Muscle strength and its association with cardiometabolic variables in adolescents: does the expression of muscle strength values matter?
2022-01-12iagoRodriguesdeLimaXuemeiSuiLuizRodrigoAugustemakdeLimaDiegoAugustoSantosSilva
iago Rodrigues de Lima · Xuemei Sui · Luiz Rodrigo Augustemak de Lima · Diego Augusto Santos Silva
Abstract
Keywords Abdominal · C-reactive protein · Hand strength · Insulin resistance · Obesity · Triglycerides
Introduction
Muscle strength has been directly associated with cardiometabolic health benefits (e.g., lower propensity to abdominal obesity, reduced blood pressure levels, improved glycemic and lipid metabolism, lower values of inflammatory markers) in adolescents [ 1- 3]. However, the beneficial relationship does not seem to be a consensus, given that the absence of association [ 1- 3], or even association between muscle strength and worse prognosis in relation to cardiometabolic variables, has been described [ 1- 3].
The discrepancy for divergent results in the interrelationship between muscle strength and cardiometabolic variables in adolescents has been attributed to the way how muscle strength values were expressed in these analyzes [ 1- 3]. This is because muscle strength can manifest in different dimensions (e.g., maximum strength, power, endurance); therefore, it can be assessed in different ways (e.g., standing or sitting, with or without propulsion, static or with support of the own body) [ 4]. Many of these studies investigating this interrelation (muscle strength/cardiometabolic variables)used absolute muscle strength values, while others considered body-volume-related indices [e.g., body weight, body mass index (BMI), fat mass] or body-length-related indices(e.g., height) in the expression of muscle strength values[ 1- 3]. However, regardless of the manifestation or way of assessment, since body size has direct impact on the associations between muscle strength and cardiometabolic variables, strategies to consider such impact have been adopted by studies [ 1- 3, 5].
During adolescence, muscle strength is directly impacted by body size and body composition components [ 4]. As concomitant with maturational development, muscle strength will increase as a function of muscle mass and size of muscle fibers, which explains the direct relationship between body size and muscle strength [ 6]. In this sense, it is hypothesized that when normalized for body-related indices or their indicators (e.g., body weight, height, BMI, fat mass) the values obtained may be more accurate compared to absolute muscle strength values in predicting health-related results [ 5].However, the best strategy to be adopted when considering the body size in the expression of muscle strength values in adolescents is currently unknown [ 1, 2].
Thus, the present study aimed to investigate the association between different muscle strength indices (absolute muscle strength, muscle strength normalized for body weight, muscle strength normalized for BMI, muscle strength normalized for height and muscle strength normalized for fat mass) with cardiometabolic variables in adolescents.
Methods
Study design and setting
This is a cross-sectional study using data from the “Guia Brasileiro de Avaliação da Aptidão Física Relacionada à Saúde e Hábitos de Vida-Etapa II”, a school-based population study which was carried out in the second semester of 2019 and included representative sample of adolescents aged 14-19 years enrolled in public high schools, living in São José, Southern Brazil. The study was approved by the Ethics Committee on Human Research of the Federal University of Santa (protocol n° 3.523.470).
To determine the sample size, the procedures suggested by Luiz and Magnanini [ 7] for a finite population were adopted. According to data from the Secretariat of Education of the State of Santa Catarina, there were 5411 students(14-19 years old) who were enrolled in the 2019 school year at 11 eligible public schools of Sao José and distributed into 186 high school classes (77.1% of students were on the day shift). In the first stage, the school density was adopted as a stratification criterion (size small, with less than 200 students; medium, with 200-499 students; and large, with 500 students or more); thus, the schools that predominated according to size were proportionally selected. In the second stage, the study period and the teaching grade were considered. All students from the selected classes were invited to participate in the study. A confidence level of 1.96 (95% confidence interval), a tolerable error of five percentage points,a prevalence of 50%, and a 1.5 design effect were adopted.We included an additional 20% to compensate for possible losses and refusals and another 20% to control for potential confounders in the association analyses [ 8]. Under these parameters, the required sample size was 1233 students. In view of the lack of financial resources, which made it impossible to collect blood information for all evaluated students,the minimal sample needed to test and identify associations of interest aimed at in this study (association between muscle strength with cardiometabolic variables) was calculated. Thus, considering the statistical analysis that would be used (multiple linear regression), the number of predictor variables (five different muscle strength indices), possible control variables to be included in these models based on information described in the literature (sociodemographic,lifestyle and sexual maturation) [ 1, 2, 9], a medium effect size (f2= 0.15) [ 10] and the desired power (1-ß = 0.95), the required sample of adolescents was 172. Thus, a sub-sample of adolescents (n= 372, based on the availability of the students themselves) had blood information collected. Of this amount, 351 students had all information regarding cardiometabolic variables (clinical and blood), muscle strength and other variables investigated in the present study.
Cardiometabolic variables
Blood pressure measurements were performed using the oscillometric method through a calibrated Omron (Kyoto,Japan) electronic and digital device model HEM 742. Blood pressure [systolic blood pressure (SBP), diastolic blood pressure (DBP)] were collected according to the recommendations of the literature [ 11]. The mean of two measurements either for SBP or DBP was then calculated.
Anthropometric factors [waist circumference (WC),height, and body weight] were measured [ 12], and the mean of 2 measurements for each of these variables was considered for analysis. WC was measured in the narrowest part of the trunk using an anthropometric tape (Sanny®,Sao Paulo, Brazil). Height was measured with a Sanny®stadiometer with tripod (Sao Paulo, Brazil) and body weight with G-tech®digital scale (Zhongshan, China). BMI was estimated as continuous variable (kg/m 2 ) [ 13].
