Design and synthesis of unique thiazoloisoquinolinium thiolates and derivatives
2021-12-29WsimAhmedZiHoHungZiNingCuiRiYunTng
Wsim Ahmed,Zi-Ho Hung,Zi-Ning Cui,b,c,*,Ri-Yun Tng,c,*
a Department of Applied Chemistry,College of Materials and Energy,South China Agricultural University,Guangzhou 510642,China
b State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources,Integrative Microbiology Research Centre,Guangdong Province Key Laboratory of Microbial Signals and Disease Control,South China Agricultural University,Guangzhou 510642,China
c Guangdong Laboratory for Lingnan Modern Agriculture,Guangzhou 510642,China
Keywords:Isoquinoline Heterocycle Zewitterionic Elemental sulfur Cycloaddition
ABSTRACT Natural isoquinolinium alkaloids possess a wide range of biological activities.The design and synthesis of mesoionic isoquinoliniums is of great importance.This paper reports the synthesis of unique mesoionic thiazoloisoquinolinium thiolates stabilized by aromatization and 1,3-dipolarization.Such compounds can be synthesized via the three component[2+2+1]cycloaddition reaction of isoquinolines with ethyl propionate and elemental sulfur in the absence of any metal catalyst and additives.Importantly,thiazoloisoquinolinium thiolates can be transformed to thioether-containing thiazoloisoquinolinium halides.A selective[4+2]cycloaddition can also be used to form S-bridged fused tetracyclic compounds with a thiothiamide ring unit and two quarternary carbon centres.Compound I-1 shows good bioactivity against the chlorophyll of duckweed (Lemna minor) with inhibition rate of 51.5 μg/mL.
Isoquinoline constitutes an important structural motif in a range of natural alkaloids and bioactive molecules[1,2],displaying a broad range of biological activities,such as anti-HIV,antimalarial,antitumor,antimicrobial,antileukemic antibacterial and insect growth retarding properties [3,4].Natural isoquinolinium salts such as magnocurarine [5],Quinocitrinines [6],chelerythrine [7],berberine [8],sanguinarine [9],and latifolians [10],have been reported to show a wide range of biological activities (Fig.1A).In the majority of these natural isoquinolinium salts,the paired anions are often halogen ions.Very few examples of mesoionic isoquinolinium salts,namely the paired cation and anion present in one molecule,have been reported [11].To the best of our knowledge,no reports for the synthesis of mesoionic thiazoloisoquinolinium thiolate have been divulged (Fig.1C,compoundI).Problems for the synthesis of such compounds include:(1) The design of a [2+2+1]cyclization system for the formation of a thiazole ring via C-H sulfurization of the isoquinoline with elemental sulphur [12].Up until now,elemental sulfur has not been used for the reaction with isoquinoline to form carbon-sulfur bonds.(2) Trapping the elemental sulfur for the formation of the paired sulfur anion along with the [2+2+1]cyclization,and (3)maintaining the stability of the zwitterinic thiolates which are used as transitional active intermediates for cyclization [13,14].These challenges prompted us to further explore the synthesis of mesoionic isoquinolinium thiolates.In 1975,Bradsher reported a cyclization of isoquinolinium derivatives to form an unstable 2,3-dihydrothiazolo[2,3-a]isoquinolin-4-ium (Fig.1B) [15].A severe limitation of this chemistry was the tendency of the cyclized product to undergo ring opening with the resonance energy of the restored aromatic system.To overcome this problem,we designed a stable model for the mesoionic thiazoloisoquinoliniums(Fig.1C,compoundI) that stabilized by the aromaticity and the 1,3-dipolarization of the ion pairs.
Herein,we report a metal-and additive-free synthesis of 1,3-dipolar isoquinolinium thiolates via the[2+2+1]cycloaddition of isoquinoline,ethyl propiolate and elemental sulfur (Fig.1C).This strategy provides the desired specimen for the synthesis of unique structures stabilized by conjugation and the 1,3-dipolarization effect.In addition,the corresponding thiazoloisoquinolinium thiolates are versatile intermediates for the synthesis of thioether-containing isoquinolinium salts and S-bridged fused tetracyclic compounds.Although polycyclic isoquinolines are well reported in the literature [16-19].We reveal for the first time,a new and unique type of S-bridged fused tetracyclic structure consisting of thiothiamide ring and two quarternary carbon centres.
