G-protein coupled receptors and synaptic plasticity in sleep deprivation
2022-01-11ShwetaParmarRamakrishnaTadavartyBhagavatulaSastry
Shweta Parmar,Ramakrishna Tadavarty,Bhagavatula R Sastry
Shweta Parmar,Ramakrishna Tadavarty,Bhagavatula R Sastry,Department of Anesthesiology,Pharmacology and Therapeutics,The University of British Columbia,Vancouver V6T 1Z3,British Columbia,Canada
Abstract Insufficient sleep has been correlated to many physiological and psychoneurological disorders.Over the years,our understanding of the state of sleep has transcended from an inactive period of rest to a more active state involving important cellular and molecular processes.In addition,during sleep,electrophysiological changes also occur in pathways in specific regions of the mammalian central nervous system (CNS).Activity mediated synaptic plasticity in the CNS can lead to long-term and sometimes permanent strengthening and/or weakening synaptic strength affecting neuronal network behaviour.Memory consolidation and learning that take place during sleep cycles,can be affected by changes in synaptic plasticity during sleep disturbances.G-protein coupled receptors (GPCRs),with their versatile structural and functional attributes,can regulate synaptic plasticity in CNS and hence,may be potentially affected in sleep deprived conditions.In this review,we aim to discuss important functional changes that can take place in the CNS during sleep and sleep deprivation and how changes in GPCRs can lead to potential problems with therapeutics with pharmacological interventions.
Key Words:G-protein coupled receptors;Metabotropic glutamate receptors;Gammaamino butyric acid-B receptor;Synaptic plasticity;Sleep deprivation;Memory consolidation
INTRODUCTION
Sleep is one of the fundamental needs of most conscious living beings.Earlier sleep was more analogous to just the idea of resting and having our mind and body recharge for the activities in our consecutive state of wakefulness.We have moved far along from this initial notion as we now know that even in the state of rest,our body is constantly working right from the molecular to the more visible physiological and psychological levels.The implications associated with sleep deprivation manifest themselves both behaviourally as well as physiologically.In a way,both these implications are correlated considering external stimuli enables an animal to act in a certain way depending on what kind of signals the brain has processed.The processing of such signals is mediated by cellular components like genes and proteins.In different neurological disorders like depression,loss of memory,psychosis,hallucination,anxiety,etc.,certain biomolecules are differentially affected.Synaptic plasticity which is thought to be a cellular correlate involved in memory consolidation and learning,is also affected following sleep deprivation.
In this review,therefore,we aim to understand and highlight the fundamental properties of sleep and sleep deprivation by keeping memory consolidation and synaptic plasticity as representative functions which get affected by the different states of sleep.In addition,any changes in G-protein coupled receptors (GPCRs) caused by sleep disturbance are emphasised.
THE DYNAMIC STATE OF SLEEP
Sleep and its significance
Sleep is indispensable for most animals.Extensive studies in this area have now led us to believe that sleep is not a static phenomenon but it is heterogenous and dynamic even if its physical nature appears completely passive.There are interconnections between the thalamus,cortex and hippocampus regions of the brain and this interplay of networks is known to be operated by stage specific oscillations that take place while we are asleep[1,2].Aserinsky was one of the first researchers whose studies overcame the concept of the cerebral cortex being dormant in sleep and gravitated the scientific community towards the reality of the brain being in an active state even while we are asleep[3].There are stages or periods in sleep which transition alternatively from an active period,which is manifested by rapid eye movements (REM) to an inactive or quiescent period,also known as the slow-wave sleep (SWS)[3].The oscillation patterns in the brain fluctuate during these two stages of sleep with high frequency and low amplitude waves being a characteristic of the REM/Late-night paradoxical sleep and the SWS portraying the contrary wave pattern[2,3].Advancement in this area has revealed many more biological functions like respiration,thermoregulation,etc.,along with the neurobiological processes of learning and memory formation in association with the alternating stages of REM sleep and SWS[4].
Disturbances in regular sleep cycles were assessed through the monitoring of EEG patterns.Waves of low amplitude and high frequency,associated with wakefulness,also occur during REM sleep.Deprivation of sleep is usually followed by a compensatory increase in REM sleep (REM rebound).This increase is interpreted to indicate an attempt in re-establishment of homeostasis in learning and memory as well as emotional balancing[5].Non-REM sleep is associated with high amplitude and low frequency delta waves (stages 3 and 4) along with stage 2 spindle activity[2,3].Sleep deprivation increases the amplitude of the waves associated with spindle activity but reduces spindle density[6-9].Upon chronic sleep restriction,the power density of theta wave frequency increases[10,11].Therefore,lack of sleep seems to result in changes in REM as well as non-REM sleep.
The functional significance of sleep guides us towards the negative implications of sleep deprivation.A balanced and sufficient sleep cycle acts as one of the major factors that determine the quality of human life.There are a wide range of environmental,psychological and physiological factors that lead to sleep deprivation.Environmental changes have influenced many aspects of our day-to-day life and sleep disturbances can arise due to an increase in surrounding noise as well as fluctuations in light and temperature[12].These factors add on to the list of causes that negatively burden the state of mind.Mental health also plays a crucial role in maintaining a regular sleep pattern since the prevalence of stress,anxiety,depression,etc.,affects regular sleep cycle which can translate into insomniac conditions.Psychosis disorders like schizophrenia or neurodegenerative diseases like Alzheimer’s have often been associated with issues related to sleep deprivation like a reduced REM sleep cycle as well as a lowered sleep spindle activity[13,14].Apart from mental disorders,pathophysiological illnesses (e.g.,cancer,diabetes,respiratory disordersetc.) also often result in sleep disturbances due to manifestations like pain or difficulty in breathing.Interestingly,lack of sleep can also increase the prevalence of such physiological disorders since an important function of sleep is the regulation of the immune system[15].Modern lifestyle changes like uneven working hours,over consumption of caffeine along with an increase in screen-time exposure have potentially interluded the quality and quantity of sleep.Homeostasis of the normal biological circadian rhythm is required for better cognition and task performance.For example,studies have implicated that night shift-workers,especially in chronic situations,experience varying levels of cognitive impairment and task performance[16,17].Drug abuse and alcohol consumption which can lead to substance use disorder have also been recognized as a growing cause of sleep disruption.For instance,alcohol consumption is indicated to suppress REM sleep which in turn causes impairment in performing procedural tasks[18].Conversely,lack of sleep affects the activity balance of important neurotransmitters like dopamine (DA) in the brain and this has been indicated to increase vulnerability to the use of drugs[19].The above mentioned negative influences of sleep deprivation are just few of the many but it helps in bringing the importance of sleep deprivation into perspective.
Without sufficient amount of sleep,the brain cannot adequately perform the processes that take place during the state of sleep.Sleep deprivation affects the ability to concentrate,intake information and mediate that information through neuronal signalling,learn as well as process memories for consolidation.Furthermore,it is affected by the signalling and expression profiles of many biological molecules including the GPCRs[20,21].These functions are related to the different stages of sleep and hence there are stage specific implications of sleep deprivation.For example,lack of REM sleep has been directed towards to an impairment in the development and expression of emotional and spatial memories[22,23].Additionally,disruption of SWS is suggested to reduce attention span,affect motor activity and task performance[24].Since learning and task performance are dependent on the memory processing of various responsible stimuli,the concept of memory consolidation has been highlighted to understand the significance of sleep and its associated cellular machinery.
