Recognition and Cell Imaging of Zn2+by Coumarin Derivative Fluorescence Sensor
2022-02-17YINJiQiuANYongLinJIAXianChaoJIAOYang
YIN Ji-Qiu AN Yong-Lin JIA Xian-Chao JIAO Yang*,
(1State Key Laboratory of Fine Chemicals,Dalian University of Technology,Dalian,Liaoning 116024,China)
(2College of Medical Laboratory,Dalian Medical University,Dalian,Liaoning 116044,China)
Abstract:Coumarin-derived fluorescence sensor was designed and synthesized to selectively recognize Zn2+ions.The structure and fluorescence properties of the sensor were investigated by NMR,MS,fluorescence spectroscopy,and other technical methods.Sensor CANQ exhibited conspicuous fluorescent enhancement response to Zn2+ion and had the characteristic of fast response,high selectivity,and good biocompatibility.It has been applied to the imaging of Zn2+ions in MCF-7 cells.
Keywords:Zn2+ion;coumarin derivative;fluorescent sensor;recognition
0 Introduction
Trace elements play an important physiological function in the life activities of the organism and they are involved in the synthesis of biological macromolecules such as enzymes,hormones,and vitamins[1-3].Zinc,which is an essential trace element in the body,is the second most abundant transition metal ion in the human body and the concentration of zinc in human plasma is above 10 µmol·L−1,its content in the human body is only less than iron[4-7].Zinc participates in physiological processes such as neurotransmitter transmission,gene expression,enzyme activity,and DNA synthesis,which plays a key role in a variety of biological processes[8-9].Zinc metabolism imbalance will cause a series of pathological changes and eventually lead to diseases and apoptosis,such as Alzheimer′s and Parkinson′s diseases[10-12].The decrease of enzyme function related to zinc and the regulation failure of biological signals are crucial factors in the occurrence and development of tumors[13].Moreover,zinc is also widely applied in industrial,large amounts of Zn2+ions enter the water and soil,which have adverse effects on aquatic organisms and soil microorganisms to lead environmental pollutants.Therefore,rapid and effective identification and detection of Zn2+ions are of great significance to the diagnosis of related diseases,environmental pollution monitoring,and other research fields.
Compared with traditional methods(inductively coupled plasma mass spectrometry,atomic absorption spectroscopy,and electrochemistry)for detecting Zn2+ions[14-17],the fluorescent detection method has advantages of simple operation,low cost,high sensitivity,specific selectivity,and real-time detection,etc.It is favored by researchers with the suitable characteristic for direct imaging and biosensors and is currently the relatively superior assay method for Zn2+ions in living cells[18-24].In recent years,fluorescent sensors for detecting Zn2+ions were widely reported based on different fluorophores,such as rhodamine,fluorescein,BODIPY(boron dipyrromethene),cyanine,oxazoline[25-31].It is one of the current research hotspots of developing fluorescence sensors with high sensitivity and high selectivity to monitor Zn2+in biological and environmental systems.
Coumarin is a kind of lactone compound with a benzo α-pyrone core,and coumarin derivatives have good fluorescence properties with a wide application as fluorescent dyes[32].Coumarin derivatives contain C=C,C=O,and lactone in their parent ring benzopyranes,which enhance the conjugation level and rigidity of the molecular system,and they have high fluorescence quantum yields,large stocks shifts,and good photochemical stability[33-37].Coumarin fluorophore is an ideal model for constructing fluorescent chemical sensors recognizing Zn2+ion[38-41]and coumarin ring can be modified by hydroxyl,alkoxy,isoprene,carbonyl,and other substituents.Coumarin derivatives are often used in the detection of common metal ions and small molecules,and the colorimetric and fluorescent sensor provides a highly accurate quantitative analysis[42-44].Coumarin derivatives have biological activities(anticancer,anti-oxidation,and inhibiting tyrosinase[45-47])and high biocompatibility,fluorescent sensors based on coumarin derivatives have a broad prospect in the detection of metal ions in a biological system.Furthermore,quinoxaline derivative is a heterocyclic compound containing one nitrogen atom and the synthesis methods of quinoxaline derivative is an efficient,simple,and green procedure.Acenaphthene quinone is usually used as raw material at a low cost.Quinoxaline derivative is of potent biological activities and fluorescence emission of quinoxaline derivative can be easily distinguished from the environment of cells,which has been widely used in tumor cell imaging[48-50].
