Phthalide-derived oxaspiroangelioic acids A-C with an unprecedented carbon skeleton from an aqueous extract of the Angelica sinensis root head
2021-12-29YouzheChenChengboXuWeipingWangXiaoliangWangQinglanGuoJiangongShi
Youzhe Chen,Chengbo Xu,Weiping Wang,Xiaoliang Wang,Qinglan Guo,Jiangong Shi
State Key Laboratory of Bioactive Substance and Function of Natural Medicines,Institute of Materia Medica,Chinese Academy of Medical Sciences and Peking Union Medical College,Beijing 100050,China
Keywords:Umbelliferae Angelica sinensis Phthalide-derived analogues Oxaspiroangelioic acids A-C
ABSTRACT Three phthalide-derived analogues,oxaspiroangelioic acids A-C (1-3),were isolated as minor components of an aqueous extract of the Angelica sinensis root heads (guitou).Oxaspiroangelioic acids A and B were racemates separated into enantiomers by chiral HPLC.Their structures including absolute configurations were determined by spectroscopic data analysis,single crystal X-ray diffraction,exciton chirality method and electronic circular dichroism (ECD) calculation.These compounds share an undescribed carbon skeleton,for which biosynthetic pathways are proposed.Compound 1 and its enantiomers showed almost identical activity inhibiting Tandem of P domains in a weak inwardly rectifying K+ channel 1 (TREK-1).
Danggui,the dried whole root of Angelica sinensis (Oliv.) Diels(Umbelliferae),is a popular traditional Chinese medicine for the treatment of various diseases [1].It is also used as food/dietary supplement in the Western countries [2,3].In traditional Chinese medicine,different parts of the root are described to have different medicinal functions.The root head(guitou)is hemostasis to induce blood up going,the root body (guishen)nourishes blood to guard heart,and the root tail (guiwei) activates blood to induce blood down going [1].Extensive chemical and pharmacological studies were previously performed on crude extracts of the whole roots,and around 100 chemical constituents,including phthalides,phenylpropanoids,coumarins,lignans,alkynes,terpenes,sterols,alkaloids,fatty acids and polysaccharides [2-10],have been documented.Ferulic acid,Z-ligustilide and polysaccharides are considered as the main active constituents of the dried whole root(danggui).However,there is no specific investigation on the root head(guitou).Thus,as part of our program to access the chemical diversity of traditional Chinese medicines,focusing on the minor constituents[11-19],we investigated an aqueous extract of guitou,to keep relative consistency with classic utilization method of water decoctions of the drug materials.In a previous paper,we reported a pair of novel neolignan enantiomers from the extract[20].A continuation of the study has resulted in isolation and structural determination of three phthalide-derived analogues with a novel carbon skeleton,oxaspiroangelioic acids A-C (1-3)(Fig.1),from the same extract.Oxaspiroangelioic acids A and B(1and2) were racemates that were separated into enantiomers(+)-/(-)-1and (+)-/(-)-2,respectively,while oxaspiroangelioic acid C (3) was optical pure.Herein,are detailed isolation(Supporting information),structural elucidation,plausible biosynthesis and bioactivity of1-3.
Racemate1was obtained as colorless crystals.Its IR spectrum indicated the presence of carbonyls (1702 and 1667 cm-1) in the molecule.The molecular formula was determined as C16H22O4with six degrees of unsaturation by(-)-HR-ESIMS at m/z 277.1444[M--H]-(calcd.for C16H21O4,277.1445) and NMR spectroscopic data(Supporting information Table S1).The1H NMR spectrum of1in acetone-d6exhibited proton signals ascribable to a conjugated trisubstituted double bond at δH7.43 (dd,J=4.8 and 3.0 Hz,H-7)and two inequivalent propyls attaching to olefinic carbons at δH2.48/2.11 (t,J=7.2 Hz,H2-2′/H2-9),1.65/1.46 (hex,J=7.2 Hz,H2-3′/H2-10)and 0.96/0.88(t,J=7.2 Hz,H3-4′/H3-11),along with partially overlapping signals attributable to three vicinal coupling methylenes between δH2.44 and 1.69(6H,m,H2-4,H2-5 and H2-6).The13C NMR and DEPT spectra of1showed 16 carbon signals(Table S1)corresponding to the above proton-bearing units and six quaternary carbons,including five sp2carbons and an oxygen-bearing sp3carbon.When compared with those of the previously reported constituents from A.sinensis [3-8],these spectroscopic data demonstrated that1was a phthalide analogue having an additional four carbon unit to give an abnormal structure,which was further determined by 2D NMR experiments (Fig.2).
