Treatment of pediatric mild persistent asthma with low-dose budesonide inhalation suspension vs. montelukast in China
2022-01-12inChenDeYuZhaoLiXiangJianGuoHong
in Chen 1 · De-Yu Zhao 2 · Li Xiang 3 · Jian-Guo Hong 4
Abstract
Keywords Allergic rhinitis · Asthma · Budesonide · Child · Montelukast
Introduction
Asthma is a common chronic airway disease in children that is characterized by variable symptoms of wheezing,shortness of breath, chest tightness, and/or cough [ 1]. Globally, the prevalence of asthma reaches 11 and 14% in children aged 6-7 and 13-14 years in 2007, respectively [ 2].The prevalence of childhood asthma has been increasing in China in recent years. Two national epidemiological surveys, conducted among children aged 0-14 years in 2000 and 2010 by the Childhood Asthma Collaborative Group of China, indicated that the cumulative prevalence of asthma in urban children in China increased from 1.97% in 2000 to 3.02% in 2010 [ 3, 4]. The pooled current prevalence of childhood asthma in the mainland of China in 1990,1991-2000, 2001-2010, and 2011 were 0.959, 1.914, 2.600,and 4.043%, respectively, showing a remarkably increasing trend [ 5].
Asthma may lead to airway remodeling and a progressive loss oflung function if not treated effectively [ 6]. Although a number of safe and efficacious asthma controller agents are available for childhood asthma, the management of asthma in children also could be challenging because children may lack adherence and/or the successful operation of inhalers[ 7- 10]. Current guidelines, including those from the Global Initiative for Asthma (GINA) and the Chinese Medical Association, recommend inhaled corticosteroids (ICSs) as the preferred treatment for childhood persistent asthma [ 1,11]. In previous meta-analyses comparing montelukast vs.ICS agents, ICS therapy was found to be generally more effective than montelukast in mild-to-moderate persistent asthmatic children under controlled clinical conditions [ 12,13]. However, the results from other comparative studies have shown that montelukast is comparable with budesonide inhalation suspension (BIS), a widely used ICS with a favorable therapeutic ratio, in the treatment of mild persistent asthma in children over a 1-year period and over a 12-week period, respectively [ 14, 15]. In fact, there are limited studies comparing BIS vs. montelukast in real-world settings where treatment adherence and persistency remain suboptimal. Therefore, the purpose of this study was to evaluate the effectiveness of montelukast vs. BIS as monotherapy in the management of childhood mild persistent asthma in real-world settings in China, thus providing pediatricians with real-world experiences in the daily clinical practice.
Methods
Data sources
This cross-sectional observational study of asthma control was a retrospective questionnaire-based analysis of 4223 patients, aged 2-16 years, with mild persistent asthma from 42 tertiary hospitals across all regions of the mainland of China [ 16]. The indicators of asthma control and costs in 393 pediatric patients, aged 2-14 years, with mild persistent asthma (as defined by requiring GINA step 1 or 2 treatments) and who were on montelukast or low-dose BIS were compared using this database [ 1].
The inclusion criteria were as follows: (1) age ≥ 2 years old, both male and female; (2) diagnosed with asthma ≥ 3 months, having symptoms of asthma (wheeze or cough) or having used asthma medication within the last 12 months; (3) Informed consent from both the guardian and those who were aged 8 years old and above. The exclusion criteria were as follows: (1) diagnosed with bronchiolitis, cystic fibrosis, chronic lung diseases or pneumonia; (2)could not give an informed consent due to cognitive impairment or other reasons.
Data of patients were collected from medical charts,including disease and treatment duration. Children were diagnosed with asthma according to the 2008 edition of the Chinese national guidelines “Guidelines for the Prevention and Treatment of Bronchial Asthma” and the “Guidelines for the Diagnosis and Prevention of Bronchial Asthma in Children”. Cough-variant asthma was diagnosed according to the 2009 edition of “Guidelines for the Diagnosis and Treatment of Cough”. Diagnosis was made based on clinical history, pulmonary functions tests or airway responsiveness tests [ 17].
This study was approved by the Ethics Committee of the Beijing Children’s Hospital, Capital Medical University(approval number: 2012-70). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.Written informed consent was obtained from all individual participant’s guardians included in the study.
Study variables
Sociodemographic and clinical data, including family history of allergic diseases, concomitant diseases, asthma symptoms, duration and treatment, were collected from medical charts. In addition, asthma related events in the previous four weeks (e.g., frequency, limitation on activity,sleeping disturbance, and use of reliever), and the extent of limitation of physical activities during the past 12 months were collected through self-reporting by children or their legal guardians. Pulmonary function data, including peak expiratory flow (PEF) and forced expiratory volume in one second (FEV1), were collected.