The determination of fat mass was preceded by the calculation of the percentage of body fat (%BF), based on triceps and subscapular skinfold measurements [ 12]. Based on skinfold evaluation, %BF was estimated using a general predictive equation [ 14], with specific constants developed for Brazilian children and adolescents (by sex, according to age and ethnicity/race) [ 15]. After the %BF determination,the fat mass was obtained by the equation: fat mass = (body weight × %BF)/100 [ 15].
Venous blood samples were collected early in the morning after at least 8 hours of fasting. High-sensitivity C-reactive protein (hs-CRP, mg/L) was determined by quantitative turbidimetry method. Lipid profile [cholesterol(CHOL, mg/dL), triglycerides (TRG, mg/dL), high-density lipoprotein cholesterol (HDL-C, mg/dL), low-density lipoprotein cholesterol (LDL-C, mg/dL)], fasting glucose(FBG, mg/dL), and fasting insulin levels (FIL, mU/L) were determined by colorimetric test, while homeostatic model assessment for insulin resistance (HOMA-IR) was calculated as previously described [ 16], as follows: HOMAIR = (FBG × 0.0555 × FIL)/22.5. TRG, hs-CRP, and HOMAIR were transformed to their natural logarithm (ln) due to their non-normal distribution.
Muscle strength measures
Muscle strength was assessed by handgrip, measured using Saehan manual dynamometer (Seoul, South Korea). During evaluation, participants remained standing with arms outstretched at the side of the body, with the hand and dynamometer not touching the body [ 17]. The test was performed on both hands alternately, three times, and the best result of each hand was summed and recorded in kilograms force (kgf) [ 17]. In view of the absence of reference test/measurement for determining muscle strength levels, and divergent information regarding the impact attributed to body composition parameters on muscle strength values in children and adolescents [ 1- 5], in the present study,muscle strength was analyzed through five distinct ways(1) absolute muscle strength (sum of the values obtained in both hands-kgf); (2) muscle strength normalized for body weight (sum of the values obtained in both hands and normalized for body weight-kgf/kg); (3) muscle strength normalized for BMI {sum of the values obtained in both hands and normalized for BMI-[kgf/(kg/m 2 )]}; (4) muscle strength normalized for height (sum of the values obtained in both hands and normalized for height-kgf/height); and(5) muscle strength normalized for fat mass (sum of the values obtained in both hands and normalized for fat masskgf/fat mass).
Sociodemographic, lifestyle and sexual maturation
Sociodemographic, lifestyle and sexual maturation were included as control variables due to the relationship with cardiometabolic variables and muscle strength [ 1- 3, 9]: sex(male/female), age was collected in years; socioeconomic level was evaluated by questionnaire that evaluates the purchasing power of the adolescents’ families [ 18]. Through the information collected by this instrument, a score that varies from “E” (lower purchasing power) to “A” (higher purchasing power) was generated.
Physical activity level was assessed by a validated questionnaire for Brazilian population [ 19]. Physical activity level was assessed by the following question: during the past 7 days, how many days were you physically active for at least 60 minutes a day (consider the time you spent in any kind of physical activity that increased your heart rate and made your breathing faster for some time)? Adolescents who responded to perform physical activity for at least 60 minutes, seven days a week, were considered as meeting recommendations for physical activity [ 20]. Additionally, those aged 18 years or over were considered meeting recommendations for physical activity performed at least 150 minutes of moderate-intensity physical activity throughout the week or at least 75 minutes of vigorous-intensity physical activity throughout the week, or an equivalent combination of moderate- and vigorous-intensity activity (physical activity) [ 20].
Information regarding muscle strengthening exercises were investigated by a question from Health Risk Behaviors Project (COMPAC) [ 21]. The COMPAC questionnaire presented favorable face and content validity, Kappa index equal to or greater than 0.27 and intraclass correlation by thematic unit from 0.51 to 0.96 [ 21]. The following question was adopted: during a typical week, on how many days do you exercise to improve the tone and strength of your muscles such as weight training and gymnastics? Adolescents who responded get engaged in muscle strength exercises on three or more days per week were considered as meeting recommendations for muscle strength exercises [ 20]. Additionally, school children aged 18 years or over were considered meeting recommendations for performance of muscle strength improvement exercises when they performed muscle strength exercises for at least two times per week [ 20].
The questions regarding eating habits and smoking came from the “Fantastic lifestyle” questionnaire, translated and validated for the Brazilian population [ 22]. Information regarding eating habits was collected by questions related to a typical week: do you have a balanced diet? A balanced diet was composed of cereals and grains (5-12 servings per day), fruits and vegetables (5-10 servings per day), meats and meat products (2-3 servings a day), milk and dairy products (3-4 servings up to 16 years and 2-4 portions over 16 years) [ 17]. Response options with relative frequency and often were considered as frequent response. Smoking was assessed by the follow question: do you smoke cigarettes?Individuals who responded “never smoked” were considered negative for smoking, and those that responded more than 10 per day, 1-10 per day, none in the last six months, none last year were considered as positive for smoking.
The question regarding alcohol use was as follows [ 19]:during the last 30 days, on how many days did you drink five or more alcoholic drinks in a single occasion (a dose corresponding to one can of beer, a glass of wine, a shot of whiskey, rum, vodka, etc.)? Those who answered at least once were considered positive response for alcohol consumption.
Sexual maturation was assessed according to Tanner’s criteria [ 23] through the use of figures indicating maturational development adopted in a sample of Brazilian schoolchildren [ 24 ]. In the present study, adolescents were classified as prepubertal, pubertal and post-pubertal [ 23, 24].
Data analysis
All analyses were performed considering sampling weights and the survey design. Data analysis was conducted in the statistical software Stata 13.0 (StataCorp LP, College Station, Texas, USA). Mean and standard deviation were used to describe symmetric continuous variables, and median and interquartile range (p25-p75) for asymmetric variables. Categorical variables were presented as percentages (%). Pearson correlation coefficient was adopted to verify the relationship between the variables used.