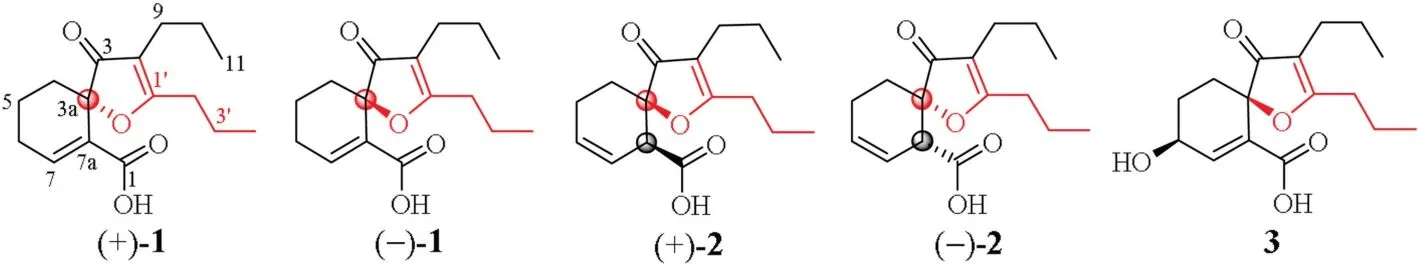
Fig.1.Structure of compounds (+)/(-)-1,(+)/(-)-2 and 3.
Analysis of the HSQC spectrum assisted assignment of the proton-bearing carbon and corresponding proton signals in the NMR spectra of1.The1H-1H COSY spectrum showed homonuclear coupling cross-peaks of H2-4/H2-5/H2-6/H-7,H2-9/H2-10/H3-11 and H2-2′/H2-3′/H3-4′,proving the presence of three vicinal proton coupling fragments (Fig.2,bold lines).In the heteronuclear multiple bond correlation (HMBC) spectrum,the three-bond correlations from H2-4 and H2-6 to the quaternary sp2carbon(C-7a,δC129.1),from H2-5 and H-7 to the quaternary sp3carbon(C-3a,δC83.4);and from H-7 to the sp2quaternary carbon (C-1,δC165.6) (arrows in Fig.2),along with their chemical shifts,demonstrated that there was a disubstituted cyclohexene-carboxylic acid moiety in1.The HMBC correlations from H2-10 and H2-2′to the sp2quaternary carbon(C-8,δC114.3)and from H2-9 and H2-3′to the other sp2quaternary carbon(C-1′,δC184.6)indicated that the two propyls connected to the two ends of a vinyl unit(C-8 and C-1′),respectively.In addition,the HMBC correlations from H2-4 and H2-9 to the remaining ketone carbonyl(C-3,δC205.8)inserted the carbonyl between C-3a and C-8.To satisfy requirements of the molecular formula as well as substitution and chemical shifts of C-3a and C-1′,the two carbons must share an oxygen to form an oxaspiro[4,5]decane-carboxylic acid motif in1.Thus,the planar structure of1was elucidated as an undescribed phthalide derivative as shown.Crystallization of1in n-hexane afforded single crystals.Follow up X-ray diffraction analysis proved that1was a racemate with the P-1 space group,an ORTEP drawn crystal structure with the relative configuration shown in Fig.3.
