两种双核稀土配合物的光致变色和光磁效应
2022-01-24李港美韩松德王国明
徐 斐,李港美,韩松德,王国明
(青岛大学化学化工学院,青岛 266071)
1 Introduction
Photochromic materials not only exhibit apparent color change,but also accompany the variation of chemo-physical properties[1,2].Among the various investigated photomodulated functionality,photomagnetism features potentiality in many fields like information storage,medical service,etc.[3,4].Although assorted achievements have been made in expanding the available family of photomagnetic materials and understanding the photoresponsive mechanism[5,6],the generation of desirable photomagnetic materials,particularly those with improved photomagnetic temperature[7—10],is still a huge challenge to scholars.
One of the feasible strategies to enhance the photomagnetic temperature is to utilize the photogenerated radical at ambient conditions[10,11].The presence of photogenerated radical could enrich the paths of magnetic exchange interactions,resulting in diverse captivating magnetic performance in terms of tuning the magnetic intensity and changing the way of magnetic coupling.It has been demonstrated that the hybrid photochromic materials based on photoinduced electron transfer(ET)are promising candidates to quest materials with photomagnetism at room temperature[10—18].Therefore,the key is to construct suitable donor-acceptor system to obtain phototriggered radicals driven by ET.
Hitherto,researchers mainly concentrate on two effective strategies to construct photomagnetic materials based on ET mechanism in photochromic materials system.The most widely explored one is to link paramagnetic metal ions or building units with photoactive ligands like pyridinium-derivatives under suitable conditions[19—21].The resultant hybrid structure with intrinsic electron donor-electron acceptor(ED-EA)characteristic may present ET-triggered photochromism and photomagnetism.The modern organic synthesis provides possibilities to obtain various photoactive units to generate targeted photochromic materials[22],which provides good possibilities to construct ET-induced photochromic materials with photomagnetic performance.Another strategy is to assembly suitable nonphotochromic EDs-and EAs-moieties to yield complexes bearing ED-EA structure and expected photochromic and photomagnetic functionality,with examples of photochromic complexes driven by nonphotochromic rigid triangular tripyridine-derivatives[14—17].The kernel of this strategy is to develop suitable EAs.It has been demonstrated that the polypyridine-derivatives are prospective EAs[23—32].It is well known that the good conjugation of the polypyridine-based EAs is helpful to stabilize the photogenerated radicals at room temperature.Compared with the broadly investigated polypyridines with twisting angles between adjacent pyridine units[23—34],the coplanar 1,10-phenanthroline(phen)may be a desirable EA to better stabilize the photogenerated radicals.
Herein,we utilize the photoinduced ET strategy to produce photochromic and photomagnetic hybrid materialsviagrafting organic phen to inorganic and paramagnetic lanthanide nitrate system according to the following considerations(Fig.1):(1)the lanthanide ions are promising units for fabricating molecule-based materials bearing captivating magnetic or optical functionality[35—37];(2)the inorganic nitrate groups(NO3-)as the familiar counter-ion in lanthanide salts are potential EDs;(3)the coplanar phen moieties are preferable EAs[38].As anticipated,the resultant dinuclear complexes[Ln2(NO3)6(phen)2](Ln=Gd for 1 and Dy for 2)with ED-EA structure exhibit photochromic and photomagnetic performance.Their structures and photoresponsive properties will be discussed in the following sections.
Fig.1 Generation of photochromic and photomagnetic hybrid materials based on the assembly of lanthanide nitrate and phen
2 Experimental
2.1 Reagents and Instruments
The starting materials lanthanide nitrate,phen andN,N-dimethylacetamide(DMA)were used as received.The detailed information for instruments are given in the Supporting Information of this paper.
2.2 Synthesis of[Ln2(NO3)6(phen)2]
The starting materials,Ln(NO3)3·6H2O[Gd(NO3)3·6H2O(0.0600 g,0.133 mmol)/Dy(NO3)3·6H2O(0.0600 g,0.131 mmol)],phen(0.100 g,0.555 mmol)and DMA(5 mL),were placed into a Teflon-lined autoclave(25 mL).After stirring for 20 min,the reactants were heated at 145°C for 3 d and then naturally cooled to room temperature(RT).The output isca.40%based on phen.Elemental analysis(%)calculated for compound 1(C24H16Gd2N10O18,Mr=1046.94):C 27.53,H 1.54,N 13.38;experimental:C 27.36,H 1.82,N 13.15.IR,ν͂/cm-1:3418(m),2983(w),2358(w),1602(s),1430(s),1096(m),837(m),723(m),665(m).Elemental analysis(%)calculated for compound 2(C24H16Dy2N10O18,Mr=1057.44):C 27.26,H 1.53,N 13.25;experimental:C 27.47,H 1.88,N 13.53.IR,ν͂/cm-1:3418(m),2983(w),1602(s),1430(s),1202(w),1030(m),837(m),723(m),665(m).
