pH-Responsive amorphous room-temperature phosphorescence polymer featuring delayed fluorescence based on fluorescein
2021-12-29GuanghuiWangZifeiWangBingbingDingXiangMa
Guanghui Wang,Zifei Wang,Bingbing Ding,Xiang Ma
Key Laboratory for Advanced Materials and Feringa Nobel Prize Scientist Joint Research Center,School of Chemistry and Molecular Engineering,East China University of Science and Technology,Shanghai 200237,China
Keywords:Room temperature phosphorescence pH-Response Amorphous Fluorescein Thermally activated delayed fluorescence
ABSTRACT Numerous researchers have paid attention to achieve metal-free phosphorescence by exploring new structures or new mechanisms.Herein,a facile way is introduced to endow a common fluorescence dye,tetrabromofluorescein (4Br-Flu),some fabulous optical characteristics such as dual emission including thermally activated delayed fluorescence,room-temperature phosphorescence(RTP),and the excellent pH-sensitivity.Shortly,4Br-Flu with good light-emitting properties is composed into the polymer system.The multiple bromine atoms promote the spin-orbit coupling effect and facilitate triplet excitation.Especially,the hydrogen bonding network of the polymer restricts the molecular motion of 4Br-Flu so that the system can emit long-wavelength RTP when 4Br-Flu is doped into polyvinyl alcohol or co-polymerized with acrylamide.Due to the reversible transformation of protonation and deprotonation,the 4Br-Flu based polymer responded to acid and alkali like a phosphorescent switch which makes it an excellent hydrogen chloride/ammonia gas leak detector in dry environment.
Recently,room-temperature phosphorescence (RTP) compound as one of the most promising light-emitting materials has aroused worldwide attention attributing to its excellent luminescence properties and potential application in in vivo imaging [1,2],anti-counterfeiting materials [3],molecular switches [4,5],and so forth [6-8].Meanwhile,pure organic materials emitting RTP is excellent in low cost and low toxicity when compared with other conventional inorganic compounds such as expensive metal complexes [9].Especially,researchers have contributed a lot to the progress of achieving amorphous RTP materials when developing crystal RTP materials is limited by harsh conditions in processes of single crystal cultivation[10-17].Amorphous polymers with hydrogen bond networks can suppress non-radiative transition which will facilitate RTP emission[18-23].Therefore,developing new amorphous polymer with RTP is of great importance.Tetrabromofluorescein (4Br-Flu) as promising light-emitting monomers are introduced into the polymer system.4Br-Flu is the first reported organic compound with thermally activated delayed fluorescence (TADF) [24].On the one hand,the heavy atoms and the aromatic carbonyl group of 4Br-Flu will facilitate the spin-orbit coupling (SOC) effect to enhance the intersystem crossing (ISC) of singlet excited state (S1) to triplet excited state (T1) so that the phosphorescence quantum yield is improved [25].On the other hand,the different ionic states of 4Br-Flu probably endow the new polymer based on 4Br-Flu with pH-response characteristic,which could be applied in the production environment because there are colours and structures changes (Scheme 1).
Herein,4Br-Flu and 3-bromopropene were reacted together,and the dark red crude product (intermediate1) was purified by column chromatography using dichloromethane and methanol as the developing solvent (Scheme S1 in Supporting information).Through Ma’s previous work [26,27],polyacrylamide is a good rigid polymer matrix that can effectively fix the luminophore,and these hydrogen bonds formed by the intertwining of the chains network will further strengthen this rigidity.Naturally,intermediate1and acrylamide were co-polymerized at a molar ratio of 1:50 to result polymerP1as a dark pink powder.
Scheme 1.Colours changes and corresponding structures changes of P1.