Rhinitis was defined as a heterogeneous disorder with runny nose itching, sneezing and/or nasal congestion, occurring during multiple several consecutive days for more than 1 hour on most days and not caused by a cold or the flu[ 18]. Allergic rhinitis was defined as IgE-mediated rhinitis confirmed by a serum-specific IgE or skin prick test [ 18].
Asthma controller adherence was assessed by measuring refill rate for prescriptions in the past 3 months and was categorized into four groups: complete adherence, good adherence, poor adherence, and nonadherence (based on rate of refills: > 90, 70-89, 50-69, < 50%, respectively). The reasons for nonadherence were asked and recorded.
The level of asthma control was evaluated by the 5-item asthma control test (ACT) for children aged 12 years old and up [ 17, 19, 20] and 7-item Childhood Asthma Control Test(C-ACT) for those aged 4-11 years [ 21, 22], respectively.Both Chinese versions of questionnaires have been validated in other studies [ 19, 22]. The asthma was considered as controlled if ACT or C-CAT > 19 and uncontrolled if ≤ 19. In addition, the level of asthma control was assessed by the GINA criteria for children aged 2-3 years, which was classified into controlled, partly controlled and uncontrolled [ 1].
Statistical analysis
For statistical description, continuous variables were presented by the mean and standard deviation (SD), or median and first and third quartiles (Q1 and Q3). Categorical variables were described by the frequency and percentage. The unpaired t test was used to compare continuous variables that were normally distributed, Mann-WhitneyUtest was used to compare continuous variables that are not normally distributed, and the chi-squared test was used to compare categorical variables. Differences between treatment groups were considered significant ifP< 0.05. All statistical analysis was performed using the software SPSS 16.0 (Chicago,IL, USA).
Results
Subjects
A total of 393 children, aged 2-14 years, with mild persistent asthma were enrolled in this study. The subjects treated with montelukast were comparable to those treated with BIS with respect to their age, gender, weight, height, education level of their parents or caregivers, smoking habits of their parents or caregivers, and number of first-degree relatives with asthma (Table 1). The subjects in both groups also had a similar asthma duration since diagnosis and treatment duration. The BIS group had higher percentages of children with a history of eczema (P= 0.009) and predicted FEV1 < 80% (P= 0.028), compared with the montelukast group. However, PEF was not statistically different between groups (P= 0.160). In addition, there were no significant differences in the major reasons for the outpatient visit between groups (P= 0.370).
The past 3-month period, 60.42% of the patients in the montelukast group and 56.21% of the patients in the BIS group (P= 0.042) had ≥ 90% compliance. Meanwhile, 15%of the patients in the montelukast group and 25.49% of the patients in the BIS group had < 70% compliance (Table 1).
Patient-reported outcomes and asthma control assessment
The montelukast group exhibited better asthma control in the past 4-week period, including lower percentages of children who had asthma symptoms more than twice a week(P= 0.021), had night waking or night coughing due to asthma (P= 0.022), or required reliever medication more than twice a week (P< 0.001). Asthma control was similar in the montelukast and BIS groups for indicators, which included experienced asthma symptoms and activity limitation due to asthma (Table 2).Compared with the BIS group, the montelukast group had a lower percentage of children with an ACT/C-ACT score ≤ 19 (P= 0.015). Moreover, the caregivers reported a significantly better exercise tolerance in their children who received montelukast vs. those who received low-dose BIS in the past 12 months (P< 0.001).
Asthma-related medical costs
Caregivers reported that medical expenditures attributable to asthma were significantly higher for those children who received low-dose BIS vs. those who received montelukast in the past 12 months [4000 (2000, 7000) yuan/RMB vs. 5000(3000, 10,000) yuan/RMB] (P= 0.001, Fig. 1.). In addition,caregivers were more likely to spend ≥ 10,000 RMB if the children were prescribed with BIS rather than montelukast.In contrast, more than half of caregivers (53.6%) caring for children prescribed with montelukast spent < 5000 RMB.
Allergic rhinitis comorbidity-related medications
A higher proportion of asthma patients with concomitant allergic rhinitis, with a similar severity as defined by the Allergic Rhinitis and its Impact on Asthma classification,was found in the montelukast group (121, 43.33%) vs. the BIS group (60, 39.21%) (Table 3). In patients with allergic rhinitis comorbid with asthma, 89 out of 121 (73.6%)and 26 out of 60 (43.3%) were on allergic rhinitis medication in the montelukast group and in the BIS group,respectively (P= 0.355). A unified treatment strategy for asthma and allergic rhinitis was adopted by 47.22% of children in the montelukast group (Table 3). Differences were found between the montelukast and BIS groups in terms of their choices for allergic rhinitis medication, such as oral H1-receptor blockers, nasal steroids, Chinese patent drugs,vasoconstrictors, and others (P= 0.008, Table 3).
