Breast cancer in schizophrenia could be interleukin-33-mediated
2022-01-11MilicaBorovcaninKatarinaVesic
Milica M Borovcanin,Katarina Vesic
Milica M Borovcanin,Department of Psychiatry,University of Kragujevac,Faculty of Medical Sciences,Kragujevac 34000,Serbia
Katarina Vesic,Department of Neurology,University of Kragujevac,Faculty of Medical Sciences,Kragujevac 34000,Serbia
Abstract Recent epidemiological and genetic studies have revealed an interconnection between schizophrenia and breast cancer.The mutual underlying pathophysiological mechanisms may be immunologically driven.A new cluster of molecules called alarmins may be involved in sterile brain inflammation,and we have already reported the potential impact of interleukin-33 (IL-33) on positive symptoms onset and the role of its soluble trans-membranes full length receptor(sST2) on amelioration of negative symptoms in schizophrenia genesis.Furthermore,these molecules have already been shown to be involved in breast cancer etiopathogenesis.In this review article,we aim to describe the IL-33/suppressor of tumorigenicity 2 (ST2) axis as a crossroad in schizophreniabreast cancer comorbidity.Considering that raloxifene could be tissue-specific and improve cognition and that tamoxifen resistance in breast carcinoma could be improved by strategies targeting IL-33,these selective estrogen receptor modulators could be useful in complementary treatment.These observations could guide further somatic,as well as psychiatric therapeutical protocols by incorporating what is known about immunity in schizophrenia.
Key Words:Interleukin-33;Schizophrenia;Breast cancer;Neurodegeneration
INTRODUCTION
The initial question is whether patients with schizophrenia are resistant or more susceptible to developing breast cancer.Recently,it has been reported that the prevalence rate of schizophrenia of 0.3%-0.7% in the world population remains stable[1].There is a possibility that some mechanisms underlying the pathogenesis of schizophrenia also have beneficial or even protective properties for the onset of some somatic disorders,while others lead to somatic comorbidity and mortality.
Schizophrenia is a neurodegenerative disease with a complex pathogenesis and pathophysiology.This type of disorder is characterized by a chronic course,exacerbations,and progression leading to neurodegeneration over time.Genetic predisposition,individual and environmental factors,and specific immune responses have a significant impact on disease onset and clinical presentation.Moreover,genome-wide association studies in schizophrenia have revealed similar genetic backgrounds with some immunological properties and genetic overlap with breast carcinoma[2,3].
Scientific advances in the last decade have led to the recognition that interleukin-33(IL-33) and neuroinflammation play a role in some aspects of neurodegenerative diseases.It is possible that IL-33 has various biological activities at different stages of the disease.It is necessary to consider disease progression with exacerbations and neurodegeneration,and to discuss the impact of IL-33 on somatic disturbances.
The alarmin molecule IL-33 as a marker of innate immunity was first studied in different phases of schizophrenia,but also in breast carcinoma patients[4].In summary,we will try to further elucidate the possibility of the involvement of IL-33 in the onset of breast cancer in schizophrenia patients.
BREAST CANCER RISK AND SCHIZOPHRENIA
Disturbingly,approximately 2.1 million new cases of breast cancer were diagnosed worldwide in 2018,accounting for nearly one in four cancers in women and resulting in approximately 630000 deaths[5].Previous epidemiological studies suggest that breast cancer is more common in patients with severe mental disorders than in the general population[6,7].Among mental illnesses,a higher incidence of breast cancer has been observed in schizophrenia than in mood disorders[8].Patients with schizophrenia have an increased risk of breast cancer,but not cancer overall[9,10].The mortality cancer risk in schizophrenia is estimated to be 40% higher than in the general population and 51% higher than in individuals without schizophrenia[11].