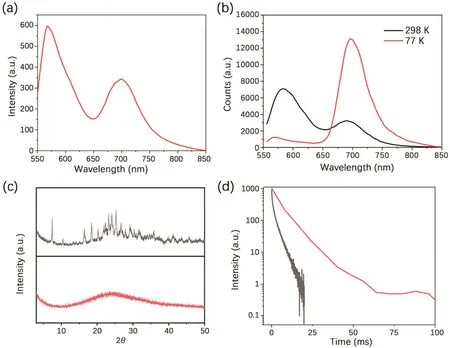
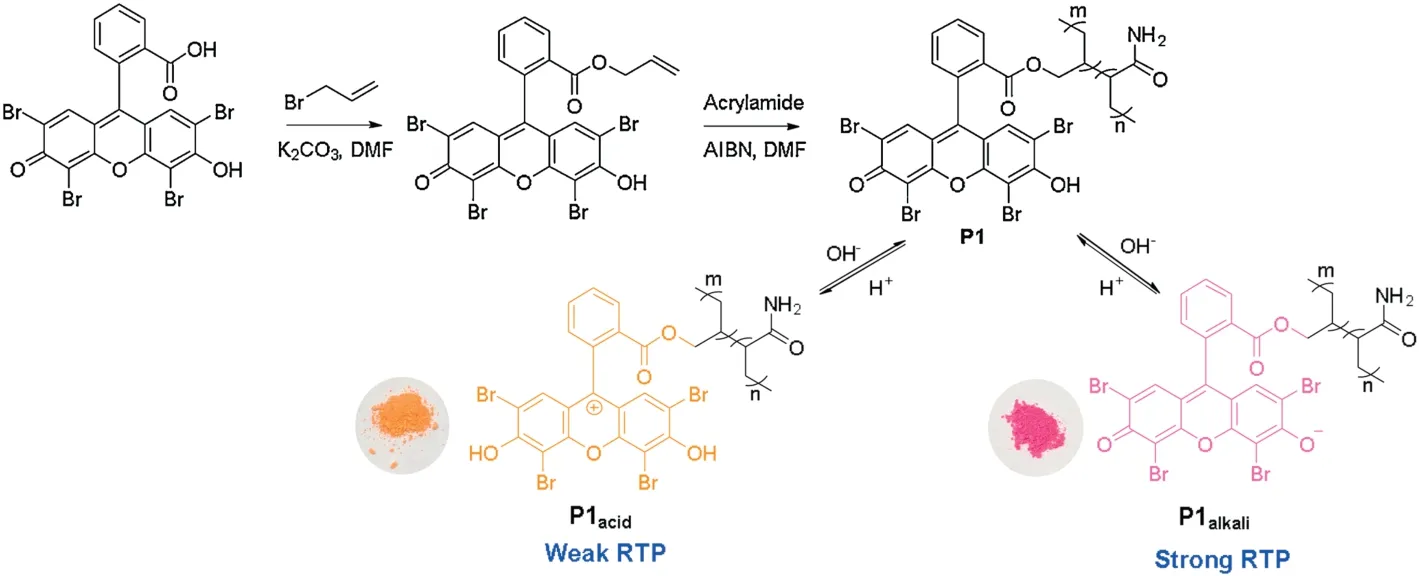
Fig.1.(a)Emission spectra of P1(red line)in phosphorescent mode(λex=540 nm,voltage=650 V).(b)Emission spectra of P1(red line)in 298 K(black line)and 77 K(red line)in phosphorescent mode(λex=540 nm).(c)XRD patterns of intermediate 1(black line)and P1(red line).(d)Lifetime of phosphorescence of P1 at 77 K(red line)and 298 K (black line).
An optical test was performed on the solid powder of the product polymerP1(Fig.1).First,XRD of both theP1and intermediate1was carried out respectively to prove thatP1is the amorphous material while intermediate1is crystal(Fig.1c).Then photophysical properties of intermediate1and polymerP1were shown in Fig.1a and Fig.S3(Supporting information)that the solid powder of intermediate1hardly emits RTP in the phosphorescence mode and the possible reason is that the internal movement of the molecule is too violent,which causes non-radiative loss of triplet.The polymerP1exhibited double emission peaks at 570 nm and 700 nm after being excited by visible light at 540 nm.Since the delayed emission at 570 nm of polymerP1was the same as the fluorescence emission (Fig.S2 in Supporting information),it was attributed to TADF emission.In order to further verify this hypothesis,the intensity tests were carried out on the emission peaks at 570 nm and 700 nm ofP1at different temperatures(Fig.1b).The luminous efficiency of TADF was specifically decreased with the decrease of temperature,which is exactly the opposite of that of phosphorescence.The lifetime of the 700 nm emission increased with the decrease of temperature(Fig.1d),which is 6.94 ms at 77 K while the lifetime is 1.85 ms at 298 K(Fig.S1 in Supporting information).Therefore,the emission at 570 nm belongs to TADF while the emission at 700 nm is attributed to RTP.