Herein,a fluorescent sensor was designed based on coumarin derivative to realize the purpose of Zn2+ion detection.Coumarin aldehyde derivative reacted with the amino compound by forming a Schiff base to introduce quinoxaline derivatives into the design of sensor CANQ.The fluorescent sensor CANQ could selectively recognize Zn2+ion in CH3CH2OH/Tris-HCl solution and the fluorescence intensity of sensor CANQ gradually enhance with the adding of Zn2+ion.Sensor CANQ was applied to MCF-7 cell biological imaging,which verified the possibility of sensor CANQ imaging application.
1 Experimental
1.1 Materials and instruments
All chemical reagents were obtained from commercial sources and used without further purification except the solvents which were purified by classical methods.DMEM medium and fetal bovine serum(FBS)were purchased from Invitrogen.The reactions were monitored by TLC and were observed by a handheld UV analyzer(254 and 365 nm).1H NMR spectra were measured on a Bruker AVANCEⅢ400 MHz NMR spectrometer and Varian DLG 400 MHz NMR spectrometer.Mass spectrometry was carried out on a Thermo Scientific-LTQ Orbitrap XL spectrometer and UPLC-Q-TOF MS.The fluorescence spectra were recorded by the FS920 fluorescence spectrometer.The absorbances of the CCK-8 assay were measured by BIORAD xMark Microplate Spectrophotometer.Confocal imaging of cells was recorded with OLYMPUS FV1000 confocal microscopy.
1.2 Synthesis
The fluorescence sensor CANQ was synthesized by a five-step reaction.The detailed synthetic steps are shown in Scheme 1.
1.2.1 Synthesis of compound 1[51]
4-Diethylaminosalicylic aldehyde (1.93 g,10 mmol)and diethyl malonate(3.2 g,20 mmol)were mixed in an ethanol solution(30 mL).1 mL piperidine was added under stirring and the solution was refluxed for 6 h.After ethanol was removed by vacuum distillation,20 mL concentrated hydrochloric acid and 20 mL glacial acetic acid were added and stirred for 6 h.The reaction solution was cooled down to room temperature and was poured into 100 mL ice water.The pH value was adjusted to 5 with sodium hydroxide and the gray precipitation formed gradually.The solid was filtered and the crude product was recrystallized from toluene to obtain 1.70 g compound 1(Yield:78.3%).1H NMR(400 MHz,CDCl3):δ 7.53(d,J=9.3 Hz,1H),7.24(d,J=8.8 Hz,1H),6.56(dd,J=8.8,2.3 Hz,1H),6.48(d,J=2.1 Hz,1H),6.03(d,J=9.3Hz,1H),3.41(q,J=7.1 Hz,4H),1.21(t,J=7.1 Hz,6H).HRMS:m/z Calcd.for[C13H15NO2+H]+218.117 6;Obs.218.117 5.
1.2.2 Synthesis of compound 2[52]
POCl3(4 mL)was added to 4 mL N,N-dimethylformamide(DMF)dropwise in an ice bath and stirred for 30 min at room temperature under an argon atmosphere.3 g(13.8 mmol)compound 1 was dissolved into 20 mL DMF,and the solution was slowly added into the DMF solution containing POCl3.The reaction solution was stirred for 12 h at 60℃.Then the solution was poured into 200 mL ice water,and NaOH solution(20%)was added dropwise to adjust the pH value to 7.The obtained orange precipitate was filtered and washed with water several times.The crude product was dried,and was separated and purified by column chromatography.1.9 g compound 2 was obtained(Yield:56.2%).1H NMR(400 MHz,CDCl3):δ 10.11(s,1H),8.25(s,1H),7.41(d,J=9.0 Hz,1H),6.64(dd,J=9.0,2.5Hz,1H),6.48(d,J=2.3Hz,1H),3.48(q,J=7.1Hz,4H),1.26(t,J=7.1 Hz,6H).HRMS:m/z Calcd.for[C14H15NO3+H]+246.112 5;Obs.246.112 7.