Discussion
This retrospective questionnaire-based analysis indicated that both montelukast and BIS provided acceptable asthma symptom control in children aged 2-14 years with mild persistent asthma. In real-world settings, compared with montelukast, children are more commonly prescribed with BIS if they have a history of eczema or a predicted FEV1 < 80%.However, children exhibited a better compliance when usingmontelukast. Better outcomes were observed for asthma control at four weeks and caregiver-reported exercise tolerance in the past 12 months for the montelukast group compared with the BIS group. Also, montelukast was associated with lower medical expenditures attributable to asthma. Thus, this study revealed the real-world treatment of pediatric mild persistent asthma patients with low-dose BIS vs. montelukast in China, providing useful information for clinical choice.
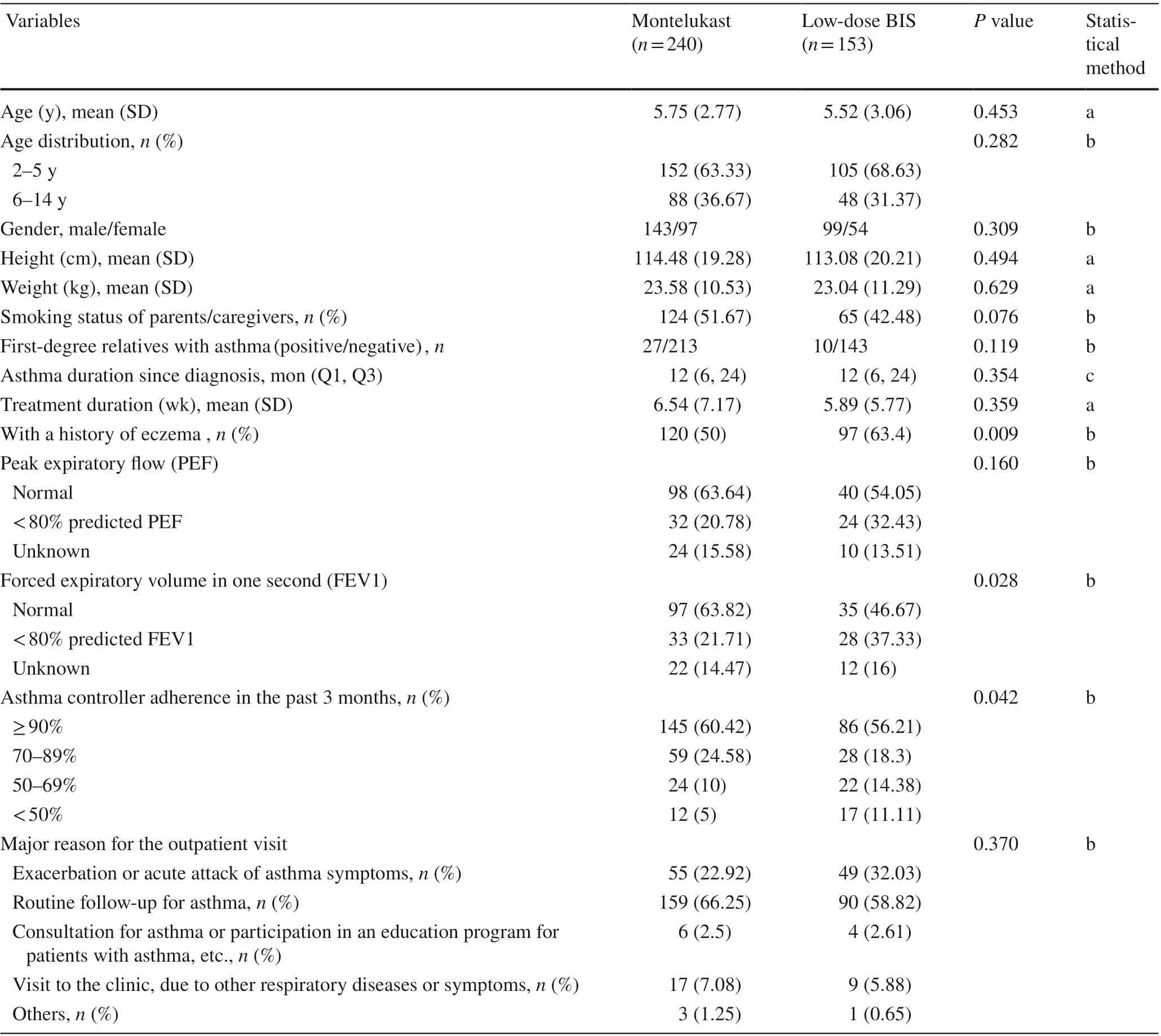
Table 1 Baseline demographics and asthma characteristics
In contrast to our findings, previous studies have reported better outcomes for asthma control with an ICS compared with montelukast [ 23- 25]. This inconsistency may be explained by two factors. First, this study included observational, real-world data in a general population of children with mild persistent asthma, which provides more representative patterns of medication use and asthma control in real life than clinical trial populations. Better adherence to therapy with montelukast than BIS may have partially contributed to the observed outcomes. The higher compliance to montelukast may be due to the convenience of oral administration and the absence of corticosteroids exposure[ 26]. Thus, it is suggested that BIS compliance should be strengthened in clinical practice. Second, disease severity at study entry might be different between the two treatment groups, as the BIS group contained higher percentages of children with a history of eczema and a predicted FEV1 < 80%, compared with the montelukast group. It is also possible that because of the variability of the disease itself, in some patients, asthma may have improvedspontaneously over time. Particularly, subjects included in this study on the basis of asthma exacerbation (montelukast:22.92% vs. BIS: 32.03%,P= 0.37).