Although the aim of the study was to investigate the association between muscle strength indices and association with cardiometabolic variables, prior to the beginning of these analyses, multiple linear regression was used to test the association of each body-related index (exposure variable)with each cardiometabolic variable (outcome variable) to investigate the independence of muscle strength indices and body-related indices in the associations of interest in the present study (association between muscle strength indices and cardiometabolic variables). In these analyses, body-related indices not analyzed as the exposure variable, as well as the muscle strength indices were included as covariates, and the results presented as regression coefficients (ß) with their respective standard error (SE). Variance values of the estimated regression coefficients (variance inflation factor, VIF)were also presented.
To investigate the association between different muscle strength indices and cardiometabolic variables, separate multiple linear regressions considering each of the five muscle strength indices were performed for each dependent variable (cardiometabolic variables). Results were presented asßwith their respective SE and VIF. Results for lnTRG,lnHOMA-IR and lnCRP were back-transformed to the exponential form (EXPß) and should be interpreted as a risk ratio compared with the mean value of the respective variable. In view of the possible effect of sex and age on muscle strength variation [ 1- 3, 9], interactions between these factors in the association with cardiometabolic variables were tested in the regression models. APvalue < 0.05 for the interaction term was considered as indicative of heterogeneity of the associations. Predicted adjusted means of the cardiometabolic variables and interaction between expressions of muscle strength values with sex and age were then estimated and presented graphically [ 25]. In the analysis in which statistical significance for interaction was identified, the results were stratified by sex (male/female) and age group (14-16 years,17-19 years-additional strategies for grouping the sample in relation to the age group were tested; however, due to the heterogeneity of this information, the way adopted was the one that best suited such distribution data not shown in figures or tables).
A post hoc analysis of power was conducted using the sample size of 351 students (155 and 167 students for sex and age, respectively, in the stratified analysis) and an equation of ten predictor variables was used as a reference. The recommended effect sizes used for this assessment were as follows: small (f2= 0.02), medium (f2= 0.15), and large(f2= 0.35) [ 10]. The alpha level used for this analysis wasP< 0.05. The post hoc analyses revealed the statistical power for this study was approximately 0.40 for detecting a small effect, whereas the power exceeded 0.96 for the detection of a moderate to large effect size in both scenarios (stratified or not). Thus, there was more than adequate power (i.e., power* 0.80) at the moderate to large effect size level.
Results
A total of 351 students (male 44.4%, age 16.6 ± 1.0 years)with complete information were included in the present study. Detailed information regarding body-related indices,cardiometabolic variables, muscle strength, sociodemographic, lifestyle and sexual maturation indices used in the present study are available in Table 1.
All muscle strength indices investigated were significantly correlated with height, body weight, BMI and fat mass (Pvalue < 0.05 for all, Table 2). Regarding the results between muscle strength and cardiometabolic variables, all muscle strength indices were correlated with WC, SBP and CHOL (Pvalue < 0.05 for all, Table 2). Finally, all bodyrelated indices (height, body weight, BMI and fat mass) were correlated with WC, lnTRG and lnCRP (Pvalue < 0.05 for all, Table 2). Detailed results for the correlation between the investigated variables (body-related indices, muscle strength indices and cardiometabolic variables) can be identified in Table 2.
Results for the association between body-related indices(height, body weight, BMI and fat mass) with cardiometabolic variables are available in Table 3. Fat mass was directly associated with CHOL, HDL-C, LDL-C, lnTRG, lnHOMAIR and lnCRP, regardless of muscle strength indices and other body-related indices (height, BMI and body weight)entered as covariates in the adjusted models. With the exception of the model that included muscle strength normalized for fat mass as a covariate in the adjusted analysis (model5), BMI was directly associated with WC when adjusted for muscle strength indices, height, fat mass and body weight.Height was directly associated with SBP in an analysis adjusted for absolute muscle strength or muscle strength normalized for body weight, fat mass, BMI and body weight.Similarly, BMI was directly associated with SBP when the possible effect attributed to absolute muscle strength (model 1) or muscle strength normalized for body weight (model 2), height, fat mass and body weight were controlled for.Finally, fat mass was directly associated with WC in an analysis adjusted for muscle strength normalized for fat mass(model 5), height, BMI and body weight (Table 3). Sensitivity analyzes investigating the possible effect attributed to absence (Supplementary Table 1), or inclusion of one(Supplementary Table 2) or two body-related indices (Supplementary Table 3) as covariates in the adjusted models are available as supplementary material.