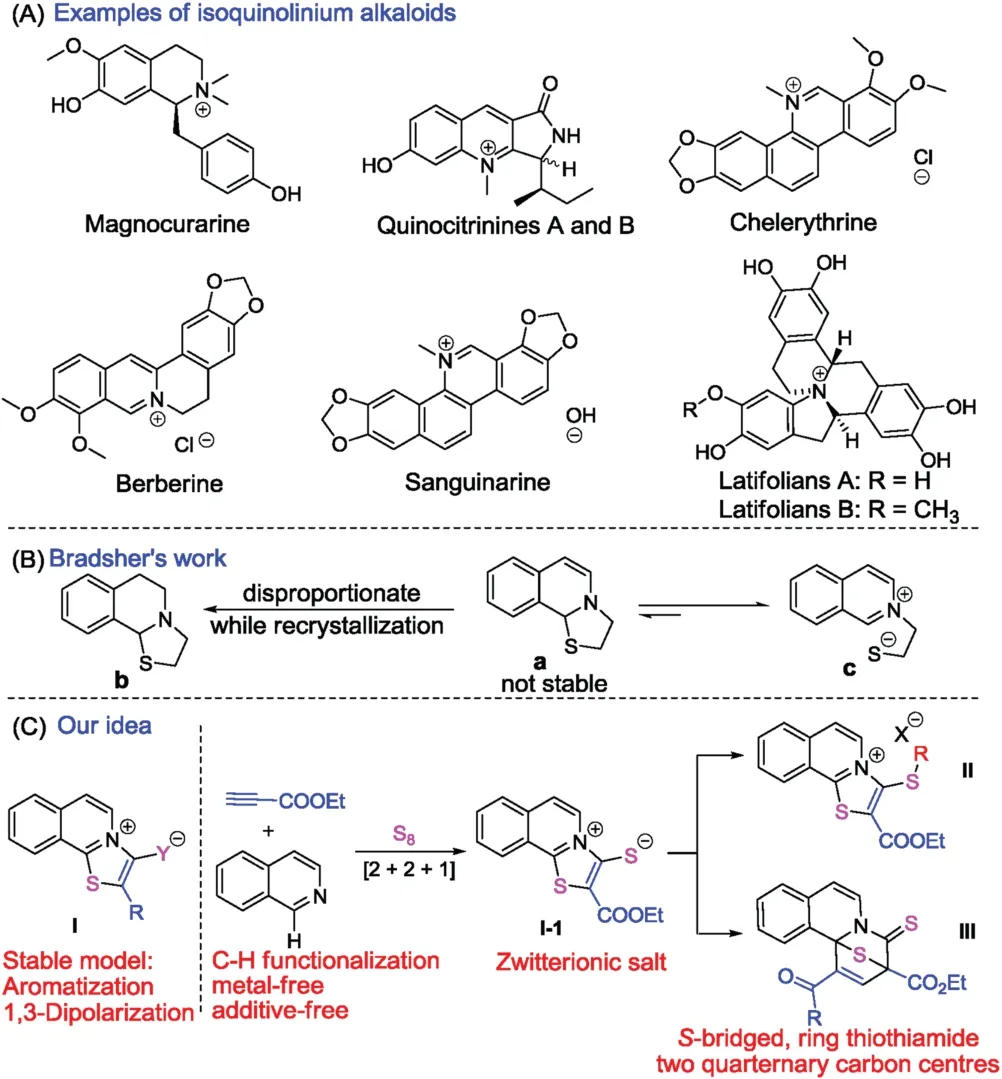
Fig.1.(A) Examples of isoquinolinium alkaloids.(B) Bradsher ’s work on the cyclization of quaternary ammonium salts.(C) Our design for the synthesis of thiazoloisoquinolinium and S-bridged fused tetracyclic compounds.