Separation of1by semi-preparative chiral HPLC (Fig.S1 in Supporting information) obtained (+)-1{+55.5 (c 0.29,MeOH)} and (-)-1{-59.2 (c 0.29,MeOH)},with the1H NMR spectra identical to that of1before separation (Supporting information),but the circular dichroism(CD)curves mirrored each other(Fig.S8 in Supporting information).The CD spectra displayed typical exciton coupling Cotton effects (CEs) at λmax267.5 and 300.5 nm,arising from coupling between n-π*transition moments of the two chromophores in the structures.By application of the exciton chirality method [21],the positive and negative exciton coupling CEs of (+)-and (-)-1predicted 3aS-and 3aR-configurations (Figs.S29 and S30 in Supporting information),respectively.The prediction was supported by consistency of the experimental CD and calculated electronic circular dichroism(ECD) spectra between (+)-1and 3aS-1as well as between (-)-1and 3aR-1,respectively (Fig.S8).Therefore,the structures of (+)-and (-)-1were determined and trivially designated as (+)-(3aS)-and (-)-(3aR)-oxaspiroangelioic acids A,respectively.
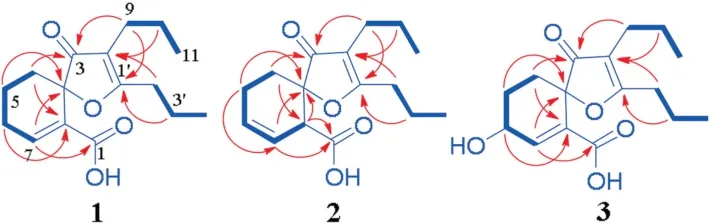
Fig.2.The 1H-1H COSY (thick lines) and main two-and three-bond HMBC correlations (red arrows,from 1H to 13C) of 1-3.
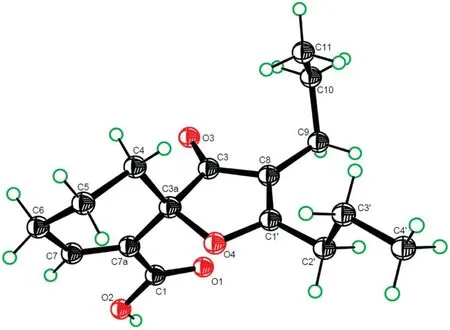
Fig.3.An ORTEP drawn crystal structure of 1.
Racemate2was an isomer of1as shown by spectroscopic data.Comparison of the NMR spectroscopic data of the two compounds(Table S1) revealed that one methylene and one quaternary sp2carbon in1were replaced by a sp2methine[δH5.85(m,H-6)and δC127.3(C-6)]and a sp3methine[δH3.56(brs,H-7a)and δC46.3(C-7a)]in2,respectively.In addition,C-4 and C-7 in2were shielded significantly by ΔδC-4.0 and -24.6,whereas C-1 and C-5 were deshielded by ΔδC+5.2 and +3.6,respectively.These variations suggested that the double bond at C-7 in1migrated to C-6 in2.The suggestion was proved by 2D NMR data analysis of2,especially by the1H-1H COSY cross-peaks of H2-4/H2-5/H-6/H-7/H-7a and the HMBC correlations of H2-5/C-3a and C-7(Fig.2).In addition,in the1H NMR spectrum of2,H-7a appeared as a broad singlet,indicating that the dihedral angle between H-7 and H-7a was nearly perpendicular and that the cyclohexene ring had a preferential half/twist chair conformation with H-7a occupying a pseudo-axial position.This was supported by a NOE cross-peaks between H-7a and H-4A(Fig.S26 in Supporting information).The racemate2was separated by semi-preparative chiral HPLC (Fig.S2 in Supporting information) into (+)-2{+150.0 (c 0.08,MeOH)} and (-)-2{-145.0 (c 0.08,MeOH)}.Because the relative configuration could not be deduced from the above spectroscopic data,the ECD spectra of two pairs of the possible enantiomers of2were calculated (Figs.S9 and S12 in Supporting information).The calculated ECD curves of the(3aR,7aS)-and(3aS,7aR)-enantiomers were fully consistent with the measured CD spectra of (+)-and(-)-2(Fig.S9),respectively.In contrast,in the low wavelength region the calculated ECD curves of the (3aR,7aR)-and (3aS,7aS)-enantiomers completely differed from the measured CD spectra of(+)-and (-)-2(Fig.S12).Thus,stereochemistry of (+)-and (-)-2was assigned as 3aR,7aS and 3aS,7aR,respectively.The assignment was consistent with that predicted by the exciton chirality method (Figs.S43 and S44 in Supporting information).Therefore,the structures of (+)-and (-)-2were determined and named(+)-(3aR,7aS)-and(-)-(3aS,7aR)-oxaspiroangelioic acids B,respectively.