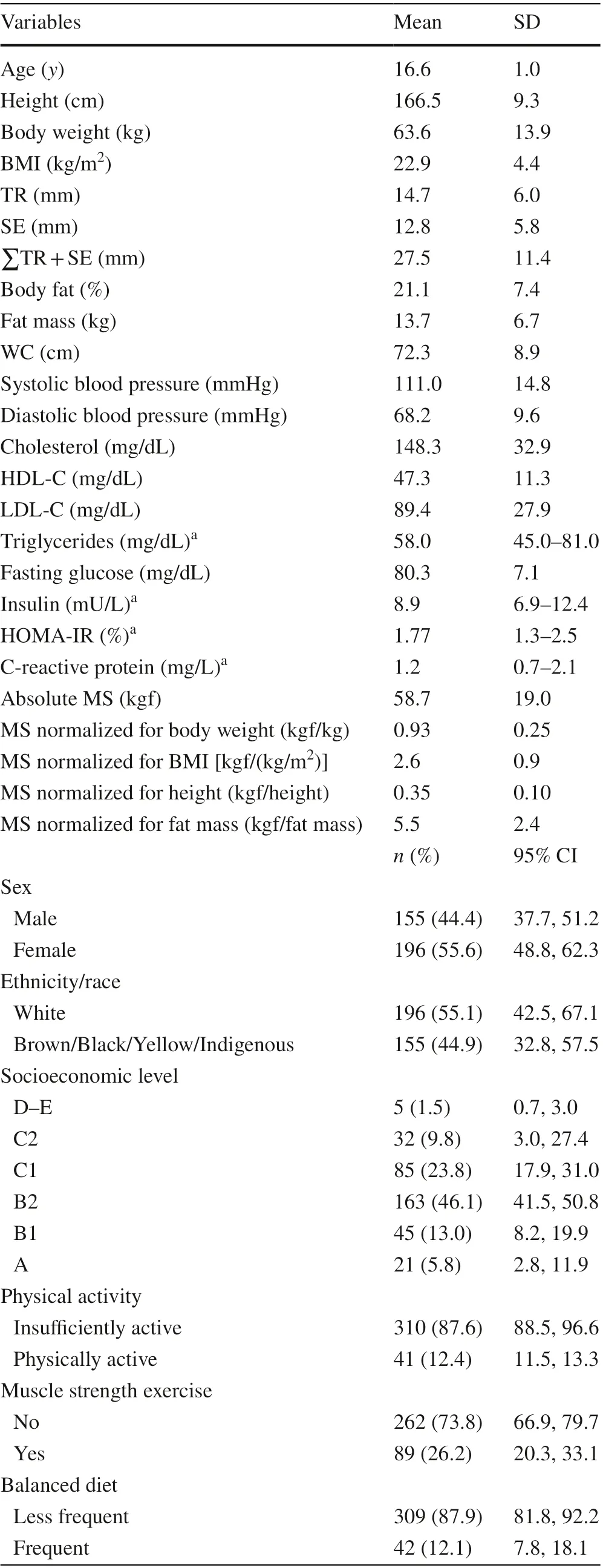
Table 1 Descriptive characteristics of participants with complete data for all outcomes investigated in this study ( n = 351)
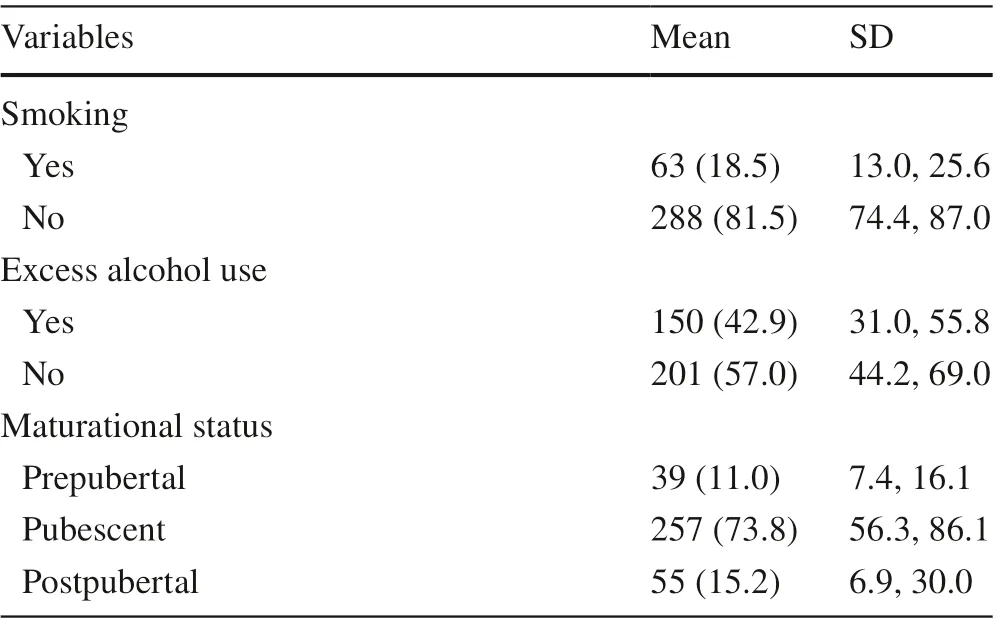
Table 1 (continued)
Absolute muscle strength and/or muscle strength normalized for height were directly associated with WC and DBP,and inversely associated with HDL-C (Pvalue < 0.05). Muscle strength normalized for body weight, muscle strength normalized for BMI and muscle strength normalized for fat mass were inversely associated with WC (Pvalue < 0.05). When normalized for fat mass, muscle strength was inversely associated with lnTRG and lnCRP (Pvalue < 0.01) (Table 4). In addition,interactions between sex and age with muscle strength indices in some of these associations were identified (Table 4).
Sex-stratified analyzes demonstrated interaction between muscle strength (all five muscle strength indices)and lnTRG (Pvalue < 0.05, Supplementary Fig. 1). Interactions between muscle strength normalized for fat mass with sex were found in the association with SBP, lnHOMA-IRand lnCRP (Pvalue < 0.05, Supplementary Fig. 2). In addition, interactions between muscle strength normalized for BMI with sex (Supplementary Fig. 2) and age (Supplementary Fig. 3) in the association with WC and CHOL respectively, were identified (Pvalue < 0.05). Moreover,interactions between muscle strength normalized for height with sex (Supplementary Fig. 2) and age (Supplementary Fig. 3) in the association with HDL-C were reported(Pvalue < 0.05).