Our study began with the synthetic analysis of mesoionic thiazoloisoquinolinium thiolates (Scheme 1).In order to obtain mesoionic compounds,a terminal alkyne bearing an electronwithdrawing group is preferable for the addition with isoquinoline.Such an addition may produce an unstable mesoionic species.We reasoned that the anion in the mesoionic species can be trapped by the electrophilic elemental sulfur to form an aromatic conjugated 1,4-zwitterionic intermediateA.At this stage,similar to Bradsher’s work[15],the loss of the stable non-aromatic intermediateBmay occur.The enol-type intermediateAmay be transformed to a ketone-type intermediateC,which reacts with sulfur to form a 1,3-zwitterionic intermediateD.We speculated that further oxidative cyclization could produce intermediateE,the elemental sulfur may play the role as an oxidant.And the aromatization is the predominant driving force leading to the formation of the stable 1,3-zwitterionic isoquinolinium thiolateFfrom intermediateE.
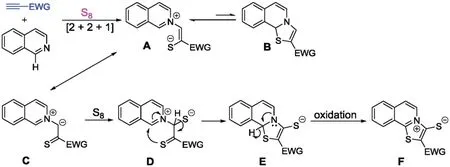
Scheme 1.A plausible mechanism for the synthesis of 1,3-zwitterionic thiazoloisoquinolinium thiolates.
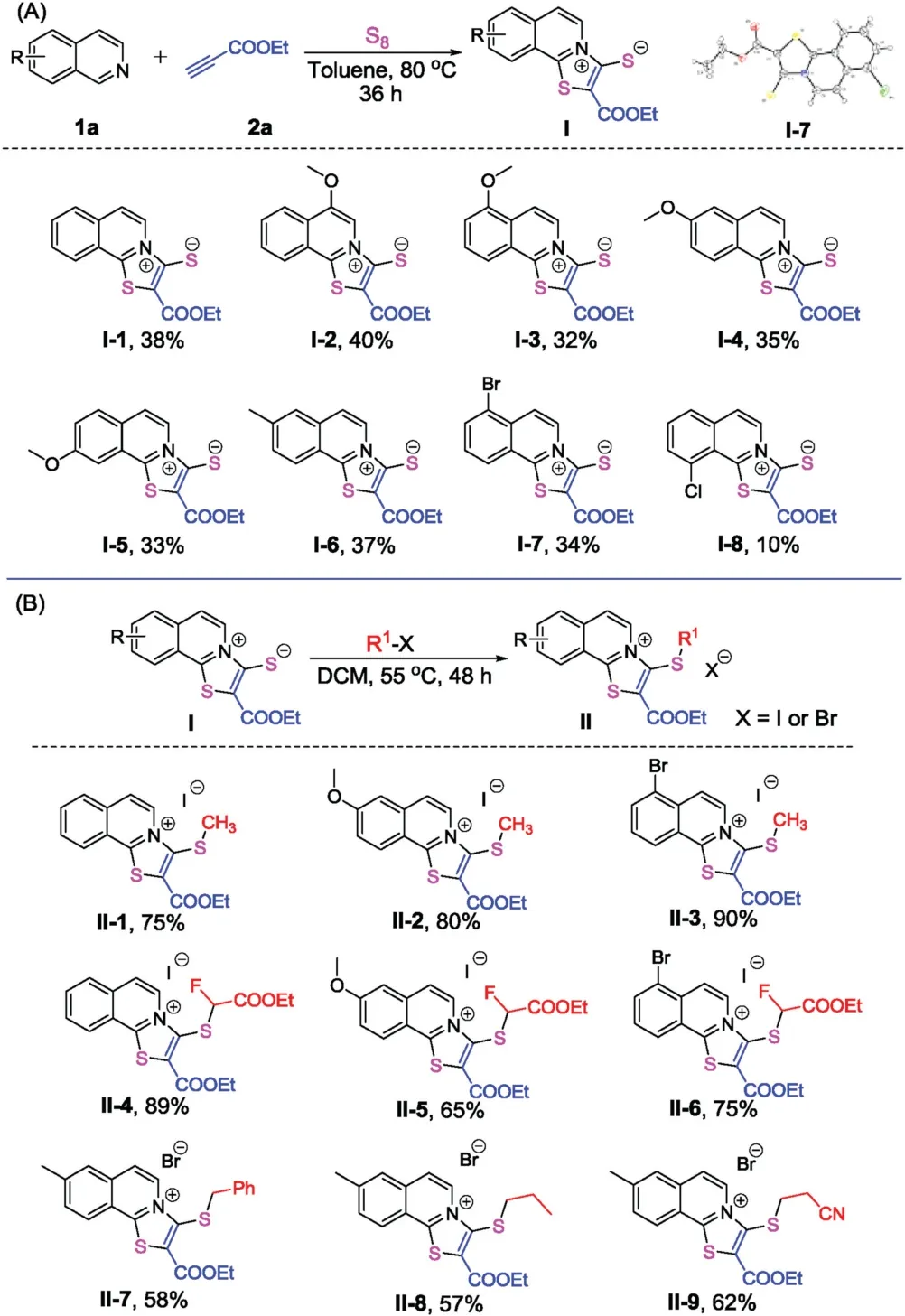
Scheme 2.Synthesis of thiazoloisoquinolinium thiolate and thiazoloisoquinolinium halides.
Based on the above analysis,ethyl propionate was selected to activate the isoquinoline for the [2+2+1]cyclization.In the cyclization process,the in situ formed paired anion can be trapped by elemental sulfur to form a mercapto anion.Thus,aprotic solvents are preferable for such a transformation.In the reaction of isoquinoline with ethyl propionate and elemental sulfur at room temperature in different aprotic solvents (e.g.,DMF,CH3CN,DCE,dioxane and toluene),the target product was detected by LCMS in all cases.Toluene was found to be the most effective solvent for the transformation.After extensive