkali metal adsorption is an effective method to influence the optical absorption capacity and photocatalytic activity of MoSi2N4monolayer because of the enhanced visible light absorption.

Figure 7.(a) Shows the optical absorption coefficient α(ω) as a function of wavelength for pristine and alkali-metal-adsorbed MoSi2N4.(b) Band alignments of MoSi2N4 monolayer for photocatalytic water splitting and carbon dioxide reduction.The band edges are given with respect to the NHE potential (volts).
The phenomenon of enhanced absorption is consistent with the electronic structure(shown in figure 2).The bandgap of the alkali-metal-doped MoSi2N4monolayer is smaller than that of the pristine MoSi2N4monolayer.Meanwhile, the Naadsorbed MoSi2N4monolayer with the smallest bandgap corresponds to the most significant optical absorption coefficient.Furthermore, the absorption edges of alkali-metaldoped MoSi2N4monolayer systems shift to lower energy(higher wavelength), and then the redshift occurs.Due to the sharp increase in infrared absorption, alkali-metal-adsorbed MoSi2N4monolayer systems can also be used for longwavelength optoelectronic devices, such as infrared lightemitting diodes and infrared detectors.
The photocatalytic properties [35, 36] of pristine and alkali-metal-decorated MoSi2N4monolayers for water splitting and CO2reduction were investigated.The relative positions of the band edges are one of the most vital criteria for the photocatalytic process, and the CBE (conduction band edge) must lie above the H+/H2reduction level, while the VBE (valence band edge) must lie below the O2/H2O oxidation level.The CBE and VBE can be calculated according to the following relations found in [37, 38]:
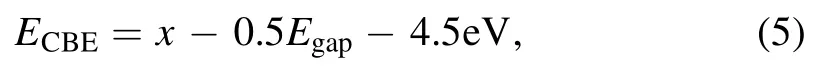
and
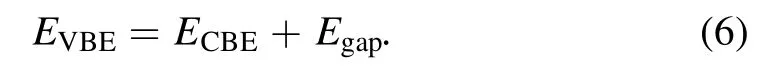
Where x [39] is the geometric average value of the electronegativity of the constituent atoms, 4.5 eV is the free energy of the electrons relative to the vacuum level.
Based on the above calculations, the redox potential of pristine and alkali-metal-adsorbed MoSi2N4monolayers for water splitting and CO2reductions relative to the NHE(normal hydrogen electrode) were calculated and are shown in figure 7(b).The result reveals that the pristine MoSi2N4monolayer can be used for photocatalytic water splitting.In addition, the EVBMand ECBMof alkali-metal(Li-, Na-, and K)-adsorbed MoSi2N4monolayer systems are more positive and negative than the oxidation level and the reduction level of water, respectively; hence, they are active for both the photo-oxidation and photo-reduction of water.By comparison,the EVBMof the K-decorated MoSi2N4monolayer is upshifted ∼0.15 eV over the pristine MoSi2N4monolayer,while the ECBMis almost similar to that of the pristine MoSi2N4monolayer.This indicates that the reduced ability of H+is maintained for the K-decorated MoSi2N4monolayer with an increased tendency to generate oxygen.On the other hand,the CBE and VBE positions of Na(Li)-adsorbed MoSi2N4monolayer systems approach the H+/H2reduction level and O2/H2O oxidation level, inducing Li- and Na-adsorbed MoSi2N4monolayers exhibit a higher potential tendency for water splitting.Based on the overall consideration of the redox potential and photon absorption coefficient,we believe that the Na-adsorbed MoSi2N4monolayer will show the best performance for photocatalytic water splitting.
Then, for the photocatalytic CO2reduction, the ECBMof the Na-adsorbed MoSi2N4monolayer lies below the CO2/HCOOH reduction level, which indicates that it cannot complete the process of reducing CO2to HCOOH.However,the Na-adsorbed MoSi2N4monolayer can reduce CO2to other substances like HCHO,CO,CH4,and CH3OH.Besides,the ECBMof both Li- and K-adsorbed MoSi2N4monolayer systems are more negative than the CO2/HCOOH reduction level.Thus, the electrons in ECBMof the Li(K)-adsorbed MoSi2N4monolayer have enough potential to transfer CO2to HCOOH, HCHO, CO, CH4, and CH3OH.Compared with Li and K dopants,the Li-adsorbed MoSi2N4monolayer shows a much better ability to reduce CO2.