1.2.3 Synthesis of compound 3[53]
Acenaphthylene-1,2-dione(182 mg,1.0 mmol)and methyl 3,4-diaminobenzoate(216 mg,1.3 mmol)were taken into a 100 mL round-bottom flask,and 50 mL glacial acetic acid was added to dissolve them.The solution was heated to 145℃and was refluxed for 3.5 h.After the reaction was cooled,the precipitate formed.The solid was filtered and the crude product was recrystallized from DMF.After most of the solids were separated,suction filtration was performed,and a small amount of methanol and ether was added for washing.158 mg compound 3 was obtained(Yield:50.6%).1H NMR(400 MHz,CDCl3):δ 8.83(d,J=1.8 Hz,1H),8.35(dd,J=6.9,2.3 Hz,2H),8.28(dd,J=8.6,1.9 Hz,1H),8.15(d,J=8.6 Hz,1H),8.08-8.04(m,2H),7.80(dd,J=8.1,7.2 Hz,2H),4.02(s,3H).HRMS:m/z Calcd.for[C20H12N2O2+H]+313.097 2;Obs.313.097 3.

Scheme 1 Synthesis route of CANQ
1.2.4 Synthesis of compound 4[54]
Compound 3(312 mg,1 mmol)was dissolved in 15 mL ethanol.1 mL 80% hydrazine hydrate was added dropwise and the mixture solution was stirred and refluxed for 12 h.After cooling and standing in an ice bath for 2 h,the mixture was filtered.The solid was washed with cold ethanol(20 mL×3),and was dried to obtain 187 mg compound 4(Yield:59.9%).1H NMR(400 MHz,DMSO-d6):δ 10.17(s,1H),8.68(d,J=1.5 Hz,1H),8.49(d,J=6.9 Hz,2H),8.36(dd,J=8.2,1.7 Hz,2H),8.28(d,J=8.5 Hz,1H),8.26-8.22(m,1H),8.02-7.96(m,2H),4.66(s,2H).HRMS:m/z Calcd.for[C19H12N4O+H]+313.108 4;Obs.313.107 1.
1.2.5 Synthesis of compound CANQ
Compound 2(245 mg,1 mmol)was dissolved in 50 mL ethanol and stirred,then compound 4(312 mg,1 mmol)was added,and 3-5 drops of glacial acetic acid were added.The reaction was refluxed for 12 h.After the reaction was completed,the solution was cooled to 0℃to form precipitate.After filtration,the solid was washed with cold ethanol(20 mL×3)and dried to obtain 432 mg CANQ(Yield:80.1%).1H NMR(400 MHz,DMSO-d6):δ 12.21(s,1H),8.84(s,1H),8.59(s,1H),8.48-8.43(m,2H),8.37(s,1H),8.34(d,J=8.3 Hz,2H),8.31(s,2H),8.01-7.93(m,2H),7.63(d,J=8.9 Hz,1H),6.73(d,J=8.8 Hz,1H),6.55(s,1H),3.53-3.46(m,4H),1.14(t,J=6.9 Hz,6H).HRMS:m/z Calcd.for[C33H25N5O3+Na]+562.185 0,Obs.562.183 2.