Sleep and memory consolidation
Memory is the ability of living beings to retain information that they have acquired through their various day to day experiences and activities.The brain consolidates memories in different stages starting first from acquiring the memory through learning experiences,encoding and then consolidating those memories to be recalled or retrieved upon stimulus[25].Interestingly,even the processed memories can become transient overtime and a reconsolidation of those memories is required for which sleep is essential[26].While we sleep,the brain is actively carrying out its functions and ‘offline-reprocessing’ of memories appears to be one of them[3].The presence of the rapid and spontaneous oscillations in the cortical networks of the brain during wake and sleep periods have been known to get triggered upon sensory stimuli[27,28] which can get incorporated in the brain in form of a memory.This gradual incorporation of acquired memories into the different regions of the brain is more crucial for long term memory than the short-term memory,which points towards a quicker mechanism of action[29,30].REM sleep and SWS are generally associated with the consolidation of long-term implicit (non-declarative) and explicit (declarative)memories,respectively[31-33],both working in a double-step process.In support of the alternate nature of the REM sleep and the SWS,work done on the memory consolidation function of these stages has revealed that SWS also has its part to play in reactivation and redistribution of the spatio-temporal patterns of the neurons which are observed during the encoding stage of memory function when we are awake[31,34-36].When there is distraction or disruption of the periods immediately after the learning or teaching stimulus,the formation of memory is interrupted and consequently the task reperformance associated with that memory is affected[29,30].Now,since memory is processed during the state of sleep,it appears that disruption of this state during sleep deprivation can also affect memory consolidation in the brain.For instance,there have been studies demonstrating how lack of sleep can cause weak recollection of visual stimuli or how motor skill learning improvements are dependent on a good night-time sleep[37-39].
The discussed oscillation and wave patterns during the state of sleep are at least partly a result of the electrical impulses that are transmitted between neuronsviathe synapses.This transmission modifies the synapses and hence synaptic plasticity is an important consideration while studying about the various functions of sleep including memory consolidation.To better understand this correlation,we will first discuss the regions and cells of the brain that are associated with sleep dependent functions and how these regions communicateviasignal transmission.
The cortical hippocampal dialogue
As mentioned earlier,activities in the brain during the state of sleep are in conjunction with changes in the oscillation and wave-patterns in specific regions of the brain.These complex wave-sequences form a characteristic component of the neocortical and the thalamic system,which co-ordinate in order to exhibit the various functions of sleep,including memory consolidation.Although the mechanisms that are involved in the functions and regulation of memory consolidation are yet unclear,various cellular processes in brain regions have been studied as potential candidates.Amongst these,the hippocampus is one of the main centers for processing sleep dependent memories through neurons like granule cells and pyramidal cells[40].The significance of the hippocampus and its sub-regions may be attributed to its function of independently giving the neuronal networks the ability to process multimodal information and building an integrated system that can represent the newly acquired information for its consequent storage in the higher centers of the brain in the form of 'memories'[41].Inspired by this finding,a two-staged memory consolidation concept was put forward which essentially highlighted the events wherein there occurred a transmission of information between the hippocampus (short-term storage) and the neocortex (longterm storage) through the exploratory theta burst stimulations of the granule cells and sharp waves of the pyramidal cells[42].While the hippocampus is concerned with high frequency oscillations and sharp wave ripples in the CA1 and CA3 regions,the neocortex shows slow oscillations and spindles during SWS[43].During SWS,the signal from the hippocampus to the neocortex is transmitted through the CA1 neurons whereas during REM sleep the information enters the hippocampus from the neocortex through the CA3 neurons;thus,causing the CA1 neurons to hyperpolarize the CA3 neurons through the entorhinal cortex[43].This rhythmic communication of oscillations gives rise to coupling as well as consistent spike timing relationship between the neocortex and the hippocampus which further strengthens the information processed for learning and memory consolidation[43-45].
As discussed above,there seems to be a pattern of relay of information that is associated with sleep dependent functions between the hippocampus and the neocortex.Since the relayed signals go through synapses,any plasticity at the junctions can significantly alter the above mentioned patterns.Therefore,in this review,a discussion on the phenomenon is made.
THE ROLE OF SYNAPTIC PLASTICITY IN SLEEP AND LEARNING
The active nature of brain during the state of sleep so far has been advocated by multiple studies on the specific regions of the brain which primarily show distinct wave patterns and relay information through signal transmission.Transcending from the Hebbian[46] theory,the strength of these signals has been associated with the continuous firing of action potentials from a pre-synaptic neuron to a post-synaptic neuron.It has appeared that a signal gets stronger when it is repeatedly transmitted from one synapse to another and this is where the phenomenon of synaptic plasticity comes into play.When there is continuous firing of signals between neurons,there are changes that take place at the synapses of these cells,which means that the cellular components at these junctions also undergo changes and consequently affect the strength of the signal being transmitted.This concept can be appreciated by looking at instances wherein it appears that by repeatedly performing a task,we get better and better at it.In a similar way,a repeated stimulus to the brain results in repeated signal transmission from one neuron to another and this can have the potential to strengthen that signal[46].This takes place through synaptic plasticity which entails molecular as well as electrophysiological changes at the synapses.The electrophysiological changes can be characterized by long term depression (LTD) and long term potentiation (LTP),each relating to the persistent weakening and strengthening of the synapses,respectively.Initial work done in this area revealed that at the excitatory synapses,there occurred a use-dependent and long term strengthening of the synapse upon high frequency stimulation of the pre-synaptic fibres in the hippocampus,which was termed as LTP[47].Analogous to the Hebbian[48] findings,experimental data at cellular level has indicated that synaptic plasticity in the form of LTP in the hippocampus is input specific and can be associative in the sense that if activity in inputs coincides with a depolarization of the post-synaptic neuron,the active synapse gets strengthened[48-50].The post-synaptic depolarization is thought to remove a Mg2+block of the N-methyl-D-aspartate (NMDA) receptor coupled ionic channel allowing a Ca2+influx which activates protein kinases that sets up the expression of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors on the dendritic spines at synapses leading to an increase in response to the released glutamate,the transmitter[51].The post-synaptic depolarization is also suggested to facilitate the release of a retrograde messenger that causes an increase in glutamate transmitter release and,thus,an increase in synaptic transmission[49].Upon stimulation of synapses at lower frequencies than those that induce LTP,the induction of LTD also involves an influx of Ca2+ions,although the surge of Ca2+ions is comparatively lower than that in case of LTP[52].The difference in the level of Ca2+influx results in activation of phosphatases that are responsive to lower concentration of Ca2+ions.This subsequently leads to a dephosphorylation of AMPA receptors and a reduction in their activity which decreases the response to the released glutamate and causes an overall reduction in synaptic efficacy[53].Therefore,the phosphorylation of AMPA receptor through kinases in LTP is counteracted by the phosphatases during LTD which leads to an interference and reversal of LTP.This interplay promotes modulation of synaptic plasticity which as explained in the section below,appears to be crucial for memory processing and learning.The comparative details of LTP/LTD associated molecular events are summarized in Table 1 and are also discussed further in subsequent sections of this review[54-66].