The aspects of genetics,as well as epigenetic disturbances due to unhealthy lifestyle and harmful habits should be considered in this comorbidity.Recently,the genetic overlap of schizophrenia and breast cancer has been extensively reported[12].A study by Luet al[3] reported the phenotypic and genetic positive association of schizophrenia and breast cancer,estimated the percentage of genetic overlap to be 0.14 [95%cumulative incidence (CI):0.09-0.19],and identified a shared locus at19p13(GATAD2A) as a significant risk factor for the development of both diseases.A polymorphism of the breast cancer resistance protein transporter (ABCG2) was associated with a 4-fold higher probability of a relevant reduction in positive and negative schizophrenia syndrome[13].This suggests a potential genetic overlap between the two diseases.
In recent years,epidemiological evidence of a controversial association between cancer and neurodegenerative diseases has been increasing.Common etiological factors may play opposing roles in the pathogenesis of neurodegeneration and breast cancer[14,15].Characteristic brain pathology leads to neuronal cell death and neurodegeneration over time,whereas cancer is dominated by the process of unlimited cell proliferation[16,17].Evidence suggests that common biological mechanisms such as oxidative stress,metabolic dysregulation and inflammation underlie both diseases,all of which could promote apoptosis and cell proliferation[18-20].
Some immune-metabolic specifiers observed in patients with schizophrenia,such as diabetes,hyperinsulinemia,insulin resistance,and hyperlipidemia,may also be involved in the development of breast cancer[21-23].Inflammatory changes that occur in patients with schizophrenia could also be marked as a cancer risk factor[24](Figure 1).IL-33 is significantly higher in serum,indicating tumor recurrence,which also indicates poor prognosis in patients with breast cancer[25-27].Similarly,higher serum levels of IL-33 are measured in schizophrenia during psychotic episodes[4].
Figure 1 Interleukin-33/suppressor of tumorigenicity 2 axis as a crossroad in schizophrenia-breast cancer comorbidity.
So far,many contributing risk factors have been considered for the onset of breast carcinoma,and they seem to overlap with those considered lifestyle-related qualifiers in patients with schizophrenia.Behavioral habits such as smoking,alcohol consumption,obesity,physical inactivity and stress exposure are considered hazardous in the etiology of breast carcinoma[28-31].
It is particularly important to acknowledge gender specificities in schizophrenia.Gender differences in breast carcinoma risk have been observed in people without mental illnesses,but also in patients with schizophrenia.Taiwanese women with schizophrenia were observed to have a 1.94-fold higher risk of breast cancer than nonschizophrenic controls[32].In addition,hormonal disturbances have been implicated as a risk factor for schizophrenia and breast cancer.According to previous findings,estrogen levels in women decrease during certain periods of life,such as during menstruation,after childbirth,and menopause,which can often lead to an exacerbation of schizophrenia symptoms and resistance to antipsychotic treatment[33,34].At the same time,estrogen intermediates carcinoma development[35].
IL-33 ROLE IN NEUROINFLAMMATION AND NEURODEGENERATION
IL-33 is a multifunctional cytokine,and an alarmin,a damage-associated molecular pattern (DAMP),an endogenous molecule released into the extracellular space upon cellular stress and damage.DAMPs can correct altered physiological states and regulate homeostasis at low concentrations,propagate inflammatory reactions at high concentrations,or even lead to trauma and activation of surrounding cells and recruitment of distant cells in excessive release[36].The role of DAMPs in neurodegenerative diseases has already been recognized in sterile inflammation.As recently summarized by Pandolfoet al[37],acute or chronic stress may trigger sterile inflammation associated with DAMPs and may be an etiopathogenetic mechanism for affective disorders.
In recent years,there is increasing evidence for an important immunomodulatory role of IL-33 in neurodegenerative diseases.IL-33 is a multifunctional cytokine that acts intracellularly as a nuclear factor and extracellularly as a cytokine[38].By binding to the trans-membranes full length receptor (sST2),IL-33 exerts its biological activity through the IL-33/suppressor of tumorigenicity 2 (ST2) signaling pathway[39].In contrast,the soluble form functions as a decoy receptor and limits the biological activity of IL-33[40].IL-33 is highly expressed in the brain and released from astrocytes and oligodendrocytes,while the ST2 receptor is expressed in glial cells[41].It has a dual function and may exert pro-inflammatory or anti-inflammatory effects in the central nervous system (CNS)[42,43].Up-regulated expression of IL-33 in peripheral cells contributes to blood-brain barrier disruption[44].By binding to the ST2 receptor,IL-33 promotes microglial activation and proliferation and enhances production of different cytokines,leading to an acute inflammatory response[45].IL-33 signaling is associated with regulatory T and B cell responses[46,47].By inducing microglial and macrophage polarization to an anti-inflammatory type 2 phenotype and phagocytosis,IL-33 exerts a neuroprotective and reparative role in the CNS[45,48].