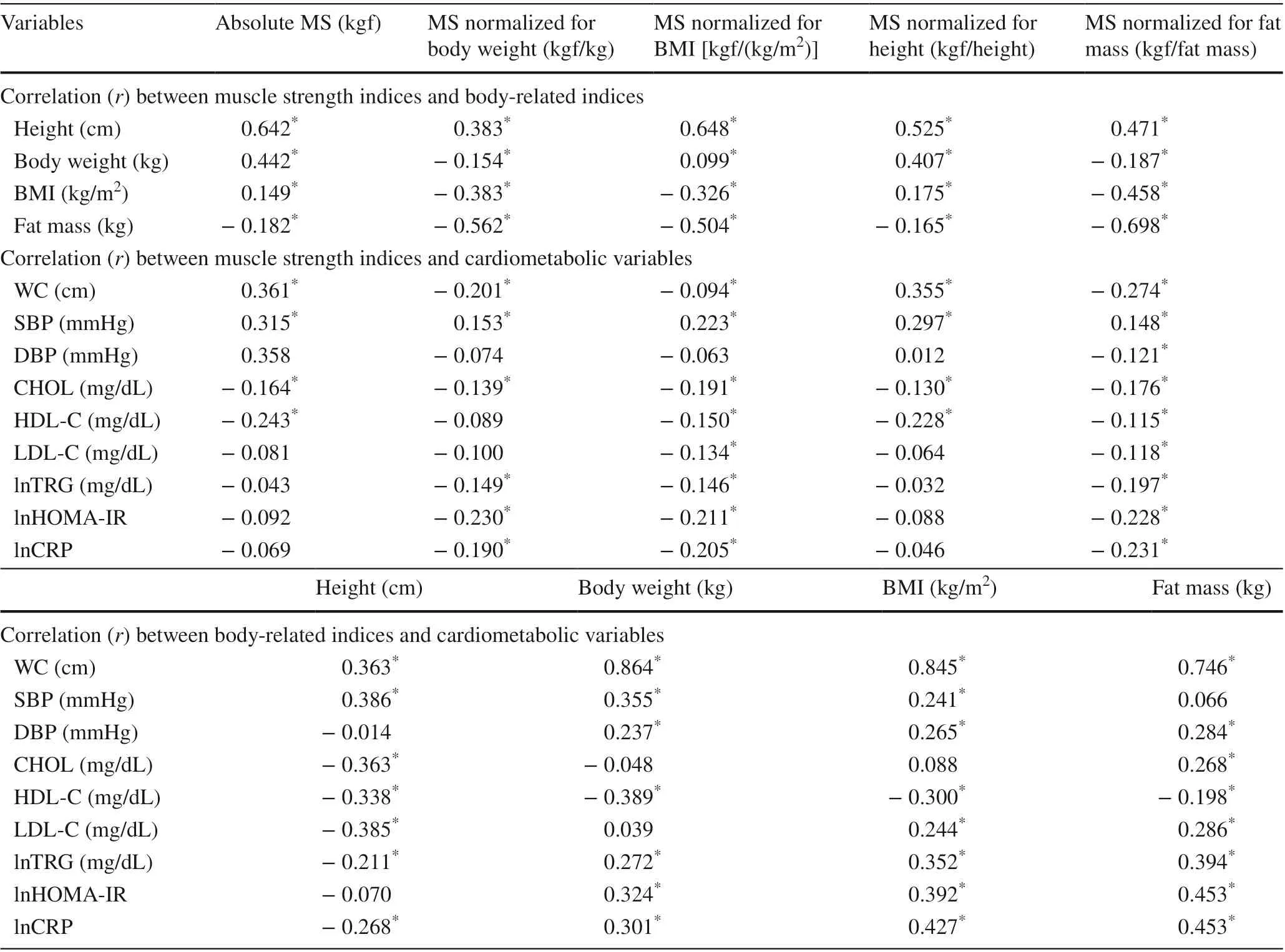
Table 2 Correlations between muscle strength indices, body-related indices and cardiometabolic variables analyzed in the present study( n = 351)
Adjusted results for the association between muscle strength indices and cardiometabolic variables according to sex and age group are described in Table 5. When normalized for body fat, muscle strength was associated with lower concentrations of lnTRG and lnHOMA-IR among male and female, and lower values of lnCRP only among male (Pvalue ≤ 0.05).
Discussion
According to the findings of the present study, the adoption of absolute muscle strength or muscle strength normalized to height when compared to muscle strength indices that included body-volume-related indices can lead to confusion in the interpretation (direction and magnitude) of the associations. Absolute muscle strength and/or muscle strength normalized for height were directly associated with WC and DBP, and inversely associated with HDL-C. Muscle strength normalized for body-volume-related indices (body weight, BMI or fat mass) were inversely associated with WC. In addition, muscle strength values normalized for fat mass were inversely associated with lnTRG and lnHOMAIR among male and female, and inversely associated with lnCRP only among male.