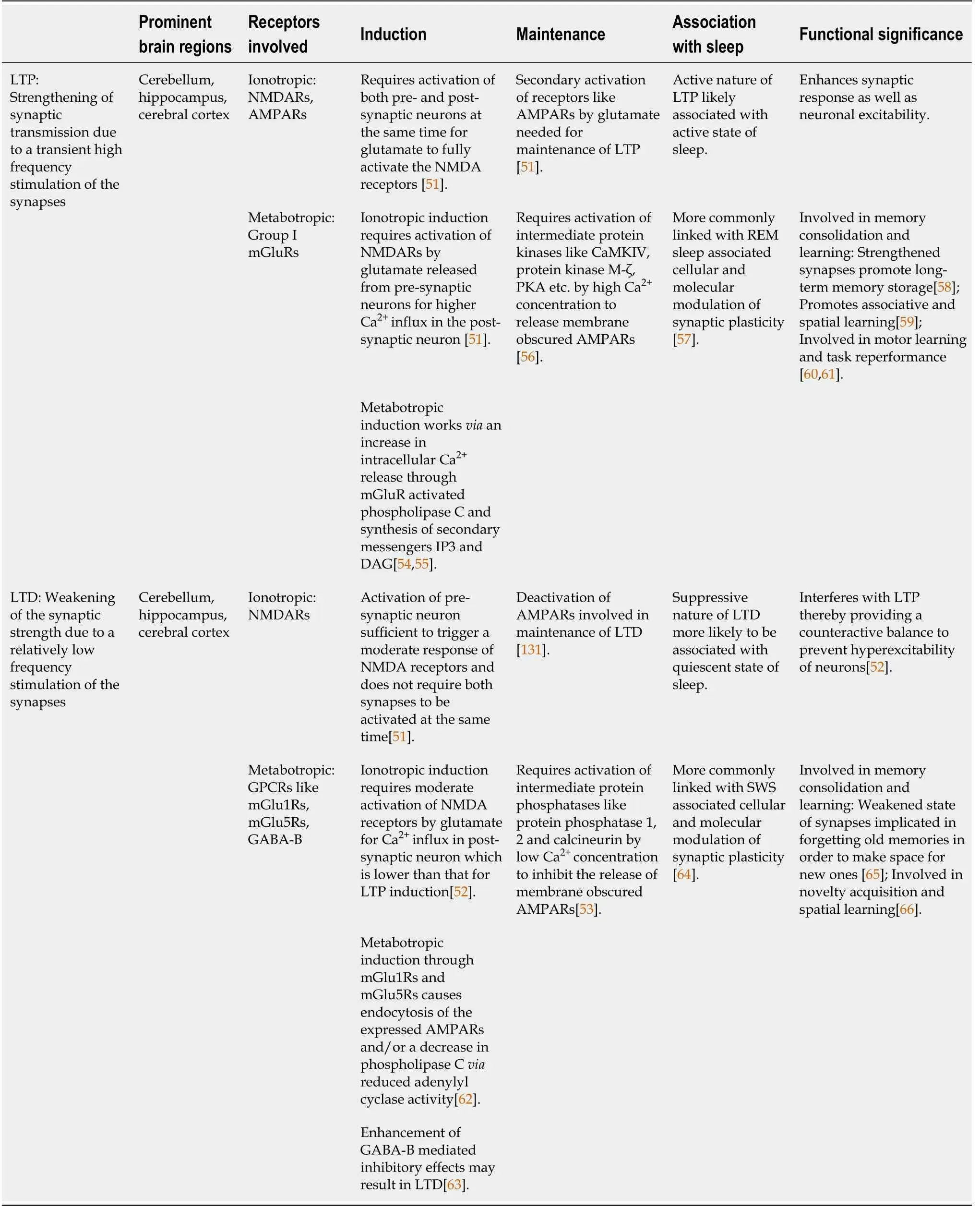
Table 1 Comparative features of long term potentiation and long term depression and their implications in sleep and associated functions
The process of learning and memory consolidation that takes place while we are asleep can also be attributed to further molecular changes that can take place following the synaptic strengthening.A disruption of these due to sleep deprivation can,thus,have a negative consequence.
LTP and LTD as cellular correlates for learning and memory formation
In spite of having a breakthrough discovery about LTP and LTD causing long-lasting modification in the synapses in the 1970s[47,67],the functional implications of LTD and LTP in learning and memory processing are still under a lot of conjecture.While the initial studies relied more on the active state of synapses through LTP as the focal model for learning,subsequent studies also implicated the role of LTD by highlighting the fact that a consequent increase and decrease in signal strength is what allows the phenomenon of synaptic plasticity to function without getting worn out[52,58,59,68].For instance,Tsumoto[65]’s work highlights LTD in cerebral cortex being responsible in 'forgetting' certain memories in order to make place for the new ones.Looking back at Hebbian[46] and the follow-up findings,the role of synapses and the existence of synaptic plasticity as cellular correlates of learning and memory consolidation become apparent.As discussed in the previous sections,learning and memory consolidation are a result of collective cellular communications through the processing of the learning stimuli.The cortical-hippocampal dialogue is thought to drive these learning stimuli forward in the brain to encode,process and store the information acquired through those stimuli.Several lines of studies demonstrate the correlation of memory acquisition with the processing of spatial learning to LTP and LTD[65,66,69].Furthermore,exploratory behaviours and learning are known to promote information acquisition[70] and experimental studies on rats that were exposed to hole board exploration resulted in events that reversed LTP upon exposure to a novel environment with inductive LTD mechanisms being activated.These rats showed habituation upon a second exposure to that environment indicating LTD’s possible role in remembering the previously acquired information of that environment[66].On the contrary,work done on adult rats has also revealed the significance of LTD and LTP by demonstrating that the preservation of LTP is hampered upon an increase to LTD susceptibility and this also interrupts the CNS functions including learning and information retention[71,72].Therefore,there are debatable influences of LTD on learning but its role in novelty acquisition and spatial learning[66,73] deserves further investigation.
What exactly is harnessing this LTP and LTD in these regions of the brain and how these activity dependent synaptic changes relate to memory consolidation and learning? In this review,an attempt is made to find some answers.An early hypothesis put forward by Lynch and Baudry[74] on the biochemistry of memory involved an examination of calcium proteinase-receptor interaction.They demonstrated that in the forebrain sub-synaptic membranes,an influx of Ca2+causes a long lasting increase in the number of glutamate receptors through its activation of the enzyme calpain which is a proteinase.The activated form of this enzyme degrades the membrane-anchored cytoskeleton protein fodrin and as a result,exposes the obstructed glutamate receptors.The effects of this Ca2+proteinase-receptor interaction could functionally modify neuronal circuits as well as showed similar biochemical effects that are seen post learning[74].This and other related studies examining molecular mechanisms were,therefore,examined to find correlations between synaptic strength and memory consolidation.
Most work done on excitatory neurons like the hippocampal pyramidal cells reveal that tetanic stimulation of inputs can readily induce LTP that lasts for hours,days and can even be permanent,making it a good cellular correlate of memory and learning[75-77].In vivoandin vitroinduction of LTP and LTD through external theta burst stimulations in model organisms[47,49,78] has revealed the various cellular components that present themselves as functional entities in the process of memory formation and learning.It is already well established that receptors like metabotropic glutamate receptors (mGluR),ionotropic NMDA and AMPA receptors are mediators for LTP and LTD through the increase or decrease in the Ca2+concentration,respectively[51,79,80].Both ionotropic (e.g.,NMDA,AMPA receptors) and metabotropic (e.g.,mGluR) receptors are known to be involved in synaptic plasticity but project different mechanisms of action which are employed for mediating excitatory and/or inhibitory signals[81].The ionotropic receptors are heteromeric compounds that consist of a ligand binding site and pore forming channel that combines the function of the receptor and ion channel into a single unit for the mediation of postsynaptic potentials.On the other hand,metabotropic receptors do not comprise of an ion channel but mediate their effects on other ion channelsviaintermediate effector molecules and secondary messengers.Ionotropic receptors like NMDA and AMPA receptors get activated upon binding of the neurotransmitter glutamate on the ligandbinding site and this leads to an opening of the ion channel subunit of the receptor which is permeable to Na+and K+ions to induce an inward current causing depolarization through the excitatory post-synaptic potential (EPSP) that lasts for a few milliseconds[51].NMDA channel is permeable to the above cations and also to Ca2+which affects the downstream signalling cascade that regulates synaptic plasticity[82].Glutamate can also bind to the mGluRs which leads to an activation of signal transduction molecules like the receptor associated G-protein that gets detached from the receptor and directly activates the nearby ion channels like NMDA receptors or promotes the action of various effector molecules that indirectly activate other ion channels.mGluRs can also regulate the intracellular Ca2+levels through membrane proteins and secondary messengers.The influx of ions through these receptors therefore results in post-synaptic potentials that have a slower induction and longer response time ranging from a few milliseconds to even much longer times[83].LTP is known to be predominantly mediated by the ionotropic receptor NMDA when high frequency stimulations result in a build-up of EPSPs due to the activation of AMPA receptors causing the removal of a Mg2+block of the NMDA channel[51,58].However,metabotropic receptor mediated LTP has also been observed with mGlu1 and mGlu5 receptors wherein mGluR activation can lead to LTP induction through NMDA receptors as well asviathe modulation of intracellular Ca2+levels[84,85].As mentioned before,LTD arises upon a lower frequency stimulation of the excitatory synapses than needed to induce LTP and also involves NMDA receptors but with a resulting deactivation of the AMPA receptors.LTD mediated by metabotropic receptors like mGluRs usually involves secondary messengers that modulate intracellular Ca2+levels[62].Other metabotropic GPCRs like gamma-amino butyric acid-B receptors (GABA-B Rs) can also induce LTD due to their inherent nature to transmit inhibitory signals[63].The details of these molecular and cellular features of synaptic plasticity will be discussed more in the coming sections.Since activity dependent synaptic plasticity and memory consolidation occur sequentially,cellular receptors and other associated molecules that mediate LTP and LTD consequently find themselves to be associated with learning and memory processing[61,86].