In Alzheimer’s disease (AD),soluble decoys trap IL-33 already produced at lower levels,and its concentrations in serum and cerebrospinal fluid are significantly reduced[49].IL-33 could exert a neuroprotective effect by inducing innate immunity to reduce soluble amyloid β levels and amyloid plaque deposition[50].Carlocket al[51] in 2017 pointed out that deficiency of IL-33 caused tau abnormality and late-onset neurodegeneration in the cerebral cortex and hippocampus,accompanied by memory impairment.A recent study suggested that IL-33 gene mutations affect susceptibility to late-onset AD,which in turn confirms that IL-33 may exacerbate neuroinflammation and cognitive decline[52,53].
As a neuroinflammatory and neurodegenerative disease,multiple sclerosis (MS) is associated with increased expression of IL-33 in the periphery,white matter,and plaque areas of the MS brain[54-57].However,the exact role of IL-33 has not been fully elucidated,and data in the current literature are inconsistent.Previous studies have supported the involvement of IL-33 as a proinflammatory cytokine in disease pathology[56].However,several recent observations suggest a dominant neuroreparative role of IL-33 in MS.Studies on experimental autoimmune encephalomyelitis(EAE) reported a detrimental effect of IL-33 treatment on EAE severity.The identification of ST2 expression by oligodendrocytes indicates an important role in the myelination process during CNS development and the repair phase of MS[57,58].
Recently,the protective effect of IL-33 in some acute neurological states has been reported.For example,Miaoet al[59] in 2021 highlighted that increased IL-33 levels might reduce brain damage in patients with intracerebral hemorrhage.The reviewed results support the evidence of elevated IL-33 serum levels in the acutisation of schizophrenia and depression,suggesting the involvement of this axis in the processes of relapse and recurrence of mental disorder[37].
IL-33 ROLE IN CANCEROGENESIS
IL-33 exerts its function by binding to the ST2 receptor,expressed on T helper (Th) 2 cells,but skewing toward Th1 cytokines has been found in MS[60,61].IL-33 has been categorized as a promoter of Th2 immunity by inducing the production of IL-4,IL-5 and IL-13,M2 polarization of macrophages,and eosinophil recruitment[62].The Th2-related cytokine can act as a pro-tumorigenic factor by limiting anti-tumor immunity and promoting extracellular matrix remodeling,but the localization of IL-33 is crucial for accurately distinguishing its effect in tumor biology[63].
IL-33 stimulates innate type 2 lymphoid cells (ILC2s),leading to the release of proinflammatory cytokines and type-2 inflammation[64].An association with allergic disease has been observed in patients with autism spectrum disorder,and an immune pattern initiated by IL-33,ILC2 and mast cells has been confirmed[65].To date,there are no data on the role of ILC2s in schizophrenia,and these findings in autism spectrum disorder as a neurodegenerative disease may serve as a basis for further investigation in psychosis.ILC2s are the largest subset in the lung and skin associated with pro-allergic and antiparasitic immunity.Dysregulation of their signaling circuitry may accelerate fibrotic responses and have predominantly carcinogenic activities[66].In the breast cancer model,increased endogenous IL-33 was observed during cancer progression,further facilitating the intratumoral accumulation of immunosuppressive IL-13-producing innate lymphoid cells and promoting the growth of breast cancer and metastases in the lungs[67].Escalation of systemic IL-33 secretion could precipitate the carcinoma development in neurodegenerative diseases and schizophrenia as a representative.