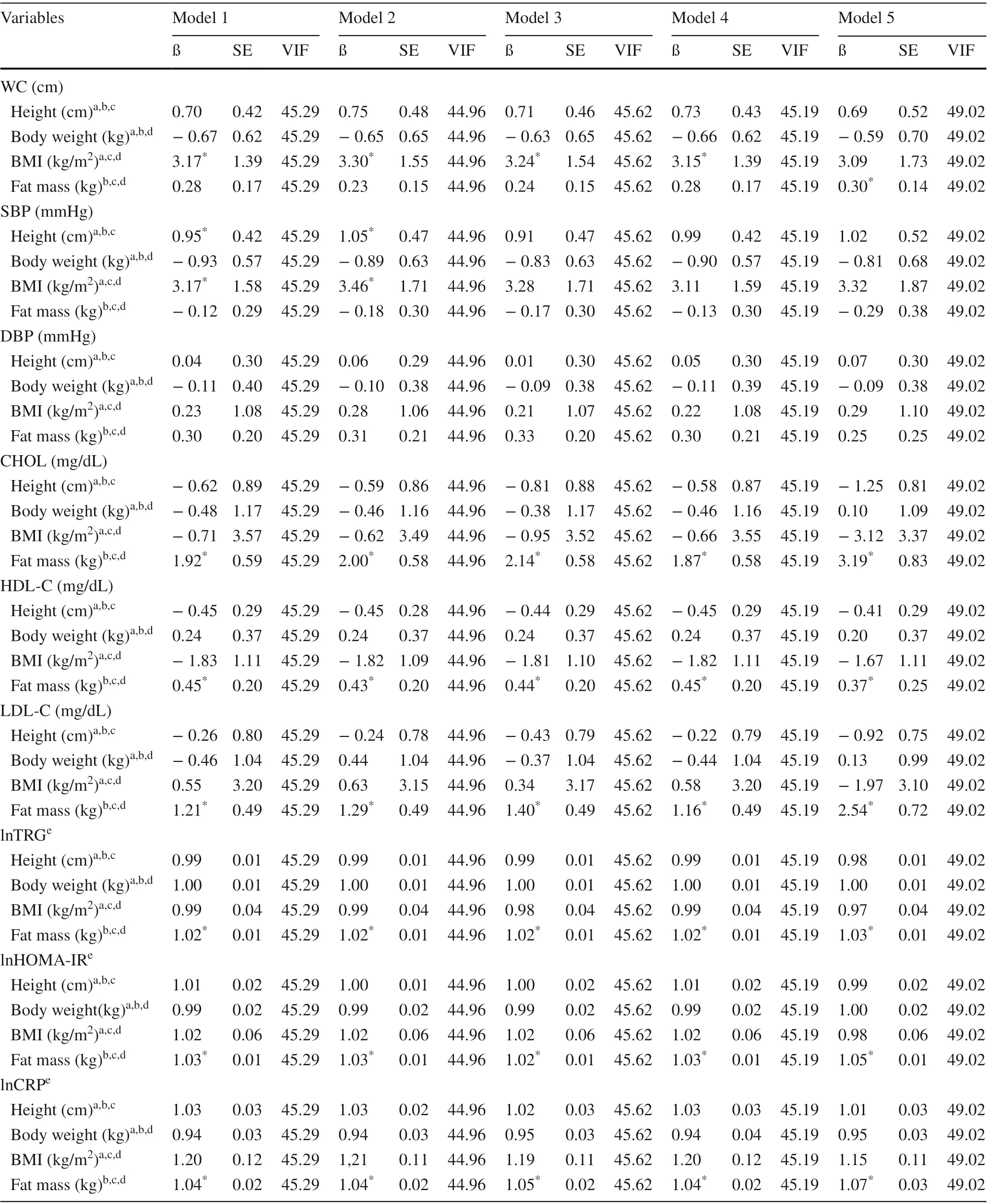
Table 3 Adjusted association between cardiometabolic variables and body-related indices among participants of the study ( n = 351)
In the present study, muscle strength indices were investigated in the association with cardiometabolic variables in adolescents because muscle strength can be affected both by the test used for its evaluation [ 1- 3, 5], and by determinants of the muscle strength itself [ 26]. With regard to the relationship exercised by the test adopted on the values of muscle strength, some tests to assess muscle strength require the need for propulsion (e.g., jumps) or body support (e.g.,pull-ups), where in fact the direct impact that body size has on performance is identified. This is because, concomitant with the increase in body dimensions, the magnitude of the relationship between muscle strength and body size tends to increase, possibly due to the increase in muscle mass and physiological muscle cross-sectional area (proportional to height and body weight) [ 4]. However, muscle strength increases at a lower rate than body size [ 4]. Thus, although it is expected that individuals with larger body dimensions(body-volume-related indices and body-length-related indices) have higher muscle strength values [ 4], these individuals will also be heavier, which will negatively impact the performance of tests involving jumps or body support [ 27],showing the need to somehow consider body size in the expression of muscle strength values. Thus, although there is no consensus on the best strategy to be adopted to consider body size in determining muscle strength [ 5], it is hypothesized that the inclusion of height and body weight simultaneously in the expression of muscle strength values can contribute to that muscle quality (instead of muscle quantity)is evidenced, and in this way more accurate results are identified [ 4]. Additionally, tests such as the handgrip strength, in which the need to jump or support the body is not required,are also directly impacted by the size of the body [ 28]. This is because handgrip strength is closely related to body mass,and the values of muscle strength identified by handgrip strength will be higher among taller and heavier individuals, compared to those lower and lighter [ 4]. In addition,it is hypothesized that in response to successive exposure to optimal efforts, both muscle strength and muscle mass will develop [ 29]. Thus, if the same individual is subjected to the same effort (amount and extent of this activity), the muscle strength will be proportional to the body weight, as it will act as a load [ 29]. For this reason, in addition to the impact attributed to the test used to assess muscle strength,body-related indices should be considered in the expression of muscle strength values [ 4]. Another aspect that should be considered is the fact that although muscle strength isdirectly impacted by muscle mass, fat mass can moderate the magnitude of muscle mass/muscle strength interrelation[ 26, 30], in which it is possible that by normalizing muscle strength for fat mass, possible differences in muscle strength identified in obese compared to non-obese individuals can be minimized. Additionally, based on the premise that adolescence is a period of changes in body structures [ 23], and that muscle mass plays an important role in maintenance of growth development [ 31], it is speculated that the normalization of muscle strength for height allows to include such development in the structure and body composition,and that the use of this technique can result in more reliable muscle strength values. Such assumption is based on the fact that an increase in fat-free mass throughout puberty occurs similarly to an increase in height-at least in the shape of the growth curve [ 31].