3 Results and Discussion
3.1 Structural Description
Considering their isostructural characteristics,only the structure of compound 1 is depicted in detail.The single-crystal X-ray diffraction(SCXRD)analysis show that the space group of compound 1 isP-1(Table S1,see the Supporting Information of this paper).There are one Gd3+ion,three nitrate(NO3-)groups,and one neutral phen ligand in the asymmetric unit.The nitrate groups exhibit bridging and chelating modes to coordinate with metal ions.Two pairs of symmetry-related nitrate group with theη1:η1:η0:μ2-andη2:η1:η0:μ2-bridging modes,together with one pair of symmetry-related nitrate with bidentate chelating mode,connect two Gd3+ions to form dinuclear[Gd2(NO3)6]unit,which is further decorated by chelating phen to form hybrid discrete structure with the chemical formula of[Gd2(NO3)6(phen)2][Fig.2(A)].The Ln3+ions feature the[LnO7N2]muffin geometry evidenced by SHAPE analysis(Table S2,see the Supporting Information of this paper).The distance of Ln—O(dLn-O)is in the range of 0.2347(7)—0.2579(8)nm for compound 1 and 0.2331(5)—0.2552(6)nm for compound 2 and thedLn—Nis in the range of 0.2566(10)—0.2641(9)nm for compound 1 and 0.2550(7)—0.2613(7)nm for compound 2(Tables S3 and S4,see the Supporting Information of this paper).The bond angles around Ln3+ions lie in 51.3(2)°—150.5(3)°for compound 1 and 51.47(18)°—149.5(2)°for compound 2(Tables S3 and S4).Notably,the neighboring Ln2species connect with each otherviaweak hydrogen bonds(C11—H11…O1)to form supramolecular chains[Fig.2(B)],and weakπ…πstacking interactions were observed between phen units of adjacent Ln2units with centroid-to-centroid distance of 0.37696(6)and 0.37915(4)nm for compounds 1 and 2,respectively(Fig.S1,see the Supporting Information of this paper).Supramolecular packing structure[Fig.2(C)and(D)]are formedviathe hydrogen bonds andπ…πstacking interactions.Thermogravimetic analysis(TGA)was performed to assess their thermal stability(Fig.S2,see the Supporting Information of this paper).Compounds 1 and 2 could be stable up toca.300°C.Further heating leads to apparent mass loss because of the combustion of phenligands.
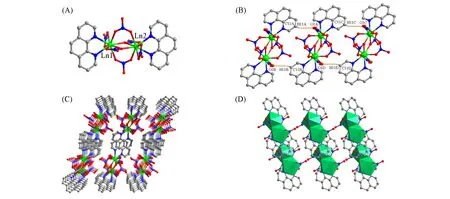
Fig.2 D iscrete dinuclear lanthanide complex(A),hydrogen⁃bond of adjacent dinuclear complex(B),ball⁃stick(C)and polyhedron(D)views of packing mode of the dinuclear structure
3.2 Photochromism and Photomagnetism
The ED-EA structural characteristic,together with the paramagnetic nature of Ln3+ion,make compounds 1 and 2 promising candidates for investigating the photochromic and photomagnetic functionality.To study the potential photoresponsive functionality,the samples of compound s 1 and 2 were irradiated by Xe-lamp.As expected,obvious coloration from colorless to pale gray for compound 1 and brown for compound 2 was observed after photoactivation for 6 and 8 min,respectively[Insets of Fig.3(A)and(B)].Besides the coloration,the time-dependent UV-Vis spectra of compounds 1 and 2 exhibit apparent absorption in the range of 360—500 nm[Fig.3(A)and(B)],indicating the appearance of phen-radicals.Their fluorescent emission plots also show time-dependent behavior.For compound 1,the broad peak around 370 nm is assigned to the phen-emission[Fig.3(C)].After photoirradiation,its fluorescent intensity gradually decreases because of the increasing amount of photogenerated radicals.For compound 2,the peak with wide shoulder around 370 nm and the sharp peak at 480 nm are assigned to the emission of phen and characteristic emission of Dy3+ion,respectively[Fig.3(D)].Thanks to the enhanced number of photogenerated radicals,the intensity at 370 nm gradually decreases after photoirradiation.Because of the overlapping between the emission of Dy3+ions and the absorption of phen-radicals,the emission magnitude of Dy3+ions progressively declines.It is well known that the sensitization of ligand plays a paramount role in the fluorescent emission of Dy3+ions.The reduced sensitization effect originated from the growing amount of photoinduced radicals is also responsible for the diminished emission intensity of Dy3+ion.The existence of phen-radicals for compounds 1 and 2 was also well certificated by comparing the EPR plots of initial samples and colored samples(Fig.S3,see the Supporting Information of this paper).Notably,the photoactivated samples could maintain their color for 6 d,implying the good stability of photogenerated radicals.In compounds 1 and 2,the coplanar phen and nitrate become EAs and EDs,respectively.The nearest distances between Nphen(EA-atom)and Onitrate(ED-atom)around[LnO7N2]are 0.2866 and 0.2912 nm for compound 1 and 0.2845 and 0.2888 nm for compound 2,which are favourable for ET and the formation of photogenerated radicals under photo-stimulus[23—32,39].After photoactivation,the energy absorbed by phen is transmitted to nitrate,resulting in the ET from Onitrateto Nphenand the resultant photocoloration.During the ET process,partial neutral phen has transformed to anionic radical,and partial anionic nitrate changed to neutral radical.However,the total valance of[Ln2(NO3)6(phen)2]does not change.The agreement of PXRD patterns of the photoactivated samples and the simulated ones(Fig.S4,see the Supporting Information of this paper),together with the accordance of IR curves of the initial samples and photoactivated samples(Fig.S5,see the Supporting Information of this paper),well demonstrate the structural stability after photoirradiation.Notably,annealing the colored samples of compound 1 and compound 2 at 145°C for 5 h could not finish the completed fading.The uncompleted fading process was also proved by the fluorescent and UV-Vis spectra of the decolored samples of compounds 1 and 2(Figs.S6 and S7,see the Supporting Information of this paper).