IL-33 IN THERAPEUTIC STRATEGIES FOR BREAST CANCER IN SCHIZOPHENIA
The first line of treatment for schizophrenia is antipsychotics,but nowadays it is surprising that these drugs could also have anticancer effects[68].Previous studies have shown that some antipsychotics may cause higher prolactin levels,called“prolactin-raising”,and thus an increased risk of breast cancer by promoting carcinogenesis and transition to invasive carcinoma[69].Breast cancer risk was higher in patients receiving first-generation antipsychotics and second-generation antipsychotics alone,as well as a combination of both,regardless of the mean exposure dose[32].However,it must be emphasized that according to the World Federation of Societies of Biological Psychiatry,breast cancer is not listed as an antipsychotic-induced hyperprolactinemia adverse effect[70].De Hertet al[71] reported that studies in patients with idiopathic hyperprolactinemia,prolactinomas,and Parkinson’s disease (PD) found no carcinogenic effects of prolactin.Hoehnet al[72] first described the possible inverse association between PD and neoplasms.This may suggest that excessive dopamine or prolactin release is not the exclusive mechanism leading to breast cancer development,but rather a milieu for more complex interactions.Our study exploring the impact of risperidone and paliperidone as prolactin-raising long-acting injections suggested a decrease in IL-33 serum levels in patients with schizophrenia in remission with possibly balancing and antitumorigenic properties[4].
The clinical presentation of schizophrenia differs in men and women and could be partly attributed to the neuroprotective properties of estrogens[73].Modulation of dopamine 2 receptor occupancy of antipsychotics can also be associated with estrogens[74].Estrogens protect women from infections and prevent mortality associated with inflammation by downregulating levels of pro-inflammatory cytokines IL-1β,IL-10,and tumor necrosis factor alpha[75].
Selective estrogen receptor modulators such as raloxifene have an antiestrogenic effect in the breast and uterus,but not in the brain and bone tissue[76].Of particular interest to us was that raloxifene was not only effective in improving schizophrenia,but even more effective in improving cognitive symptoms in postmenopausal women with low estrogen levels[77,78] (Figure 2A).This tissue-specific dual action of the drug could be simultaneously used for targeted breast cancer therapy and mental state in schizophrenia patients and specifically explored in the context of IL-33 secretion.