1.3 Fluorescence measurements
The sensor CANQ stock solution was prepared with dimethyl sulfoxide(DMSO)to a concentration of 1 mmol·L−1.The CANQ stock solution was diluted to obtain 5.0 µmol·L−1with CH3CH2OH/Tis-HCl(8∶2,V/V)solution(Tris-HCl:20 mmol·L−1,pH=7.4)and fluorescent spectrum scanning was performed.Perchlorate stock solutions(0.01 mol·L−1)of various metal ions(including the perchlorate of Pb2+,Na+,Mn2+,Ca2+,Mg2+,Ag+,Cr3+,Fe3+,Fe2+,Hg2+,K+,Cd2+,Al3+,Cu2+,Zn2+)were prepared with deionized water.The excitation wavelength was 468 nm,the emission wavelength was 522 nm,and the slit width was 2.5 nm.
1.4 Cytotoxicity assay
MCF-7 cells were seeded in 96 well plates.After the cells adhered to the wall,different concentrations(0,1,2,5,10,and 20 µmol·L−1)of sensor CANQ were added to the cell culture medium and incubated for 24 h in 5% volume fraction of CO2at 37℃.CCK-8 solution was added to each orifice and incubated on the culture plate for 1 h.The absorbance at 450 nm was determined by an enzyme marker and relative cell viability(fCV)was calculated using the equation by Graphpad prism soft:fCV=(As−Ab)/(Ac−Ab)×100%,where As,Ac,and Abrepresent the absorbance of sensor-treated cells,the absorbance of control cells,and the absorbance of the blank solution,respectively.
1.5 Cell culture and confocal imaging
MCF-7cells were cultured in a DMEM medium containing 10% FBS in a 5% volume fraction of CO2at 37℃.MCF-7 cells were inoculated into confocal dishes and cells were incubated in the culture medium.After the cells adhered to the wall,sensor CANQ and different concentrations of Zn2+ions were added to the cells and incubated.After the dishes were taken out,the medium was removed and washed three times with phosphate-buffered saline(PBS).The dishes were placed on the microscope to perform fluorescence confocal microscopic imaging to verify the practical properties of sensor CANQ in biological samples.
2 Results and discussion
2.1 Design and synthesis of sensor CANQ
The fluorescent sensor CANQ was designed by connecting 7-(N,N-diethylamino)coumarin-3-carbohydrazide and the unique properties of acenaphtho[1,2-b]quinoxaline-9-carbohydrazide.As shown in Scheme 1,sensor CANQ was synthesized by a simple Schiff base reaction,and the product was obtained as a yellow solid.The structure of intermediate and sensor CANQ was confirmed by1H NMR and MS spectra(Fig.S1-S10,Supporting information).
2.2 Optical response of CANQ toward Zn2+
The fluorescent spectra of sensor CANQ(5 µmol·L−1)were measured in a solution of CH3CH2OH/Tris-HCl(8∶2,V/V,pH=7.4)at an excitation wavelength of 468 nm.Sensor CANQ itself had a weak fluorescent signal initially and the fluorescent emission was gradually enhanced at 522 nm with adding Zn2+ion sequentially.As shown in Fig.1,the sensor had a sensitive response to Zn2+ions,and the fluorescence intensity titration curve reached a plateau after adding four equivalents Zn2+ions[55-56].To evaluate the binding ability of CANQ to Zn2+,the binding constant based on the fluorescence intensity data was calculated[57]to be 8.28×104L·mol−1,and some of the references recently published were taken to compare with this work(Table S1).