Significance of synaptic plasticity in sleep and sleep deprivation
The active state of sleep has been associated with many functions,including synaptic plasticity (LTP and LTD),cognitive learning and memory processing[87].In fact,it is also reasonable to suggest that learning paradigms that promote sleep and synaptic plasticity also enhances brain development[57,88].
The above notions have risen from earlier hypotheses that sleep affects the patterns of neuronal circuits in the brain[42,89].REM sleep is associated with long-term memory consolidation and learning events promote synaptic plasticity.Moreover,REM is required for brain development and an increase in intensive learning activity significantly enhances the duration and number of REM sleep cycles and promotes synaptic plasticity[90,91].Correlating sleep cycles and synaptic plasticity,LTP has been credited with many learning functions of the brain,especially in the hippocampus.It appears that sleep deprivation results in an inhibition or decrease in the level of LTP and an impairment in the associated learning[92,93].This trend has also been observed in rats during sleep fragmentation where in there was a decrease in LTP through NMDA receptors in the CA1 neurons of the hippocampus[94].Interestingly,the reduction in LTP is also associated with a downregulation of cortactin,which is a dendritic cytoskeleton protein,pointing towards the role of LTP in the structural modification of neurons and a disruption upon sleep deprivation[95].While the impact of sleep deprivation on LTP seems clear,the fate of LTD has not been adequately identified.However,LTD is known to protect neurons by regulating neuronal hyperexcitability and preventing a saturation of network activity[59].Additionally,LTD’s role in memory consolidation has been seen through events like novelty acquisition and spatial learning or even in making space for new memories through the concept of ‘forgetting’[65,66,73].Since these aspects of memory processing are sleep dependent,it is logical to suggest that sleep deprivation can affect LTD.In agreement with this line of thinking,an increase in hippocampal LTD following sleep deprivation was observed in rats[96].
In conclusion,sleep seems to promote synaptic plasticity,especially LTP,and memory consolidation,while sleep disturbances appear to adversely affect both.LTD seems to be enhanced following sleep deprivation and,thus,may be associated with interference with memory consolidation.
SIGNIFICANT REGULATORY MOLECULES IN SLEEP AND SLEEP DEPRIVED CONDITIONS
Molecular events in association with sleep and synaptic plasticity
After a brief introduction of a few cellular components involved in the functioning of synaptic plasticity in section II,some detailed events in relation to the state of sleep are described here.Going back to the fundamentals of electrophysiology,an electrical signal is transmitted when a neurotransmitter gets released from the pre-synaptic cell and activates its specific receptor on the post-synaptic cell.This further invites a series of downstream reactions that ultimately give rise to the required effect.Neurotransmitters and receptors are cellularly synthesized molecules and if the signal pattern changes during stages of sleep and wakefulness,changes in the expression of genes and proteins,that are precursors for these molecules,can also occur.In sleep and sleep deprivation,glutamate,acetylcholine,DA,serotonin,etc.,are important transmitters to pay attention to.Acetylcholine,for instance,is known to be correlated with the amplitude of theta burst oscillations that take place in the hippocampus.Events that lower the levels of acetylcholine can reduce the amplitude of such oscillations[97].Since sleep sensitive processes of learning,like memory consolidation demonstrate oscillatory changes,neurotransmitters like acetylcholine are significant to such brain functions.Additionally,imbalances in the levels of neurotransmitters like DA and serotonin are associated with many neurogenic issues like depression,anxiety and psychosis;all of which are symptoms often seen in patients that have been sleep deprived[98-100].
Focusing more on the receptors involved in mediating synaptic plasticity in excitatory glutamatergic pathways,the glutamate receptors present themselves as frontline players and amongst these,the ionotropic NMDA and AMPA receptors are known to depolarize/excite the post-synaptic neuron through an influx and efflux of Na+and K+ions,respectively[83].The main inhibitory neurotransmitter of the CNS is GABA and it acts upon the two types of GABA receptors:Ionotropic GABA-A/GABAC receptors and metabotropic GABA-B Rs.GABA’s contribution to modulating LTP and LTD in excitatory pathways,owing to the strategic location of its receptors to regulate input and output signals in the pyramidal neurons,is well documented[101-103].LTP and LTD are carried out by the long term excitatory and inhibitory actions of these receptors including GPCRs,which will be looked into in the next section.Ca2+has been a crucial factor and its increase has shown to depend on the activation of NMDA receptors by glutamate,Ca2+release from intracellular stores and its influx through the voltage gated calcium channels[104].The binding of the released calcium to calmodulin and then further to two other proteins:Ca2+/calmodulin dependent protein kinase type 2 (CAMK II) and calcineurin,results into LTP and LTD,respectively[104].
In continuation with the molecular changes associated with synaptic plasticity,protein synthesis and post-synaptic biochemical changes have been implicated in different phases of LTP[105].For instance,brain derived neurotrophic factor,protein kinase Mζ,calcium/CAMK II,and activity-related cytoskeletal protein,have so far been indicated in the induction or maintenance of late phase LTP[106-109].Whether these or other factors participate during sleep-induced consolidation of memory is unknown and needs future investigation.Another not so conventional molecule that is speculated to promote synaptic plasticity is nitric oxide (NO).What is intriguing about this molecule is that the enzyme which synthesizes it is activated by an increase in Ca2+concentrations which again is dependent on receptors like NMDA which promotes synaptic plasticity[57,110].More insight on such accessory molecules needs to be put forward through detailed experimental work and NO has so many other important physiological functions like decreasing vascular resistance and increasing cerebral blood flow and oxygenation rate[111] or playing a key role in mediation of an immune response against infectious diseases[112].Thus,its definitive role in sleep deprivation through synaptic plasticity can be a major contribution to this field of research.
In summary,these are just few of many cellular components which have been discussed here to help understand and appreciate the intricate network of cellular molecules in driving synaptic plasticity which also play an important part in carrying out sleep associated functions.The functions carried out by these regulatory molecules have been known to get altered during the state of sleep and sleep deprivation.This means that the genes that transcribe the proteins for these receptor molecules also undergo changes.Differential gene expression has been observed during both the states of sleep and wakefulness.In fact,modulation in the gene expressions during these two states have shown that around 10% of the total cerebral cortex transcripts are differentially expressed[113].For instance,the mRNAs for the receptors of the inhibitory neurotransmitter GABA,shows a higher expression during the state of sleep as compared to the state of wakefulness[113,114].
GPCRs
GPCRs by definition are receptors that mediate their effects after binding to G proteins which are heterotrimeric molecules made up of alpha,beta and gamma subunits that function by phosphorylating the nucleotide guanosine triphosphate to guanosine diphosphate.Structurally,GPCRs are transmembrane proteins with multiple transmembrane domains and the homology of these domains categorize them into 4 classes (A,B,C and F).About 90% of the non-sensory GPCRs are expressed in the brain and appear to play regulatory roles in various neurological processes.The expression profile of these receptors is extremely high in the hippocampus (around 300 GPCRs) and about 20 of these GPCRs display their potential role in synaptic plasticity[115].The signalling pathways that are induced by the GPCRs regulate both pre and post-synaptic components which ultimately can affect synaptic plasticity as well as the release of pre-synaptic vesicular molecules[116].They are also known to be involved in events of structural plasticity as well as cognitive development[117].GPCRs can also alter NMDA receptors by a direct action at the CA1 synapses,thus indirectly affecting NMDA receptor-mediated synaptic plasticity[118],suggesting the possibility for receptor co-expression.Furthermore,GPCRs also seem to elicit their effects intracellularly while modulating synaptic plasticity[119].The range of neuronal functions that are attributed to GPCRs needs a more coherent and concrete investigation to be well defined in relation to sleep deprivation,synaptic plasticity and memory consolidation.For the purposes of this review,two major types of GPCRs known as the mGluRs and GABA-B Rs are discussed in more detail.Other GPCRs,especially those that are important for drugs used in psychiatry,are also discussed.