Dual emission was resulted from the formation of a strong hydrogen bond network between the polyacrylamide chains,which largely inhibited the molecular motion of 4Br-Flu.At the same time,the four bromine atoms also greatly promoted the SOC process,so the RTP emission peak ofP1is formed at 700 nm.As shown in Scheme 1,fluorescein is featuring with the varied states of protonation and tautomeric form[28,29],which endows 4Br-Flu a reversible transformation of protonation and deprotonation in different concentration of acid and alkali environment so that the photophysical properties ofP1can be changed accordingly.In acidic solutions,4Br-Flu exists in the form of cations and its conjugation degree is small while 4Br-Flu exists in the hydroxyl form in alkaline solutions and the degree of conjugation increases so that the pH of the environment has an influence on both the color and luminescence directly.Thus,P1was treated with HCl solutions (pH 0.25,2.50) and NaOH solutions (pH 8.70,10.95) to adjust its corresponding TADF and RTP emission.It can be seen from Fig.2 that with the pH decreased,the intensity of the TADF and RTP emission of theP1decreased compared with the initial state ofP1in the phosphorescence mode and there was also a 5 nm blue shift of value of 0.25,the TADF and RTP emissions ofP1were almost disappeared.Subsequently,the NaOH solution was added to the acidic polymer and its pH value increased,which led to the corresponding solid polymer TADF and RTP emission intensity gradually increased again.When the pH value of the solution is 10.95,the TADF and RTP emission peak intensities of the obtained polymerP1@pH 10.95 restored to half of that ofP1,not to the original intensity,which is probably because strong alkaline environment causes the collapse of the hydrogen bonding net so thatP1cannot recover to its original structure.After the sequential treatments of HCl and NaOH solutions,the polymer was also changed from dark pink to orange and back to dark pink under sunlight (Fig.S5 in Supporting information).Then the reversible repeatability test was also carried out (Fig.3).The emission intensity of TADF and RTP was changed with the times of HCl and NaOH solution treatments,andP1had certain reversible repeatability after five cycles of test.In a word,polymerP1has achieved an acid-base response at good repeatability when treated with acid-base solutions of different pH value.It is noteworthy that there are few reports about fluorescein-based polymers whose RTP emission is in the red region,and the most striking thing is its responsiveness to the pH value of the environment.
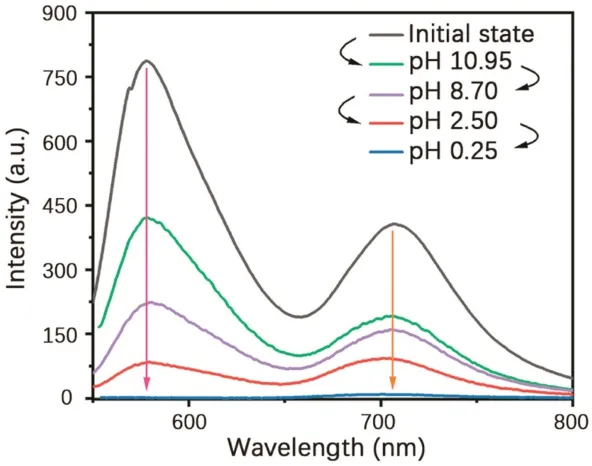
Fig.2.The corresponding emission changes of solid powder in phosphorescent mode after successively treated with HCl solutions of pH 2.50 and pH 0.25,NaOH solutions of pH 8.70 and pH 10.95 (the excitation wavelength of acidic polymer is 520 nm,and that of alkaline polymer is 540 nm).