Table 4 Adjusted association a between muscle strength and cardiometabolic variables among participants of the study ( n = 351)
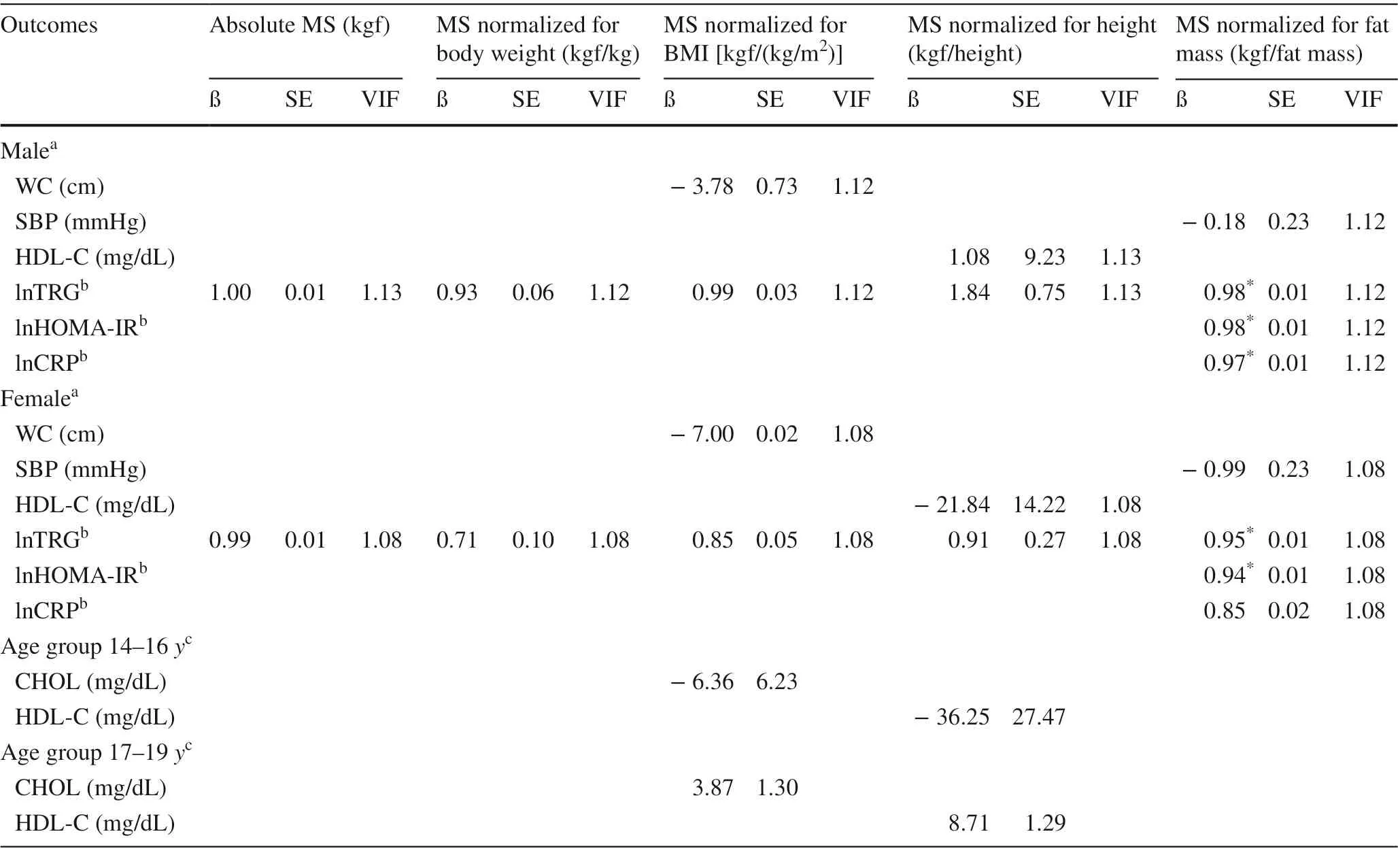
Table 5 Adjusted association between muscle strength indices and cardiometabolic variables according to sex and/or age group among participants of the study ( n = 351)
As reported in the literature [ 2, 3, 5], the results of the present study indicated that the relationship between muscle strength and WC was dependent on the body-related indices used to normalize muscle strength. Higher energy expenditure (increased basal metabolic rate and/or resting heart rate) due to the greater amount of skeletal muscle and consequently higher levels of muscle strength support the plausibility of the beneficial relationship between muscle strength and WC [ 32, 33]. However, it has been suggested that the excess weight often identified in individuals with a higher WC produces increases in fat-free mass to support this extra load, which may reflect in higher values of muscle strength [ 29]. Thus, given the diversity of aspects involved in the interrelation between muscle strength and WC, the adoption of strategies that considering body-volume-related indices when expressing muscle strength values allows the establishment of more reliable measures with regard to the association between muscle strength and WC in adolescents.
In the present study, absolute muscle strength or muscle strength normalized for height was directly associated with DBP and inversely associated with HDL-C. Although the results reported in this study are in accordance with information gathered in systematic review studies that covered the same content [ 1- 3], such findings are not a consensus in the literature, since absence of association[ 1- 3], inverse association of muscle strength with DBP, or direct association of muscle strength with HDL-C has been described among adolescents [ 1- 3]. The plausibility for the beneficial association of muscle strength with DBP and HDL-C is based on the hypothesis that the greater contractile function seen in individuals with higher levels of muscle strength, contributes to both the maintenance of the endothelium (release of nitric oxide) [ 34 ] and the improvements over muscle metabolic efficiency (e.g., lipid oxidation) [ 5,29, 35]. However, according to the findings described in this study, aspects related to the use of absolute values of muscle strength or body-related indices adopted to normalize muscle strength contributed to opposite results.