Based on the calculated bandgap variation with the number of alkali metal atoms adsorbed onto the MoSi2N4monolayer(figure 3(a)),we computed the ECBMand EVBMfor 1∼3 homogeneous alkali metal atoms adsorbed onto the MoSi2N4monolayer, as shown in table 4.According to relations (5) and (6), found in [37, 38], for a fixed bandgap value, the positions of the band edges mainly depend on the constituent atoms’ average electronegativity of the material.The electronegativity of alkali metals decreases as the atomic number increases.According to previous articles [39], theelectronegativity of one Li, Na, and K atom is 2.58 eV,2.32 eV,and 1.92 eV,respectively.With the increase of alkali metal doping concentration, the electronegative gap among Li-, Na-, and K-adsorbed MoSi2N4monolayers increases.As a consequence, the band edges of K-adsorbed MoSi2N4monolayer become more negative than that of Li- and Naadsorbed MoSi2N4monolayers.It can be seen that the EVBMof the 3K-adsorbed MoSi2N4monolayer is more negative than the O2/H2O level, resulting in the oxidation process of water impossible.

Table 4.The x,Egap,ECBM,and EVBM(which are the variables in the calculation formulas of CBE and VBE) for pristine and 1∼3 homogeneous alkali metal atoms adsorbed onto MoSi2N4 monolayers.
However, the data also reveals that 1 ∼3 Li(Na)-adsorbed MoSi2N4monolayer can be used for photocatalytic water splitting.Besides, the CBE and VBE positions of the MoSi2N4monolayer adsorbed by different concentrations of Li(Na) atoms are closer to the H+/H2reduction level and O2/H2O oxidation level compared to the pristine MoSi2N4monolayer,respectively.Thus,Li and Na with higher doping concentrations can also improve the efficiency of photocatalytic water splitting.Interestingly,with the increase of the concentration of Li dopant, the photocatalytic efficiency increases.Conversely,for Na dopant,the reduction ability for water splitting increases with the increase of doping concentration, while the oxidation ability shows the opposite trend.Therefore, Li and Na’s adsorption plays a significant role in improving the photocatalytic efficiency of MoSi2N4monolayer, which will substantially expand the potential application of MoSi2N4monolayer in the field of photoelectrochemistry.
4.Conclusions
In this study, the structural, electronic, optical, and photocatalytic properties of alkali-metal(Li, Na, and K)-adsorbed MoSi2N4were investigated using first principles calculations.The negative adsorption energy reveals the stability of the alkali-metal-adsorbed MoSi2N4monolayer structure.The electronic structure analysis shows that the pristine MoSi2N4monolayer is an indirect bandgap semiconductor (bandgap is∼1.89 eV).By contrast, the bandgaps of the Li-, Na-, and K-adsorbed MoSi2N4monolayers are 1.73 eV, 1.61 eV, and 1.75 eV, respectively.Besides, the bandgap of the MoSi2N4monolayer significantly decreases as the number of doped alkali metal atoms increases.The bandgaps of 3Li-,3Na-,and 3K-adsorbed MoSi2N4turned to 1.42 eV, 1.46 eV, and 1.47 eV.
The work function of the MoSi2N4monolayer is 4.80 eV.It is noteworthy that the work function was significantly reduced due to the adsorption of alkali metals.One Li-, Na-,and K-adsorbed MoSi2N4monolayer systems display ultralow work functions of 1.50 eV, 1.43 eV, and 2.03 eV.Furthermore, the optical investigations indicate that alkali metal adsorption substantially increases the dielectric constant, and the visible light absorption range and coefficient of MoSi2N4are also increased.Through the photocatalytic study,Li-and Na-adsorbed MoSi2N4monolayers can be applied as a promising photocatalyst for water splitting due to enhanced photo-absorption ability and proper redox potential.While the Li-adsorbed MoSi2N4monolayer shows the best potential applications for photocatalytic CO2reduction.In conclusion,due to their unique physical and chemical properties, alkalimetal-adsorbed MoSi2N4monolayers are outstanding potential candidates for novel photocatalytic materials and optoelectronic devices.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant No.11 774 054,12075036) and the talents and high-level paper cultivation plan from the School of Optoelectronic Engineering, Yangtze University.
杂志排行
Communications in Theoretical Physics的其它文章
- Impact of Joule heating and multiple slips on a Maxwell nanofluid flow past a slendering surface
- Generating a dynamical M2 brane from super-gravitons in a pp-wave background
- Uncertainty relation of successive measurements based on Wigner–Yanase skew information
- Quantum uncertainty relations of Tsallis relative α entropy coherence based on MUBs
- Performance of passive decoy-state quantum key distribution with mismatched local detectors
- Quasi-exactly solvable decatic model description of nuclei near the X(5) critical point