mGluRs
The mGluRs belong to the class C of GPCRs.They are categorized into three groups based on their signal transduction pathways.Group I (mGlu1R and mGlu5R),Group II(mGluR2 and mGluR3) and Group III (mGluR4,mGluR6,mGluR7,mGluR8)[120].The subdivisions are based on the difference in their physiological activity and structure.The group I receptors display a post-synaptic location and are known to actviathe Gq protein through the activation of phospholipase C protein and the synthesis of the secondary messengers such as inositol-1,4,5-triphosphate (IP3) and diacylglycerol(DAG).The general pathway involving these secondary messengers results in the release of intracellular Ca2+stores which is a prerequisite for the induction of synaptic plasticity[54,55].These receptors are also involved in the modulation of neuronal excitability in the hippocampus pyramidal cells wherein they activate cationic conductance through the reduction in resting K+current and inhibition of Ca2+ion channels[121,122].On the other hand,mGluRs were also seen to induce IP3-mediated release of Ca2+from intracellular stores through stimulation of CA3 pyramidal neurons.Unlike the Group I mGluRs,Group II and III mediate their functionsviathe Gi/Go protein.They are negatively coupled to adenylyl cyclase and inhibit the production of cyclic adenosine monophosphate Cyclic adenosine monophosphate(cAMP)[123] which results into activation of K+channels and inhibition of Ca2+channels.These two groups have a very distinct pre-synaptic localization pattern with Group II being predominant in extra synaptic sites whereas Group III in the synaptic sites.Additionally,the Group III receptors also appeared to be segregated in correlation to their target post-synaptic neurons[124].
GABA-B Rs
The metabotropic GABA-B Rs are widely known for their inhibitory action in the CNS and like mGluRs,also belong to the class C GPCRs.Structurally,these are obligate heterodimers made up of GABA-B1 and GABA-B2 receptor subunits which are genetically co-expressed and are homologous to the structure of mGluRs[125,126].Gene expression studies for this receptor have shown that heterodimerization is essential for the GABA-B Rs to elicit their function since the individual expression of the GABA-B1 subunit in the target mammalian cells results in immature and low functionality receptors as compared to the heterodimer[127].These receptors show a wide cellular and sub cellular localization pattern through their expression on presynaptic,post-synaptic as well as extra synaptic regions in the brain[128].Based on work performed on rat hippocampus,the two subunits of GABA-B Rs:GABA-B1a/b and GABA-B2,appear to be more on the post-synaptic regions as compared to the presynaptic ones.Furthermore,they also displayed an abundant distribution of GABAB1a/b on glutamatergic synapses on the spines and on dendritic shafts of pyramidal cells[128].GABA-B2 on the other hand,has a dominant extra-synaptic localization.These localizations are indicative of their functions and considering the range of cellular and subcellular occurrences of these receptors,they show a promising involvement in a great number of neurogenic processes.For example,their presence at the glutamatergic terminals points towards their involvement in glutamatergic neurotransmission[128].
Functionally,GABA-B Rs induce their slow and long lasting actionviathe Gαi/o proteins through the inhibition of adenylate cyclase and like the mGluRs,they too affect the Ca2+and K+conductance[129].GABA-B Rs are known to control the calcium dependent neurological processes through the inhibition of voltage sensitive Ca2+channels[85,130].On the pre-synaptic sites,GABA-B Rs appear to be negatively coupled to Ca2+channels and block the Cav2.2 (N-type) and Cav2.1 (P and Q type)voltage gates channelsviathe action of G-proteins.They also have the ability to affect K+channel directly without the involvement of a G protein.This controls the release of neurotransmitters from the pre-synaptic neurons and thus GABA-B Rs have the potential to regulate the onset of a stimulatory signal[85,130].At the post-synaptic sites,these receptors mediate their effectsviathe G proteins through a slow hyperpolarization by inward rectification of K+channels such as the GIRK channels as well as Ca2+channels[131].GABA-B Rs are,therefore,diverse both in their structural as well as functional mediation of inhibitory signals and show immense potential to regulate synaptic plasticity in association with sleep deprivation.
Serotonin,DA and norepinephrine receptors
The serotonin,DA and epinephrine group of GPCRs are very important in psychiatry considering their ligands are responsible for a wide range of CNS functions like cognitive learning and sleep-wake behaviours and are implicated in many neurological disorders.Serotonin receptors,also known as the 5-hydroxytyrptamine(5-HTA) receptors are classified into 7 families (5-HT1 to 5-HT7),all of which are GPCRs except the 5-HT3 receptor[132].These are further divided into their respective 14 sub-types [e.g.,5-hydroxytyrptamine subtype 1A (5-HT1A) to 5-HT1F] based on their function and location[132].They exhibit their function mainly through the Gαi,G αq/11,and Gαs proteins followed by modulation of Ca2+concentrationsviathe regulation of adenylyl cyclase and cAMP or activation of phospholipase C producing IP3 and DAG[133].In the CNS,5-HT1 and 5-HT5 are associated with inhibitory signal transduction and the others are excitatory in nature;with pre-and post-synaptic localizations.For instance,the 5-HT1A receptors,which are widely implicated in depression,anxiety and learning,are found in both pre-synaptic (auto-receptors) and post-synaptic (heteroreceptors) locations[134].Additionally,structural significance in this class of receptor through dimerization with each other and other GPCRs has also been indicated as a functional prerequisite for the cellular trafficking and functioning of 5-HT receptors[135].
DA receptors are GPCRs divided into 5 subtypes (D1-D5) and exhibit their action through the Gs/olf and Gi/o proteins by activating or inactivating adenylyl cyclase,respectively[136].These receptors have a wide pre and post-synaptic distribution pattern in the CNS which contributes to the functional significance of DA[137].For instance,the D1 and D2 receptors are abundantly localized in the striatum and substantia nigra region of the brain and are involved in the nigrostriatal pathway which is responsible for the control of bodily movements[138].These are also majorly distributed in the ventral tegmental area (VTA) of the brain which is connected to the ventral striatum and this connection promotes reward-associated behaviourviathe meso-limbic pathway[139].Furthermore,along with a localization pattern observed in both pre-and post-synaptic regions,co-expression is also a general trend seen with DA receptors[140].Lastly,the GPCRs that are the target for the catecholamine norepinephrine/noradrenaline (NA) are classified into alpha-1,alpha-2,beta-1,beta-2 and beta-3 receptors and they are localized on both pre-and post-synaptic sites.Alpha-1 follows the Gq protein mediated elevation of calcium levels by activation of phospholipase C producing IP3 and DAG and the other three subtypes workviathe modulation of adenylyl cyclase pathway[141].Interestingly,co-localization patterns have also been observed between DA and NA receptors in the rat prefrontal cortex[142].Whether such co-localizations and/or dimerization have implications for pharmacological actions of therapeutic agents need further investigation.