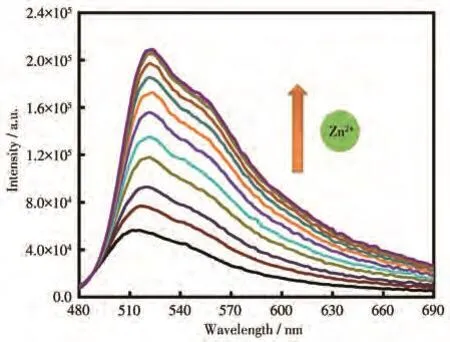
Fig.1 Fluorescence spectra of CANQ with the addition of various concentrations of Zn2+ion in CH3CH2OH/Tris-HCl(8∶2,V/V,pH=7.4)solution
Interference of other metal ions directly affects the recognition of Zn2+ions by the sensor in actual detection.So,the fluorescence response of sensor CANQ was monitored in the presence of 20 µmol·L−1various metal ions(Pb2+,Na+,Mn2+,Ca2+,Mg2+,Ag+,Cr3+,Fe3+,Fe2+,Hg2+,K+,Cd2+,Al3+,Cu2+,Zn2+)in a mixed solution of CH3CH2OH/Tris-HCl(8∶2,V/V,pH=7.4).As shown in Fig.2,the enhancement of the fluorescence signal with the addition of other metal ions was weak and the fluorescence signal decreased slightly with adding the copper(Ⅱ)ions.Sensor CANQ had considerable fluorescence enhancing with adding 20µmol·L−1Zn2+ion into the solution at 522 nm.These results reveal that sensor CANQ for detecting Zn2+ion is of high selectivity and Zn2+ion can be specifically identified without interference from other ions.
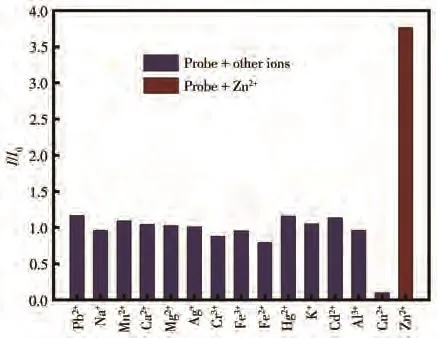
Fig.2 Fluorescence response of sensor CANQ in the presence of various cations(Mn+)in CH3CH2OH/Tris-HCl(8∶2,V/V,pH=7.4)
The good photostability of the fluorescent sensor is particularly important for the application of metal ions detection in the actual environment.As shown in Fig.3,the fluorescent signal of sensor CANQ was stable within 10 min.After adding 20 µmol·L−1Zn2+ion,the fluorescence signal of sensor CANQ enhanced fast,and the signal fluctuation was very weak within 10 min under room temperature.As illustrated in Fig.S11,in a wide pH range(from 4.5 to 9.4),the sensor CANQ had a significant fluorescence signal enhancement upon the addition of Zn2+ions.These results reveal that the sen-sor has a stable fluorescence response to Zn2+ion and it is conducive to the detection of Zn2+ion in the pH range of the physiological conditions.

Fig.3 Time-dependent fluorescent signal of sensor CANQ in the absence and presence of Zn2+ion
2.3 Response mechanism
The high-resolution mass spectrum of the sensor before and after the addition of Zn2+ions was recorded(Fig.4).The intense peak m/z at 540.203 9 can be assigned to[CANQ+H]+.The peak m/z at 758.149 4 was assigned to[CANQ−H+2DMSO+Zn]+upon addition of Zn2+ion,according to the comparison of the intense peak with the simulation-based on natural isotopic abundances(Inset of Fig.4).This result indicates that a 1∶1 stoichiometry complex was formed between Zn2+and sensor CANQ,which was well consistent with the results of Job′s plot(Fig.S12).This result can be inferred that sensor CANQ and Zn2+ion can form coordination compounds[58-59]and interaction between them may lead to fluorescence signal enhancement.
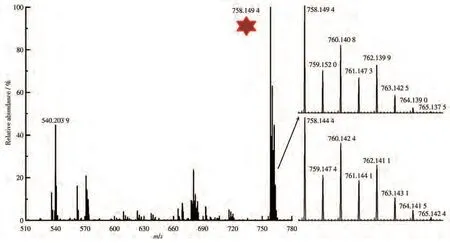
Fig.4 High-resolution mass spectrum of sensor CANQ in the presence of Zn2+ion
2.4 Cytotoxicity
Furthermore,biosafety is indispensable for fluorescent sensors and their cytotoxicity and biocompatibility must be fully demonstrated before actual application.MCF-7 cells were incubated with different concentrations of sensor CANQ for 24 h,and the cytotoxicity was measured by CCK-8 reagent.When sensor CANQ concentration reached 20 µmol·L−1,there wasn′t significant fluctuation in the cell viability compared to the control group(Fig.5).The results show that the synthesized sensor CANQ has very low cytotoxicity to cells and has excellent biocompatibility.