In view of the acid-base response characteristics ofP1,a detection agent for visually monitoring hydrogen chloride gas and ammonia gas was available (Fig.4).When a small amount of dry hydrogen chloride gas was passed intoP1,the color ofP1was changed from pink to orange.Then ammonia gas was passed into orangeP1,the color was changed from orange back to pink.It was consistent with changes ofP1after acid and alkali solution treatment.Obviously,P1can achieve pH response in the air.Making use of this feature,P1can be applied to a hydrogen chloride gas leaking detection agent while the acidifiedP1can be used as an ammonia gas leaking detection agent.In a word,the color change ofP1allowed qualitative monitoring of the hydrogen chloride gas and the ammonia gas leak by naked eyes.
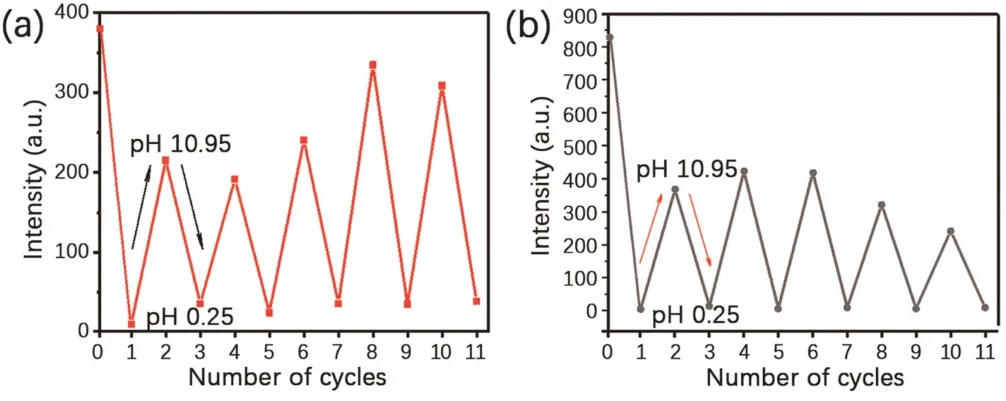
Fig.3.Reversible repeatability test of the intensity of corresponding solid powder polymers at their respective(a)RTP and(b)TADF maximum emission intensity after treated with acid/alkali solution.

Fig.4.The corresponding polymer changes in color under (a) daylight and (b)365 nm UV light after treated with HCl and NH3 gas.
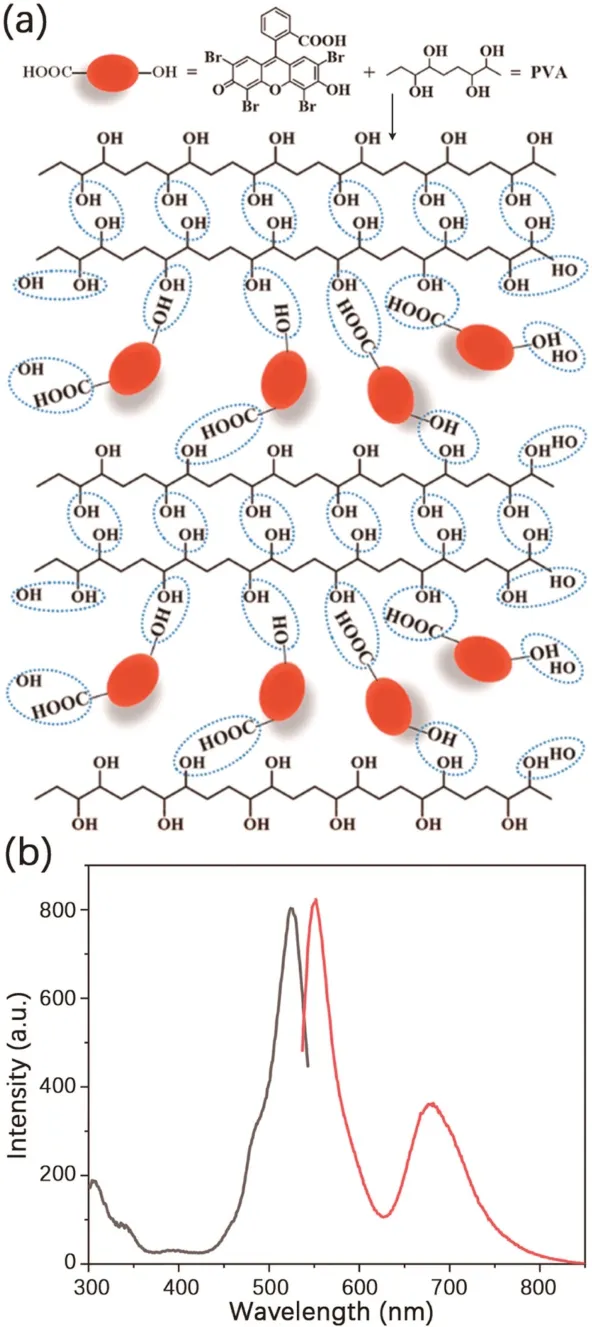
Fig.5.(a)The structural diagram and(b)time-resolved luminescence(delay time:0.1 ms) spectra of 4Br-Flu/PVA thin film (1 wt%).