Guidelines recommend tamoxifen as a standard of care for premenopausal women for 5-10 years[79].In vitrostudies could guide further investigations on the beneficial properties of the prolactin-elevating antipsychotics thioridazine and chlorpromazine in enhancing the effect of tamoxifen in tamoxifen-resistant human breast cancer cells[80].IL-33 overexpression in breast cancer cells results in resistance to tamoxifeninduced tumor growth inhibition,while IL-33 knockdown corrects this problem[81].This knockdown could be achieved through the action of antipsychotics (Figure 2B).
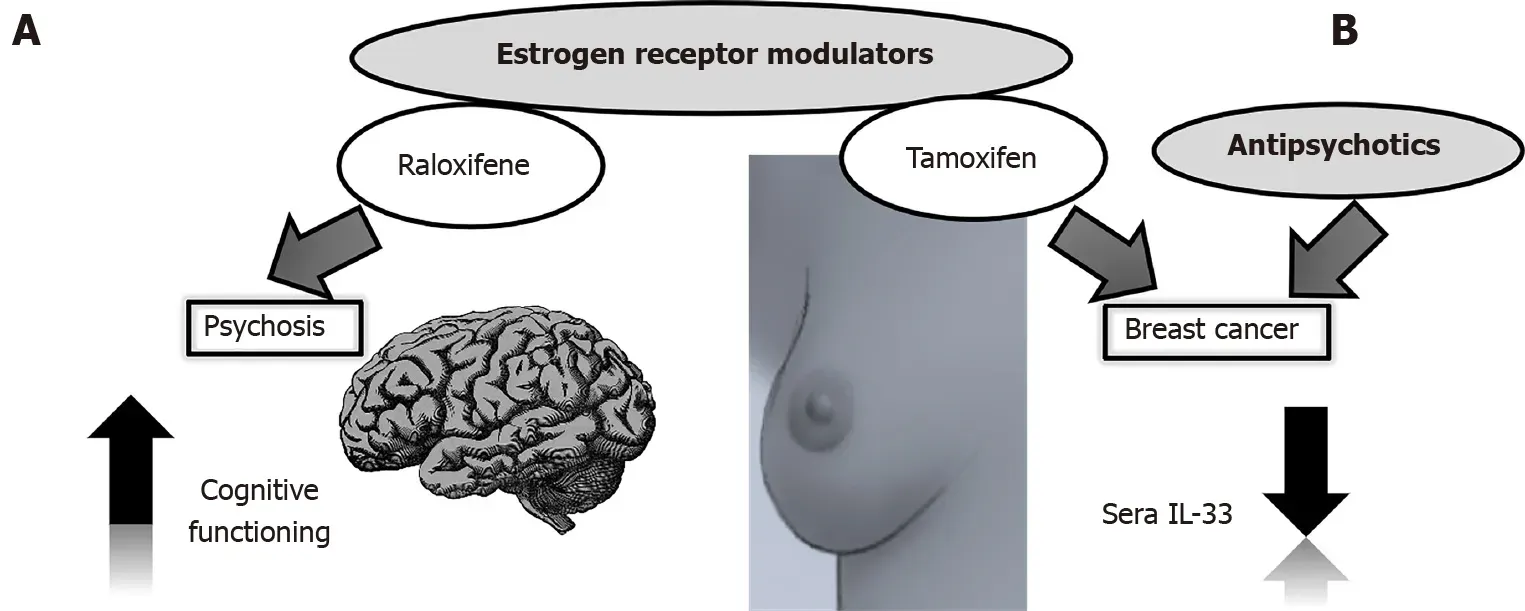
Figure 2 Estrogen receptor modulators and antipsychotics in interleukin-33 targeting strategies.
CONCLUSION
Individualized treatment is of great importance in modern medicine,as patients with schizophrenia must be treated equally.Diagnosis and treatment of somatic states in schizophrenia patients could influence behavioral changes and improve outcome.Prevention strategies for breast carcinoma onset in schizophrenia patients could be developed by understanding and recognizing the genetic background,lifestyle,and individual factors,altogether resulting in phase-specific immune dysregulation.IL-33 as a marker of innate immunity and an alarmin has been discussed in other neurodegenerative diseases.Further exploration of IL-33 as an alarmin in mental disorders should take into account gender,age at onset,duration of illness,frequency of disease acutisation,antipsychotic treatment,and a variety of comorbid somatic states including breast cancer.There appears to be an impact on the occurrence of positive symptoms and exacerbation of schizophrenia,but also on the progression of breast cancer,making IL-33 a candidate for centered therapy.We have shown that antipsychotics with their anticarcinogenic properties could be beneficial,possibly through prolactin elevation,tissue-specific estrogen-sparing drugs,and additional IL-33 downregulation.
ACKNOWLEDGEMENTS
This review was enriched by the valuable collaboration with the Center for Molecular Medicine and Stem Cell Research,at the Faculty of Medical Sciences,University of Kragujevac,Kragujevac,Serbia.We would like to thank Bojana Mircetic for language editing.
杂志排行
World Journal of Psychiatry的其它文章
- Effectiveness of cognitive behavioral therapy-based interventions on health outcomes in patients with coronary heart disease:A metaanalysis
- New-onset depression after hip fracture surgery among older patients:Effects on associated clinical outcomes and what can we do?
- Subgrouping time-dependent prescribing patterns of first-onset major depressive episodes by psychotropics dissection
- Self-compassion and resilience mediate the relationship between childhood exposure to domestic violence and posttraumatic growth/stress disorder during COVID-19 pandemic
- Psychiatric hospitalization during the two SARS-CoV-2 pandemic waves:New warnings for acute psychotic episodes and suicidal behaviors
- CPEB1,a novel risk gene in recent-onset schizophrenia,contributes to mitochondrial complex I defect caused by a defective provirus ERVWE1