Fig.3 Time⁃variable UV⁃Vis and fluorescent spectra for compounds 1(A,C)and 2(B,D)and theχM T⁃T plots of compounds 1(E)and 2(F)before and after photoirradiation
Considering the paramagnetic nature of Ln3+ion and the potential magnetic exchange interactions between photogenerated radicals and Ln3+,we investigated the photomagnetism of compounds 1 and 2.To guarantee the completed ET process,the photoactivation time for the corresponding crystalline samples(1P and 2P)was enlarged to 120 min.The mole magnetic susceptibility(χM)in the range of 3—300 K was investigated under magnetic field of 0.1 T.For compound 1,the experimental value ofχMTat 300 K is 15.79 cm3·mol-1·K[Fig.3(E)],which is in line with the theoretical one(15.75 cm3·mol-1·K)of two non-interacting Gd3+ions with8S7/2andg=2[40].During cooling,theχMTproducts drops slowly from 300 K to 20 K.Below 20 K,a fast decrease is detectable with the minimum value of 12.06 cm3·mol-1·K at 3 K.The decreasing trend of theχMT vs.Tplot manifests that neighboring Gd3+ions possess antiferromagnetic interaction.After photoirradiation,theχMTproducts of 1P at 300 K is 16.39 cm3·mol-1·K due to the presence of paramagnetic photoinduced radicals.Upon cooling,theχMTvalues of 1P decrease gradually in the range of 300—20 K,accompanied by the quick decrease in the range of 20—3 K.For compound 2,the measuredχMTproduct of 28.40 cm3·mol-1·K at 300 K agrees with the theoretical one(28.33 cm3·mol-1·K)for two isolated Dy3+ions with6H15/2andg=4/3[Fig.3(F)][35—37,40].Upon cooling,theχMTproducts progressively decline in the range of 300—55 K due to the thermo-depopulation of the Stark sublevels of Dy3+ions.Fast fall ofχMTvalues is detectable from 55 K to 3 K.After photoirradiation,theχMTvalue of 2P increases to 30.55 cm3·mol-1·K at 300 K due to the presence of photoinduced radicals.TheχMTvalues of 2P decrease slowly from 300 K to 55 K,followed by fast decrease in the range of 55—3 K.
The shape ofχMT⁃Tplots,together with the magnitude change ofχMTproducts,implies the very weak magnetic coupling between Ln3+and photogenerated radicals,which may be due to the shielding effect of 4forbitals,low quantity of photogenerated radicals,and weak magnetic coupling transmitted by nitrate.The changes ofχMTvalues for compounds 1 and 2 at high temperature regions well affirm the photomagnetic properties derived from photogenerated radicals.Notably,compounds 1 and 2 exhibit photomagnetism around RT,which is distinct from the broadly explored metallocyanate compounds with photomagnetic property at low temperature[41,42].To investigate the potential single-molecule magnet(SMM)behavior of compound 2 after illumination,we conducted the alternating current magnetic susceptibility characterizations.However,the absence of the signal for slow magnetic relaxation excludes the possibilities of SMM behavior after irradiation(Fig.S8,see the Supporting Information of this paper).
4 Conclusions
The combination of phen and lanthanide nitrate generates two hybrid lanthanide nitrates with dinuclear structure,[Ln2(NO3)6(phen)2](Ln=Gd for compound 1 and Dy for compound 2).Because of their donor-acceptor structural characteristics,compounds 1 and 2 feature photochromic performance driven by electron transfer from electron-donating nitrate to electron-accepting phen.Besides the photochromism,photomagnetic properties are also observed at room temperature.Considering the availability of paramagnetic metal nitrate and phen or phen-derivatives,this study offers a general method to synthesize hybrid materials with photochromic and photomagnetic performanceviacoordination-driven assembly of paramagnetic metal salt with phen-derivatives.
The supporting information of this paper see http://www.cjcu.jlu.edu.cn/CN/10.7503/cjcu20210337.
CCDC Numbers 2074404 and 2074405 contain the full crystallographic data of compounds 1 and 2,respectively.