Table 2 Asthma symptom control assessment in the past 4 weeks and in the past 12 months
Asthma management includes long-term treatment for asthma control as well as short-term treatment for asthma exacerbations. Thus, pediatric asthma should be considered as a life-course respiratory disease by healthcare workers and their families [ 27]. A high percentage of asthmatics present atopic sensitization and comorbidities [ 28, 29]. In this study, a significantly lower percentage of allergic rhinitis comorbid with asthma was observed in the BIS group(39.22%) vs. the montelukast group (50.42%). Children with comorbid allergic rhinitis also have been confirmed to have a significantly increased risk of uncontrolled asthma [ 16].Currently, management of pediatric asthma commonly takes place in primary care or community clinics, where the deep understanding of pediatric asthma, for instance risk factors for local families, is relatively lacking [ 27, 30]. Long-term treatment is crucial to reduce future risks related to comorbidities. Use of montelukast provided an opportunity for optimal control of rhinitis which may have beneficial effects on asthma control. In addition, a significant difference was found in asthma-related medical costs in mild persistent asthmatic children managed with either montelukast or lowdose BIS monotherapy in the past 12 months (P< 0.001).Besides the fact that daily therapy with BIS had higher costs,poorer control of asthma, as assessed by reliever usage and exercise quality, might be associated with increased costs.It is also possible that although comorbid allergic rhinitis results in greater prescription drug costs and more visits to hospitals and clinics, a unified treatment strategy for asthma and allergic rhinitis (as recommended by the Allergic Rhinitis and its Impact on Asthma initiative) might reduce, or notincrease the costs. The concurrent treatment of asthma and its comorbidities may increase the direct costs of treatment.

Fig. 1 Asthma-related medical costs in mild asthmatic children managed with either montelukast or low-dose budesonide inhalation suspension (BIS)monotherapy in the past 12 months. a Average asthmarelated medical costs (yuan/RMB). Data are expressed as median (Q1, Q3), P = 0.001; b Percentages of asthmatic children with medical costs < 5000,5000-1000, and ≥ 10,000 yuan/RMB, presented as numbers(%), P = 0.002
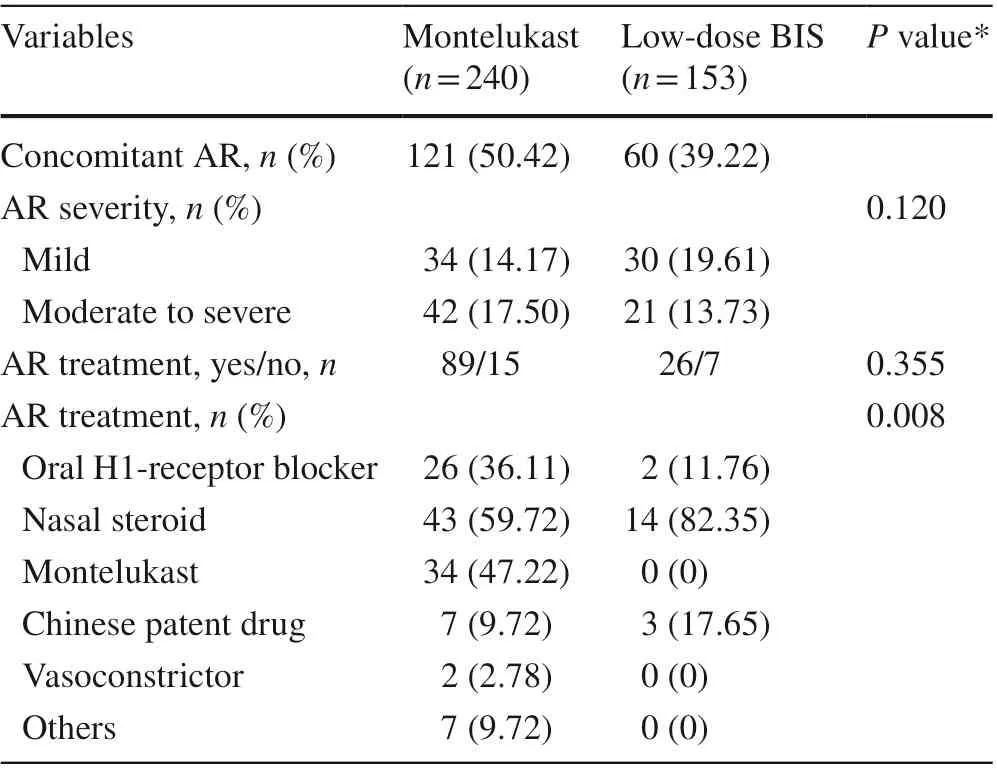
Table 3 Allergic rhinitis (AR) comorbidity and its related medication in mild asthmatic children managed with either montelukast or lowdose BIS monotherapy
This study had some limitations. As it was a retrospective questionnaire-based analysis, patient selection associated with this study was non-random and depended on the patients’ clinical visit and their willingness to be surveyed,which may have led to selection bias. Especially, there was an imbalance in disease severity of asthma observed between the two groups. In addition, the asthma control measures utilized in this study were retrospective and relied on parent reports; hence, they could be subject to recall biases. The cross-sectional nature of the study means that it lacks followup data and was not able to compare the lung function and symptoms before and after drug therapy. Thus, the results of this study should be generalized with caution. Confirmation of these findings will require further investigation in a prospective study with a larger sample size.