SIGNIFICANCE OF GPCRS IN SYNAPTIC PLASTICITY AND SLEEP DEPRIVED CONDITION
Role of GABA-B and mGluRs
As introduced above,GPCRs appear to be involved in processes that regulate the transduction of action potential especially in the cells of the hippocampus region of the brain.Both LTD and LTP have demonstrated a pattern of being modulated by such regulatory molecules.It is already well established that Type I mGluRs induce LTD through the synaptic endocytosis of AMPA receptors in the hippocampus[143].Similar lines of studies have pointed towards mGluRs in the CA1 pyramidal cells being involved in the induction of LTD through post-synaptic elevation of Ca2+levels[144].Interestingly,studies on the cerebellar Purkinje fibres have also demonstrated that activation of GABA-B Rs enhances LTD through an association with mGluRs[63].Even though the mechanism of action of these receptors may be related,many of their molecular machineries have their own distinctive features while promoting cellular LTD.For instance,in the Purkinje fibres,LTD mediatedviaGABA-B relied on the beta gamma subunits of the G-protein whereas mGluR mediated LTD depended on the alpha subunit[63].So far,it appears that these GPCRs are associated with the induction of LTD and reviewing other related studies,we know that NMDARs are the main mediators of LTP.Contrary to these findings,recent experimental work has put forward a type of LTP that is induced in the absence of NMDARs and requires the activation of Type I mGluRs[145].As for the role of GABA-B Rs in LTP,its auto receptors depress their own activity through negative feedback and promotes NMDAR mediated LTP,thus maintaining a balance between LTP and LTD[146].Considering how the hippocampus and the cortex communicate and manifest their functions of learning and memory consolidation in the state of sleep through synaptic plasticity,it is reasonable to imply that these GPCRs modulating LTD and LTP are significant for this purpose.It also seems that co-expression of GPCRs and their heteromeric subunits is a repeated trend seen in studies that show the association of GPCRs with LTD[63,125,126].The functionality of GPCR dimerization has also been put forward in earlier demonstrative studies which showed that the GABA-B1 subunit needs the GABA-B2 subunit to reach the cell surface and that GABA-B1 is responsible for agonist interaction whereas the B2 subunit works towards the G-protein activation[147,148].Furthermore,gene knock-out or elimination studies for these subunits have also indicated the dependence on dimerization for the functioning of this receptor and that in some regions of the brain,GABA-B subunit gene elimination does not affect the functionality of the receptor which implies that it is replaceable by other G-protein activating molecules[149] and thus may have the potential to associate with other GPCRs to mediate their actions.Progressing from these initial findings,recent work done on rats that were sleep deprived using gentle prodding and tapping has demonstrated that induced LTD of population EPSP in the hippocampus requires activation of mGluRs and GABA-B Rs along with an increase in Ca2+released from intracellular stores[150].In sleep deprived conditions,western blot analysis and coimmunoprecipitation studies revealed that there were elevated expression levels of mGlu1αR and GABA-B1 receptor subunit as well as enhanced co-expression and heterodimerization between mGlu1αR and GABA-B R1 subunit and mGlu1αR and GABA-B R2 subunit[150].
Role of 5-HT and other GPCRs
As introduced earlier,GPCRs for serotonin are diverse both in their localization and functions.Their effects are mediated by calcium levels,which again is crucial for synaptic plasticity.Furthermore,5-HT1A receptors are known to decrease NMDA receptor activity whereas 5-HT2A tends to increase it[151,152],thereby playing an opposing role in regulating NMDA mediated LTP.These are also co-expressed in hippocampal pyramidal cells and may direct studies towards their reciprocal action in regulating synaptic plasticity.Similar cross-talks have also been suggested between 5-HT and GABA-B receptors[153],which as already discussed,are involved in modulating LTD,especially in sleep deprived conditions.In relation to sleep associated studies,5-HT1 and 5-HT2 receptors appear to be involved in the regulation of REM sleep and sleep-wake behaviours[154].To further strengthen the significance of these receptors,experimental work has indicated that sleep deprivation resulted in a temporary increase in the expression profile of the serotonin receptor,5-HT1A through an enhanced suppressive effect of this receptor on the EPSPs recorded from CA1 pyramidal neurons[155].Studies also indicated a heterodimerization of 5-HT2AmGluR2 which is implicated in psychosis[156].However,in rat hippocampus,while there was a change in the expression profile of 5-HT1A receptor,there was no significant heterodimerization with mGluRs following sleep deprivation[155].Therefore,depending on the 5-HT receptor sub-type,it appears that sleep deprivation induces differential changes in their expression,co-localization and heterodimerization.
DA and NA receptors demonstrate somewhat analogous modulations of synaptic plasticity.In rat prefrontal cortex,DA receptors appear to modulate both LTD and LTP through glutamatergic synapses[157].In the hippocampus,opposing effect of D1/D5 and D2/D3/D4 receptors on NMDA receptor modulation,regulates LTD and LTP[158].Furthermore,D1/D5 have also been implicated in novelty acquisition through hippocampal LTD and LTP[159].The induction of synaptic plasticityviaNA receptors is more prevalent through the beta group receptors and they do so by modulating both NMDA and AMPA receptors in the pyramidal cells and perirhinal cortex[158,160].Consequently,the modulation of synaptic plasticityviaDA and NA receptors can be extended towards sleep associated functions.Studies in rats have indicated that sleep deprivation leads to a differential expression of DA receptors[161] and chronic sleep restriction results in changes in the density of NA receptors[162].Considering the range of these GPCRs and their functions,their cross-talk properties and effects during the state of sleep through synaptic plasticity require further detailed investigation.
Allosteric modulation of GPCRs
Another intriguing and related aspect of GPCRs that signifies its structural implications in synaptic plasticity is the allosteric modulation of these receptors.Binding at sites other than the active site of the GPCR,it has been suggested that allosteric modulators can selectively stimulate the homomeric and heteromeric forms of the receptor.For instance,in the CNS,heterodimerization is seen between mGlu2R and mGlu4R with allosteric modulation being targeted to the homomeric forms relative to the heteromeric receptors[163,164].Furthermore,LTP and LTD and spatial learning at the excitatory synapses in the hippocampus have appeared to be enhanced by the positive allosteric modulation of mGlu5Rs[165].Considering even GABA-B receptors demonstrate their sleep dependent synaptic changes by forming such heteromeric complexes and associations with mGluRs[150],the importance of such structural modifications and associations needs more attention with respect to sleep dependent functions.
DISCUSSIONS
Sleep is important for normal physiological and neurological functions and sleep deprivation affects majority of these functions.The negative effects of sleep deprivation can be better managed when we are able to specifically target the cellular and molecular machinery that drives these functions and,in this review,GPCRs are examined as a pivotal molecular machinery that impacts sleep associated functions through synaptic plasticity.For example,memory consolidation and learning as a model involving hippocampal-neocortical dialogue was looked at.The dialogue,in turn brings forth distinct electrophysiological patterns that are active during the SWS and REM sleep stages.Activity mediated synaptic plasticity has been widely correlated to sleep associated functions of cognitive learning,memory processing and over all brain development.GPCRs like GABA-B Rs and mGluRs are types of receptors that have shown a potential to be involved in these processes through their actions on LTD and LTP.What is even more intriguing is the fluctuation in their distribution,heterodimerization and co-localization following sleep deprivation,suggesting that these receptors can exist in one condition during normal sleep and change with sleep deprivation.GPCRs are one of the major cellular targets for drug interaction and therefore changes in the receptor expression profile can affect drug action.
For instance,the antipsychotic drug clozapine is one of the common and effective drugs used in treating disorders like schizophrenia.The cellular targets for this drug are GPCRs like 5-HT2A and DA D2 receptors with a higher affinity for the former receptor than the latter.It acts as an antagonist of DA D2 receptors in the mesolimbic pathway and although initially classified as an antagonist,clozapine is also known to be an inverse agonist of the 5-HT2A receptor present in the prefrontal cortex of the brain[166-168].Other antipsychotic drugs like olanzapine,risperidone,aripiprazole,etc.,have also been indicated to elicit their effects through these receptors[166].Interestingly,5-HT2A and mGluR2 receptors are suggested to form heterodimers which raise the possibility of testing if clozapine and other such antipsychotic drugs modulate similar complex formation[156].Moreover,drugs like clozapine are known to have sleep-inducing effects[169] and since disorders like schizophrenia and other psychiatric diseases are associated with sleep disturbances[170],potential interplay between the molecular events of sleep deprivation and actions of antipsychotic drugs needs to be investigated.Other examples of drugs that act on GPCRs include ropinirole for Parkinson’s disease which acts as an agonist on DA D2 receptors[171]and baclofen,a GABA-B receptor agonist suggested for treatment of depression and anxiety[172].Whether their actions change with sleep deprivation andvice versaalso needs to be tested.With regards to synaptic plasticity,antipsychotics and antidepressants are suggested to affect LTP upon both acute and chronic use and interestingly,these effects are different for each of these situations which may suggest differences in network behaviour with acutevschronic exposure to the drugs (see Table 2[173-211]).Moreover,their effect on LTD has not been sufficiently explored and hence similar studies on the differential effects of chronicvsacute use of such drugs on network behaviour are required with regards to LTD.Table 2 provides a brief summary on antipsychotic,antidepressant and anxiolytic drugs with their mechanisms of action and association with synaptic plasticity,learning and sleep.