screening of the reaction conditions,including temperature,addition of additives,type of sulfur agents,and loading quantities (Table S1 in Supporting information),we finally found that the reaction proceeded well in toluene at 80°C for 36 h using 3 equivalents of ethyl propionate.The target product was obtained in 38%yield.This result is acceptable for the synthesis of these unique 1,3-zwitterionic thiazoloisoquinolinium thiolates.
Further evaluation of the reaction scope shows that substrates possessing electron-donating substituents are more effective than substrates bearing electron-withdrawing groups(Scheme 2A).Electron-donating groups,such as a methoxy group at the 4-,5-,6-and 7-positions,as well as a methyl group at the 6-position,gave satisfactory results affording the corresponding products in yields of 32%-40% (compoundsI-2-I-5).When the reaction was performed with substrates bearing electron-withdrawing groups,such as chloro and bromo substituents,only 5-bromo isoquinoline was able to give the corresponding productI-7in 34%yield,while 8-chloro isoquinoline furnished its corresponding productI-8in a very low yield of 10%yield.Isoquinoline with a cyano group was not suitable for such transformation,only trace product was observed on ESI-MS.It is worth noting that the structure of the product was fully confirmed by X-ray single crystal analysis of compoundI-7.
The mercapto anion in compoundsIis a useful nucleophilic species for the construction of thioether moieties.CompoundsIcan react with methyl iodide,ethyl 2-fluoro-2-iodoacetate,and alkyl bromides respectively,affording the corresponding thiazoloisoquinolinium iodides or bromides.Nine examples are shown in Scheme 2B.These nucleophilic substitution reactions were successful in DCM at 55°C for 48 h,affording the corresponding products fromII-1toII-9in moderate to good yields(Scheme 2B).