To further explore the factor of RTP emission ofP1,1 wt%polyvinyl alcohol(PVA)thin film was synthesized for proving that RTP intensity could be enhanced by hydrogen bonding (Fig.5a).The method is to dissolve 4Br-Flu into methanol and mix with PVA aqueous solution while continuously stirring and heating.After the two components were mixed completely,it was coated on the quartz plate by dripping and dried to form a thin film.Photoluminescence of thin film was tested in phosphorescence mode.As shown in Fig.5b,when excited with 527 nm visible light,the 4Br-Flu/PVA thin film was found to emit TADF at 554 nm and RTP at 682 nm.Compared with polymerP1,the emission was almost the same but there was a 23 nm blue shift of thin film,which is probably because that the carboxyl of 4Br-Flu was substituted in polymerP1.All in all,4Br-Flu thin film with RTP emission proved that there was an interaction among the hydroxyl on the PVA chain and the hydroxyl and carboxyl of 4Br-Flu,which formed a dense hydrogen bonding network that suppressed the motion of phosphor.In fact,hydrogen bonding network contributes a lot to achieving organic RTP.
In conclusion,4Br-Flu with multiple heavy atoms and a large degree of conjugation was the basic luminophore to realize RTP in polymer system.After a simple etherification reaction and copolymerization with acrylamide to form a polymer or doping into PVA to form a film,polymers that can emit both orange-yellow TADF and red RTP at the same time was achieved.First of all,the strong hydrogen bond network formed between polyacrylamide chains can suppress the non-radiative vibration of the luminophore.In addition,the participation of four bromine atoms also strongly strengthened the SOC effect so that the polymer can achieve RTP emission.Herein,strategies such as increasing the conjugate structure of the fluorescein body [30]can be used to obtain a more red-shifted wavelength emission and applied to the field of near-infrared phosphorescent probes in the future.Meanwhile,considering how to weaken TADF to obtain phosphorescence with higher quantum yield is meaningful.More interestingly,the emission of polymer TADF and RTP will play a leading role one after the other under different temperature environments,which will provide an excellent sample for the future design of temperature-controlled TADF/RTP materials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This report was financially supported by the National Natural Science Foundation of China (Nos.21788102,22020102006,21722603 and 21871083),Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX03),Program of Shanghai Academic/Technology Research Leader (No.20XD1421300),‘Shu Guang’ project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (No.19SG26),the Innovation Program of Shanghai Municipal Education Commission (No.2017-01-07-00-02-E00010),and the Fundamental Research Funds for the Central Universities.
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2021.03.054.
杂志排行
Chinese Chemical Letters的其它文章
- Colorimetric recognition of melamine in milk using novel pincer zinc complex stabilized gold nanoparticles
- Reviving chloroquine for anti-SARS-CoV-2 treatment with cucurbit[7]uril-based supramolecular formulation
- Mechanism and selectivity of nickel-catalyzed [3+2]cycloaddition of cyclopropenones and α,β-unsaturated ketones:A computational study
- Full synthesis and bioactivity evaluation of Tn-RC-529 derivative conjugates as self-adjuvanting cancer vaccines
- Deep insight into the charge transfer interactions in 1,2,4,5-tetracyanobenzene-phenazine cocrystal
- Recent progress in carbon-dots-based nanozymes for chemosensing and biomedical applications