Compound3was obtained as a white amorphous powder with[17_TD+81.0 (c 0.21,MeOH).The spectroscopic data of3demonstrated that it was an optical active derivative of1.When compared the NMR spectroscopic data of3and1(Table S1),replacement of one methylene in1by an oxygen-bearing methine [δH4.42 (ddd,J=5.4,4.2,3.6 Hz,H-6) and δC64.8 (C-6)]and significant deshielded shift of C-5 (ΔδC+9.4) in3revealed that3was the 6-hydroxy derivative of1.The deduction was proved by the1H-1H COSY cross-peaks of H2-4/H2-5/H-6/H-7 and the HMBC correlation of H-6/C-7a.The coupling constants of H-6 (J5A,6=4.2 Hz,J5B,6=5.4 Hz and J6,7=3.6 Hz) revealed that this proton had the pseudo-equatorial orientation on the cyclohexene ring with the half chair conformation in3(Fig.S27 in Supporting information).By comparison of the measured CD and calculated ECD spectra of all the possible stereoisomers (Figs.S13 and S15 in Supporting information),the configuration of3was assigned as 3aR,6S,which was supported by prediction of the exciton chirality method(Fig.S57 in Supporting information).Thus,the structure of compound3was determined and named (+)-(3aR,6S)-oxaspiroangelioic acid C.
It is worth noting that2was unstable to generate a main product when stored under an ambient condition.Using the aforementioned methods,the product was isolated,separated into enantiomers and structurally determined(Supporting information Scheme S1) as (+)-(3aS)-and (-)-(3aR)-6-oxo-oxaspiroangelioic acids C,respectively.This indicated that2was readily oxidized by air (Scheme S1).
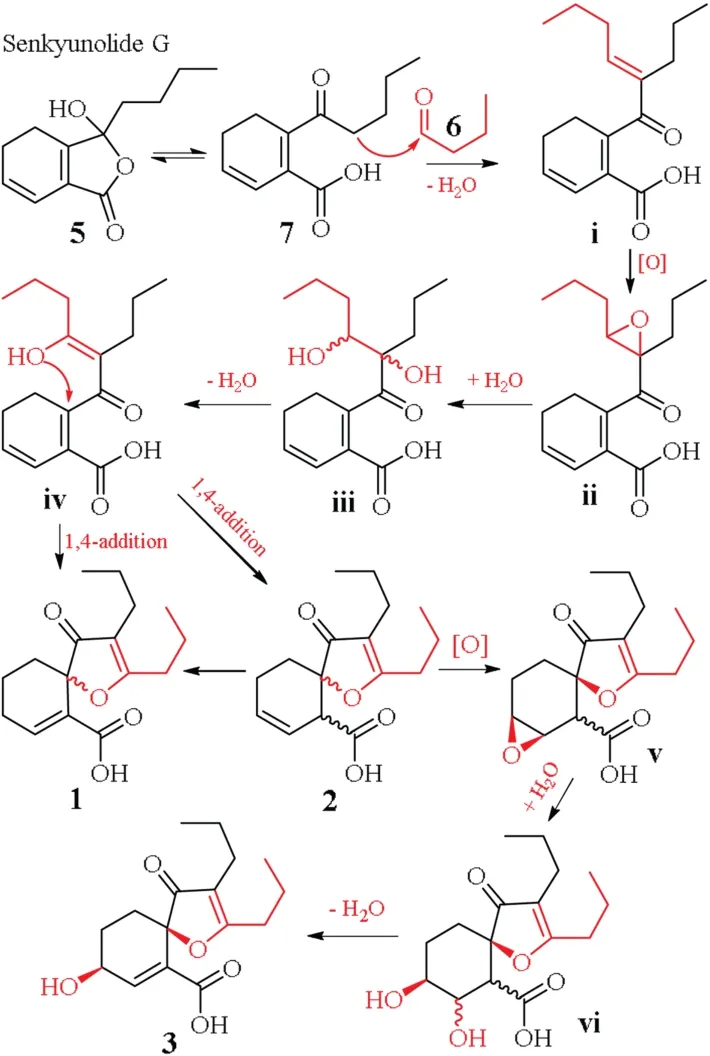
Scheme 1.The plausible biosynthetic pathway of 1-3.