The results of this study have shown that when normalized for fat mass, muscle strength was inversely associated with lnTRG and lnHOMA-IR among male and female, and inversely associated with lnCRP only among male. Although these findings are not reported in previous literature [ 1- 3],they reinforce the idea that the adoption of strategies aimed at minimizing the impact attributed to confounding aspects in determining muscle strength, such as fat mass, may reflect more sensitive measures in the relationship with cardiometabolic variables. Since muscle mass is the primary tissue for glucose and TRG metabolism [ 5], it is hypothesized that greater muscle strength and, consequently, greater amount of muscle mass may be associated with benefits to glycemic and fat function (lipids), which could reflect an improvement in HOMA-IR and TRG levels. The beneficial relationship between muscle strength and inflammatory biomarkers has been based on an important role that muscle strength plays in preventing obesity [ 36], in view of the relationship between obesity and these biomarkers [ 37]. However, the reported association between muscle strength and CRP in adolescents regardless of body fat or fat-free mass [ 38] suggests that alternative mechanisms may be involved. Studies conducted with the elderly have suggested that low-grade systemic inflammation is closely linked to loss of muscle mass and consequent muscle strength [ 39, 40], whose plausibility for this association would be related to the decline in functional capacity due to the catabolic effect on muscle mass[ 5]. However, the mechanisms that could justify the relationship between low-grade inflammation and muscle strength in adolescents are not known [ 5], therefore determining the causal chain of this interrelation in adolescents is necessary.
Although the literature has reported the direct impact attributed to body-related indices on cardiometabolic variables in adolescents [ 41, 42], in the present study, only fat mass was directly associated with cardiometabolic variables (i.e., CHOL, HDL-C, LDL-C, lnTRG, lnHOMA-IR and lnCRP) regardless of muscle strength or other variables related to body-related indices (although other body-related indices were associated with cardiometabolic markers depending on the muscle strength index inserted as a covariate, in addition to the other body-related indices-BMI and fat mass were directly associated with WC, and height and fat mass were directly associated with associated with SBP).It is hypothesized that the absence of association between the other body-related indices with the cardiometabolic variables is related to evident multicollinearity (i.e., one independent variable is highly correlated with one or more of the other independent variables in a multiple regression equation) attributed to the inclusion of numerous body-related indices as covariates [ 43]. When comparing the diagnostic parameters of the investigated analyses, this assumption is even more solid, given the high values of VIF and SE identified, compared to values identified in analyzes that did not include body-related indices, or included a reduced number of these indicators in the adjusted models (Supplementary Tables 1-3). It is worth emphasizing that, in addition to being directly associated with cardiometabolic variables,results identified in the present study indicated a direct association of body-related indices with muscle strength, in which the hypothesis that body-related indices can mediate the association between muscle strength with cardiometabolic variables in adolescents cannot be ruled out [ 44].
Despite the numerous strengths covered in this study, including the use of measured information regarding cardiometabolic variables, and the diversity of strategies adopted to express muscle strength values, some limitations must be assumed: (1)the adoption of only one instrument to assess muscle strength,which limits the investigation of the total dimensions of muscle strength (e.g., muscular endurance, muscular power) is a limitation of this study; (2) the small number of students with information for the analysis of interest, which limits the possibility of inferring the results for the entire sample of students in the city of São José, is a limitation of this study. However, the available statistical power identified to test these associations increases the reliability of the identified results.
In conclusion, muscle strength normalized for body weight, BMI and fat mass was inversely associated with WC.Absolute muscle strength and muscle strength normalized for height were directly associated with DBP and inversely associated with HDL-C. Muscle strength normalized for fat mass was inversely associated with TRG and HOMAIR among male and female, and inversely associated with lnCRP only among male. Based on the findings of this study,it is suggested that when normalized for body weight, BMI or fat mass, muscle strength may be superior to absolute muscle strength and muscle strength normalized for height in representing adequately cardiometabolic variables among adolescents.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1007/ s12519- 021- 00460-x.
Acknowledgements The authors would like to thank the team of the Clinical Analysis Laboratory of the University Hospital “Professor Polydoro Ernani de São Thiago” at Federal University of Santa Catarina, Florianópolis, Brazil, for conducting blood tests. Dr. Silva was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001 and Dr. Silva is supported in part by CNPq (302028/2018-8).
Author contributions dLTR contributed to conceptualization, methodology, software, formal analysis, investigation, resources, data curation,and writing of original draft preparation. SX contributed to conceptualization, methodology, investigation, review and editing. ALLR contributed to conceptualization, methodology, investigation, review and editing. SSDA contributed to conceptualization, methodology,investigation, review and editing, supervision, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding This study was not funded.
Compliance with ethical standards
Ethical approval The study was approved by the Ethics Committee on Human Research of the Federal University of Santa (protocol n°3.523.470).
Conflict of interest No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article. The authors have no conflict of interest to declare.
Data availability All authors approve to deposit data that support the findings of their research in a public repository.
杂志排行
World Journal of Pediatrics的其它文章
- Haploidentical hematopoietic stem cell transplantation for pediatric patients with chronic active Epstein-Barr virus infection:a retrospective analysis of a single center
- Treatment of pediatric mild persistent asthma with low-dose budesonide inhalation suspension vs. montelukast in China
- Comparison of clinical outcomes between unrelated single umbilical cord blood and “ex-vivo” T-cell depleted haploidentical transplantation in children with hematological malignancies
- Consensus statement on the epidemiology, diagnosis, prevention,and management of cow's milk protein allergy in the Middle East:a modified Delphi-based study
- Evaluation of a new frequency-volume chart for children with primary monosymptomatic nocturnal enuresis: a prospective, comparative study
- Vestibular function of pediatric patients with sudden sensorineural hearing loss: based on vertigo symptom and vestibular function testing