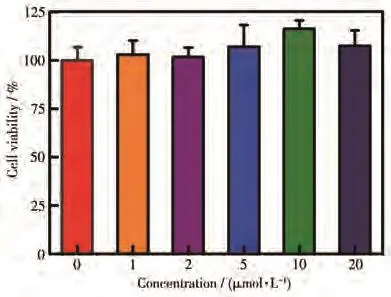
Fig.5 Relative cell viability of MCF-7 cells incubated with different concentrations of sensor CANQfor 24 h estimated by CCK-8 assay
2.5 Confocal imaging
The ability of sensor CANQ to monitor the concentration of Zn2+ions in living cells was determined,due to the fast and high selective fluorescence response.The MCF-7 cells were incubated with 5 µmol·L−1sensor CANQ and were added varying concentrations of Zn2+ion(0,5,10,and 20 µmol·L−1)for 20 min.After using the PBS to wash cells,fluorescence imaging experiments were performed with confocal laser scanning microscopy[60-61].As shown in Fig.6,the cells that were incubated with sensor CANQ alone exhibited weak fluorescence response under the fluorescence channel,and cells treated with Zn2+ion showed obvious fluorescence increasing.TPEN,which is a specific cellpermeable heavy metal chelator,effectively removed Zn2+ions in living MCF-7 cells incubated with Zn2+and sensor CANQ(Fig.S13),and the fluorescence signal of sensor CANQ decreased after adding TPEN.The results prove that sensor CANQ can be used for Zn2+ion imaging in living cells.
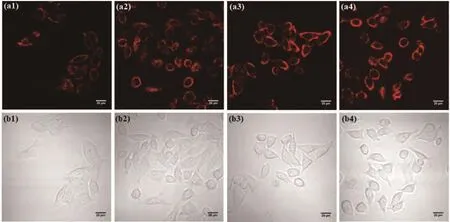
Fig.6 Fluorescence confocal microscopy images of MCF-7 cells incubated with sensor CANQ and Zn2+ion with different concentrations:(a1-a4)fluorescent images;(b1-b4)bright-field microscopic images
3 Conclusions
In conclusion,a novel fluorescent sensor CANQ based on coumarin derivative was designed and synthesized.CANQ was used to recognize Zn2+ions by fluorescence enhancement in a mixed solution of CH3CH2OH/Tris-HCl(8∶2,V/V,pH=7.4).The fluorescent emission was significantly increased at 522 nm after adding Zn2+ions.Sensor CANQ can selectively detect Zn2+among various relevant metal ions and has good photostability.Sensor CANQ also has excellent biocompatibility,and it has been successfully applied to fluorescence confocal microscopic imaging of MCF-7 cells,which provides reference experience for the detection of Zn2+ions in biological analysis.
Acknowledgments:We acknowledge SI Wen,GAO Xu,ZUO Ying-Ying for their aid in this work.
Supporting information is available at http://www.wjhxxb.cn
杂志排行
无机化学学报的其它文章
- One⁃Pot Aqueous Synthesis and Cell Labeling Application of Glutathione Capped Cu⁃In⁃Zn⁃S Quantum Dots
- Preparation and Characterization of Copper Complexes of Schiff Base Ligands Synthesized In Situ from Spiropyran Derivative
- Syntheses and Luminescence of Three Zinc Complexes Constructed by Rigid 4⁃Substituted Bis(1,2,4⁃triazole)Ligand
- 不同晶型MnO2纳米阵列的可控合成及其电催化析氧性能
- 三维花状Bi2WO6/BiOBr异质结的制备及对多种染料的降解性能
- 单分散SiO2纳米颗粒复合凝胶电解质的应用