According to the unique structures,consisting of the phthalidederived motif and the additional four carbon units,the biosynthetic pathways of1-3are proposed in Scheme 1.The abundant cooccurring senkyunolide G (5) [3]and butanal (6) are preferential precursors of1-3.Isomerization of5gives a ketene acid7,which undergoes Aldol condensation with6to yield an intermediate (i).An enzymatic epoxidation of the intermediate,followed by epoxide ring opening of the product (ii),affords a diol (iii).Subsequent dehydration ofiiigeneratesiv,which goes intramolecular cyclization via 1,4-addition to produce1and2,while1would be produced from2via double bond migration rearrangement.An enantiomer of2goes sequentially epoxidation,epoxide ring opening and dehydration throughvandvito yield3.Because3was optically active but1and2inactive,the 1,4-addition ofivmight be non-enzymatic or enzyme-uncontrolled reactions.Alternatively,1-3may be produced from Aldol condensation between (Z)-ligustilide and butanal (Supporting information Scheme S2).
In a cell-based preliminary bioassay,among the isolates,only1and (+)-and (-)-1inhibited Tandem of P domains in a weak inwardly rectifying K+channel 1 (TREK-1) with half maximal inhibitory concentration (IC50) values of 29.4,28.1 and 31.3 μmol/L,respectively (the positive control fluoxetine,IC5027.4 μmol/L) (Fig.S25 in Supporting information).Subsequent methylation of the carboxylic acid or hydrogenation of the cyclohexene ring in1(Supporting information) led to loss of activity.The result suggested that the conjugated cyclohexenecarboxylic acid moiety was required by the activity,whereas a change of chirality at C-3a had little influence on the activity.
In conclusion,compounds1-3were characterized as the minor constituents of the aqueous extract of guitou.These compounds have the undescribed carbon skeleton biogenetically deriving from Aldol condensation of phthalides and butanal.The conjugated cyclohexene-carboxylic acid moiety was essential for the TREK-1 inhibition of this group of compounds.The minor components may have contributions to clinic effects of guitou.The structural novelty enriches the diversity of phthalides and provided a model architecture for synthetic and biosynthetic chemists to obtain enough amount of the samples for further evaluation.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
Financial support from the National Natural Sciences Foundation of China (No.81630094),CAMS Innovation Fund for Medical Science (No.2017-I2M-3-010,China) and The Drug Innovation Major Project (Nos.2018ZX09711001-004 and 2018ZX09711001-001,China) is acknowledged.
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2021.03.004.
杂志排行
Chinese Chemical Letters的其它文章
- Decatungstate as a direct hydrogen atom transfer photocatalyst for synthesis of trifluromethylthioesters from aldehydes ★
- Synthesis of[6-6-6]ABE tricyclic ring analogues of methyllycaconitine
- Host-guest inclusion for enhancing anticancer activity of pemetrexed against lung carcinoma and decreasing cytotoxicity to normal cells
- pH-Responsive amorphous room-temperature phosphorescence polymer featuring delayed fluorescence based on fluorescein
- Boronic acid-containing carbon dots array for sensitive identification of glycoproteins and cancer cells
- Ultrasmall green-emitting carbon nanodots with 80%photoluminescence quantum yield for lysosome imaging