In conclusion, this study suggests that both BIS and montelukast are effective in children with mild persistent asthma,with potentially greater benefits with regard to asthma control and significant cost savings for montelukast than for BIS in real-world settings in China, where factors such as compliance are also taken into account. Subsequent studies need to further classify pediatric patients with mild persistent asthma according to specific features (e.g., comorbidities or pulmonary function status) to enable an individualized approach to treatment.
Acknowledgements The authors would like to thank all of the patients and investigators for their participation to achieve this study goal. Qi Zhong helped design the research. Lin Chen helped explain the data.Both are employees of MSD China, Shanghai, China. Editorial assistance with the preparation of this article was provided by Medjaden Bioscience Limited. This assistance was supported by MSD China,Shanghai, China.
Author’s contributions ZC and DZ contributed equally to this paper.JH conceived and designed research; JH collected data and conducted research; DZ and LX analyzed and interpreted data; ZC wrote the initial paper; ZC, DZ, LX and JH revised the paper. All authors read and approved the final manuscript.
Funding This work was supported by MSD China, Shanghai, China(grant number: 50146).
Data availability The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Ethical approval This study was approved by the Ethics Committee of the Beijing Children’s Hospital, Capital Medical University (approval number: 2012-70). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participant’s guardians included in the study.
Conflict of interest None of the authors declare any conflict of interest in this study. Authors Zhimin Chen and Jianguo Hong are members of the Editorial Board for theWorld Journal of Pediatrics. The paper was handled by the other Editor and has undergone a rigorous peer-review process. Authors Zhimin Chen and Jianguo Hong were not involved in the journal’s review of, or decisions related to, this manuscript.
杂志排行
World Journal of Pediatrics的其它文章
- Consensus statement on the epidemiology, diagnosis, prevention,and management of cow's milk protein allergy in the Middle East:a modified Delphi-based study
- Challenges and suggestions for precise diagnosis and treatment of Wilson’s disease
- Vestibular function of pediatric patients with sudden sensorineural hearing loss: based on vertigo symptom and vestibular function testing
- Evaluation of a new frequency-volume chart for children with primary monosymptomatic nocturnal enuresis: a prospective, comparative study
- Comparison of clinical outcomes between unrelated single umbilical cord blood and “ex-vivo” T-cell depleted haploidentical transplantation in children with hematological malignancies
- Haploidentical hematopoietic stem cell transplantation for pediatric patients with chronic active Epstein-Barr virus infection:a retrospective analysis of a single center