1,3-Zwitterionic thiazoloisoquinolinium thiolates are potential cycloaddition partners for the construction of fused polycyclic compounds.We reasoned that the [4+2]cycloaddition of compoundIwith ethyl propionate may occur at the nucleophilic mercapto anion and at the 2nd electrophilic position of isoquinolinium [20].Unexpectedly,the [4+2]cycloaddition selectively occurs at the α-C of the ketone possessing larger hindrance,rather than with the mercapto anion,thus a class of unique Sbridged pyrido[2,1-α]isoquinoline-4-thiones bearing two quarternary carbon centres was obtained (Scheme 3).These reactions proceeded well in DCM at 55°C over 48 h without the use of any metal catalyst or additives,affording the productsIII-1-III-6in moderate to good yields (Scheme 3).Ethyl propionate and but-3-yn-2-one were effective reaction partners for the cycloaddition.The structures of these compounds were confirmed by NMR and HRMS analysis;a characteristic peak in the13C NMR spectrum corresponding to the carbon atom of the thione is present (see NMR spectra in Supporting information).A possible reaction mechanism for the cycloaddition is also outlined in Scheme 3.CompoundIprocess a resonance 1,3-dipoleG,which is more favorable than structureIfor the [4+2]cycloaddition with a terminal alkyne.Because the location of S anion and the carbonnitrogen double bond in the structureIis trans,the energy barrier of the cycloaddition is high.Thus the reaction should undergo a Diels-Alder transition stateHto afford productsIII.
In order to confirm the hypothesis of the synthetic pathway for the synthesis of isoquinolinium thiolates(Scheme 1),we employed ESI-MS real time analysis to explore the generation of potential intermediates.MS peaks of 260 were found in the samples after reaction over 15 min and 1 h,respectively.The MS peak of 260 was designated to be intermediateIas shown in Scheme S1(Supporting information).However,the trace amounts of the intermediate cannot be isolated so their structures cannot be determined more accurately.Interestingly,in the reaction of 3-methoxy pyridine1hwith ethyl propionate and sulfur under the standard conditions,no cyclization productVwas observed,but a non-cyclized productIVwas obtained in 25% yield (Scheme S1) [21].This suggests that compoundIVcannot be transformed to compoundV.Thus,the reaction of isoquinoline with ethyl propionate and elemental sulfur probably involves an intermediateI,suggesting that the proposed synthetic pathway shown in Scheme 1 is plausible.
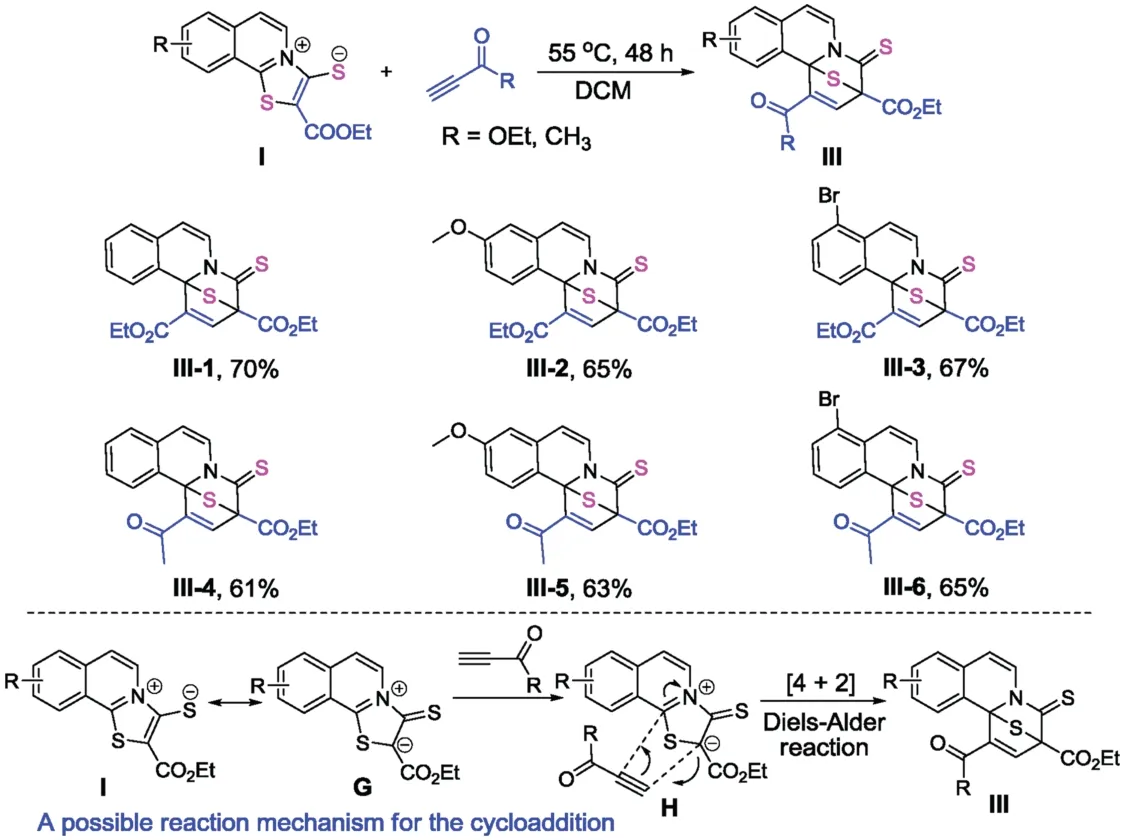
Scheme 3.Synthesis of S-bridged tetracyclic-fused rings and a possible reaction mechanism.