Table 2 Changes in synaptic plasticity,learning and sleep associated with antipsychotic,antidepressant and anxiolytic drug therapy
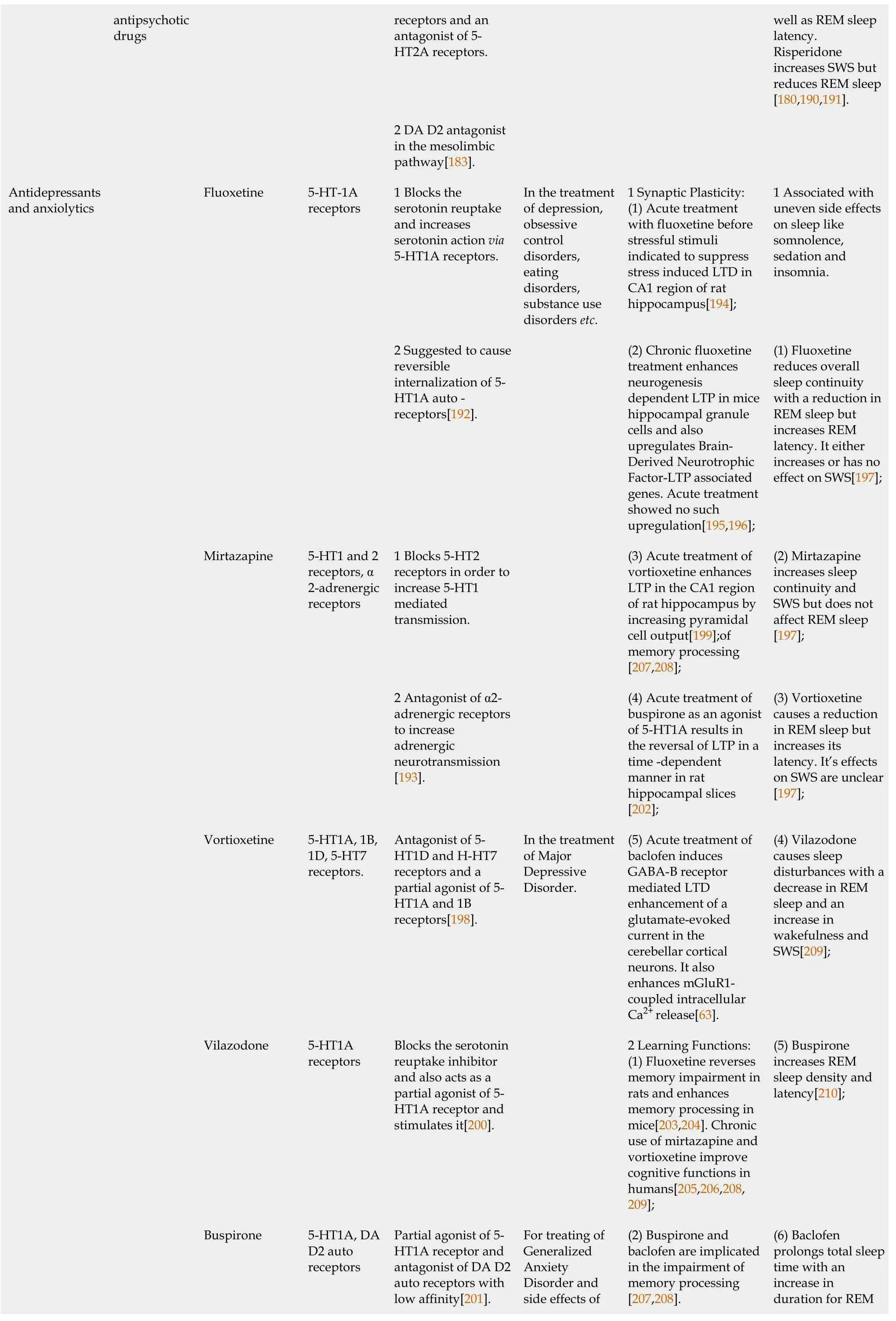
antipsychotic drugs receptors and an antagonist of 5-HT2A receptors.well as REM sleep latency.Risperidone increases SWS but reduces REM sleep[180,190,191].2 DA D2 antagonist in the mesolimbic pathway[183].Antidepressants and anxiolytics Fluoxetine 5-HT-1A receptors 1 Blocks the serotonin reuptake and increases serotonin action via 5-HT1A receptors.In the treatment of depression,obsessive control disorders,eating disorders,substance use disorders etc.1 Synaptic Plasticity:(1) Acute treatment with fluoxetine before stressful stimuli indicated to suppress stress induced LTD in CA1 region of rat hippocampus[194];1 Associated with uneven side effects on sleep like somnolence,sedation and insomnia.2 Suggested to cause reversible internalization of 5-HT1A auto-receptors[192].(2) Chronic fluoxetine treatment enhances neurogenesis dependent LTP in mice hippocampal granule cells and also upregulates Brain-Derived Neurotrophic Factor-LTP associated genes.Acute treatment showed no such upregulation[195,196];(1) Fluoxetine reduces overall sleep continuity with a reduction in REM sleep but increases REM latency.It either increases or has no effect on SWS[197];Mirtazapine 5-HT1 and 2 receptors,α 2-adrenergic receptors 1 Blocks 5-HT2 receptors in order to increase 5-HT1 mediated transmission.(3) Acute treatment of vortioxetine enhances LTP in the CA1 region of rat hippocampus by increasing pyramidal cell output[199];of memory processing[207,208];(2) Mirtazapine increases sleep continuity and SWS but does not affect REM sleep[197];2 Antagonist of α2-adrenergic receptors to increase adrenergic neurotransmission[193].(4) Acute treatment of buspirone as an agonist of 5-HT1A results in the reversal of LTP in a time-dependent manner in rat hippocampal slices[202];(3) Vortioxetine causes a reduction in REM sleep but increases its latency.It’s effects on SWS are unclear[197];Vortioxetine 5-HT1A,1B,1D,5-HT7 receptors.Antagonist of 5-HT1D and H-HT7 receptors and a partial agonist of 5-HT1A and 1B receptors[198].In the treatment of Major Depressive Disorder.(5) Acute treatment of baclofen induces GABA-B receptor mediated LTD enhancement of a glutamate-evoked current in the cerebellar cortical neurons.It also enhances mGluR1-coupled intracellular Ca2+ release[63].(4) Vilazodone causes sleep disturbances with a decrease in REM sleep and an increase in wakefulness and SWS[209];Vilazodone 5-HT1A receptors Blocks the serotonin reuptake inhibitor and also acts as a partial agonist of 5-HT1A receptor and stimulates it[200].2 Learning Functions:(1) Fluoxetine reverses memory impairment in rats and enhances memory processing in mice[203,204].Chronic use of mirtazapine and vortioxetine improve cognitive functions in humans[205,206,208,209];(5) Buspirone increases REM sleep density and latency[210];Buspirone 5-HT1A,DA D2 auto receptors Partial agonist of 5-HT1A receptor and antagonist of DA D2 auto receptors with low affinity[201].For treating of Generalized Anxiety Disorder and side effects of(2) Buspirone and baclofen are implicated in the impairment of memory processing[207,208].(6) Baclofen prolongs total sleep time with an increase in duration for REM

LTP:Long term potentiation;LTD:long term depression;SWS:Slow wave sleep;5-HT:5-hydroxytryptamine;GABA-B:Gamma-amino butyric acid-B.