The herbicidal activity of compoundI-1was tested on L.minor.The duckweed was badly withered when treated with 100 μg/mL and 50 μg/mL concentration of compoundI-1for 7 days,respectively.The inhibition rates of chlorophyll were positively correlated with the increase of the concentration,and the EC50value was 51.5 μg/mL(see the detailed experiments and results in Supporting information).These results indicate that such zwitterionic compoundsImight be a promising scaffold for the development of a new herbicide.
In summary,unique 1,3-zwitterionic thiazoloisoquinolinium thiolates have been prepared via the reaction of isoquinolines,ethyl propiolate and elemental sulfur.It is noteworthy that the reaction does not involve the use of any metal catalyst or additive.Although the obtained yields of the products are not particularly high,this approach provides a simple multicomponent process for the preparation of unique thiozoisoquinolinium thiolates,which can be further transformed to thioether-containing thiazoloisoquinolinium halides and unique S-bridged fused tetracyclic compounds bearing a thiothiamide ring and two quarternary carbon centres.Importantly,preliminary bioassay shows that compoundI-1possesses bioactivity against chlorophyll of L.minor with an inhibition rate of EC5051.5 μg/mL.This study opens up a new pathway to the synthesis of 1,3-zwitterionic polycyclic compounds and their effective transformation to S-bridged tetracyclic compounds.Further studies concerning the selective transformation of thiazoloisoquinolinium thiolates are ongoing in our laboratories and the evaluation of the biological properties for these synthesized compounds will be reported in due course.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work.We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgments
This work was financially supported by Natural Science Foundation of Guangdong Province for Distinguished Young Scholars(Nos.2019B151502052,2021B1515020107),the programs of National Natural Science Foundation of China (No.32072450),the International Science and Technology Cooperation Program in Guangdong (No.2020A0505100048),the Program of Science and Technology of Guangzhou (No.202002030295),and the open Project of the State Key Laboratory of Crop Stress Biology for Arid Areas (No.CSBAAKF2021009).
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2021.03.065.
杂志排行
Chinese Chemical Letters的其它文章
- Decatungstate as a direct hydrogen atom transfer photocatalyst for synthesis of trifluromethylthioesters from aldehydes ★
- Synthesis of[6-6-6]ABE tricyclic ring analogues of methyllycaconitine
- Host-guest inclusion for enhancing anticancer activity of pemetrexed against lung carcinoma and decreasing cytotoxicity to normal cells
- pH-Responsive amorphous room-temperature phosphorescence polymer featuring delayed fluorescence based on fluorescein
- Boronic acid-containing carbon dots array for sensitive identification of glycoproteins and cancer cells
- Ultrasmall green-emitting carbon nanodots with 80%photoluminescence quantum yield for lysosome imaging