The relation between a drug’s mechanism of action and sleep can be directly observed in drugs that are used for treating sleeping disorders like insomnia.Characterized by a lack of both quality and quantity of sleep,insomniac conditions are treated with many drugs that target GPCRs.For example,melatonin is a hormone that has been known to play a key role in the sleep-wake cycle and promoting sleep.Agonist drugs like ramelteon act on the melatonin GPCRs type 1 and 2 (MT1 and MT2) which results in reduced sleep latency in chronic insomniac patients[212].Intriguingly,the MTI and MT2 receptors have been known to form dimers among themselves as well as heterodimers with other GPCRs like the serotonin 5-HT2C receptor[213,214].Thus,differences in ligand selectivity for these homomeric and heteromeric forms of the target receptors can have important consequences if the expression profile of such GPCRs change upon sleep deprivation.Other drug targets for insomnia include neuropeptides like orexins which promote wakefulness and are synthesized by neurons in the hypothalamus region of the brain.Drugs like suvorexant act as antagonists of the orexin GPCRs type 1 and 2 (OX1R and OX2R)thereby blocking their wakefulness promoting effects[215].These GPCRs known to be associated with other GPCRs like the cannabinoid receptor type 1 (CB1) and GABA-B receptors.They form heterodimers with the CB1 receptors[216] which have been implicated in affecting memory formation and maintenance of mood.Moreover,orexin and GABA-B receptor activity are indicated to have a balancing interplay wherein inhibitory GABA-B modulates the wakefulness promoting properties in the orexin producing neurons[217].If expression profiles of GABA-B receptors change with sleep deprivation,can there be consequences for actions of orexins? In summary,changes in receptor profiles associated with sleep deprivation can have consequences for drug action and need a thorough investigation to understand the mechanisms involved so that CNS disorders can be treated with more rationally based therapeutics.
Unlike receptor redistribution and co-localization,heterodimerization can have serious implications for drug-receptor interactions and consequently,its action.On the other hand,if co-localization of different receptors leads to common transduction pathways,it can significantly affect the actions of such drugs.These consequences can vary depending on whether pre-or post-synaptic receptors are changed with sleep deprivation.Since this process is dynamic and the receptors may be in different states,an understanding of the mechanisms involved in clearly needed.Moreover,since GPCRs among 5-HT,norepinephrine,DA,etc.,may be affected,drug therapy involving antidepressants and antipsychotics should take the occurrence of receptor plasticity with sleep deprivation into account as drug-receptor interactions may be changed (Figure 1).

Figure 1 G-protein coupled receptors:Potential key-players in psychiatric therapeutics through modulation of synaptic plasticity in sleep deprivation.
FUTURE SCOPE
The diverse nature of GPCRs,both in their structure and functions has made them front line players in understanding many cellular processes.With the advent of sleep being credited with a wide range of psychological and physiological functions,and with changes happening in GPCRs following sleep deprivation,it is important to understand how the receptors and the functions they regulate,like synaptic plasticity and memory consolidation,are affected.GPCRs are one of the largest groups in the mammalian CNS.In psychiatry,most currently used antidepressants and antipsychotics act on one or more GPCR groups.Since the receptors change with alterations in sleep,unless the mechanisms involved in the change are understood,therapeutics will continue to suffer.
Potential research of cellular and molecular mechanisms
Firstly,even though LTP and LTD have both been emphasized in manifesting the neurological changes during the state of sleep and wakefulness,more work is needed with regards to LTD.In an attempt to bridge that gap,a better understanding of the inhibitory GPCRs like GABA-B receptors is highly crucial.Additionally,GABA-B and mGluRs seem to have an associative mode of action and based on the studies that are reviewed so far;it appears that co-localization and heterodimerization of these two specific receptor types occurs with sleep deprivation[150].Hence,the driving molecular factors which includes,specific genes and protein interactions,need a more coherent identification.For instance,the signalling linked with GPCRs are tightly controlled by a family of regulator of G protein signalling proteins in the brain[218].Furthermore,since metabotropic receptors (e.g.,mGluRs) affect the function of ionotropic receptors like NMDA and AMPA,similar studies can be extended for the metabotropic GABA-B receptor and its ionotropic counterparts like GABA-A and GABA-C in relation to sleep deprivation induced changes.Considering that GPCRs are involved in several CNS functions,there is a wide scope for studying the mechanisms involved in their plasticity.Another interesting area of research is the regulation of synaptic plasticity by GPCRs elicited intracellularly[119] and this could also be explored further in order to connect the transportation of GPCR subunits from intracellular compartments to the cell membrane and the processes that take place in these compartments which can give rise to heterodimers and co-localization.
Future scope for psychoneurological disorders and therapeutics
Apart from memory consolidation and learning,GPCRs are also implicated in other psychoneurological occurrences.Around 60% of drugs target membrane proteins and 30% of them are GPCRs[219].They are especially important as targets for antipsychotic and antidepressant drugs,considering some of their ligands like serotonin,DA and norepinephrine are associated with pathways that are important in psychiatry.Additionally,mGluRs and GABA-B Rs have an almost ubiquitous involvement in major cellular pathways which need to be better understood since they are affected in neurological disorders like Alzheimer’s disease,Parkinson’s disease,stress related disorders,etc.[220,221].Changes in sleep patterns are often observed in many neurological disorders and considering these major GPCRs are altered during the state of sleep,an understanding of this correlation can affect the way we approach therapeutics in this area.For example,even though there was a temporary elevation observed in the expression of the serotonin 5-HT1A receptor upon sleep deprivation[155],it is universally correlated to disorders like depression,anxiety and psychosis and changes in its expression profile upon sleep deprivation does warrant for further studies on this GPCR and its other subtypes.Additionally,related GPCRs like the DA receptor D1 are also known to interact directly with LTP inducing NMDA receptors[118] which are important in many neurological disorders.Furthermore,since the D1 and D2 DA receptors are involved in the nigrostriatal and mesolimbic pathways,changes in their expression profiles affect movements and behavioural patterns that are also implicated in Parkinson’s disease and schizophrenia[222,223].Therefore,DA GPCRs are highly potential drug targets and sleep associated changes in their expression profile needs more attention.Drugs used in psychiatry for such disorders,e.g.,aripiprazole,are known to target 5-HTA and DA receptors[224] and if the expression profile of these receptors is changing upon sleep deprivation,could it also affect the action of such drugs? Disorders like schizophrenia and depression often encompass sleep deprivation and drugs used for these conditions which target such GPCRs,may get negatively affected if receptor plasticity (co-localization and/or heterodimerization with other receptor subunits,etc.) occurs with associated sleep disorders.For example,the antidepressant drug mirtazapine exhibits its action through GPCRs by blocking 5-HT2 and adrenergic α2receptors,leading to an increase in the activation of 5-HT1A receptor mediated activity as well as NA release,respectively[193].This drug is known to have sleep-promoting properties and has been associated with problems like day-time somnolence[225] potentially disrupting the normal sleep cycle.Interestingly,there are off-label therapies that employ antidepressants like mirtazapine in treating sleeping disorders due to its sedative effect[226].Since antidepressants and antipsychotic drugs appear to directly or indirectly change activity at GPCRs,drug therapy for these disorders can affect sleep while sleep disturbances can also necessitate changes in that therapy.Therefore,sleep disturbanceinduced plasticity and cross-talk between GPCRs can have consequences for drug therapy,the mechanisms for which need to be thoroughly examined.Lastly,allosteric modulation,which is another upcoming molecular interaction that is important for drug designing,can be applied to the homo and heterodimerization of GPCRs for various conditions.Hence,studies on structural,functional,receptor co-localization and remodelling,etc.,will yield new insights into mechanisms involved and help improve therapeutics for a variety of CNS disorders,including in psychiatry.
CONCLUSION
Conclusion and future scope are included in the main text under heading "Introduction" above.
杂志排行
World Journal of Psychiatry的其它文章
- Effectiveness of cognitive behavioral therapy-based interventions on health outcomes in patients with coronary heart disease:A metaanalysis
- New-onset depression after hip fracture surgery among older patients:Effects on associated clinical outcomes and what can we do?
- Subgrouping time-dependent prescribing patterns of first-onset major depressive episodes by psychotropics dissection
- Self-compassion and resilience mediate the relationship between childhood exposure to domestic violence and posttraumatic growth/stress disorder during COVID-19 pandemic
- Psychiatric hospitalization during the two SARS-CoV-2 pandemic waves:New warnings for acute psychotic episodes and suicidal behaviors
- CPEB1,a novel risk gene in recent-onset schizophrenia,contributes to mitochondrial complex I defect caused by a defective provirus ERVWE1
