Capture and separation of CO2 on BC3 nanosheets:A DFT study
2021-12-29HouyongYangChaozhengHeLingFuJinrongHuoChenxuZhaoXiuyuanLiYanSong
Houyong Yang,Chaozheng He,*,Ling Fu,Jinrong Huo,Chenxu Zhao,Xiuyuan Li,Yan Song
a Shaanxi Key Laboratory of Optoelectronic Functional Materials and Devices,School of Materials Science and Chemical Engineering,Xi'an Technological University,Xi'an 710021,China
b Institute of Environmental and Energy Catalysis,School of Materials Science and Chemical Engineering,Xi'an Technological University,Xi'an 710021,China
c College of Resources and Environmental Engineering,Tianshui Normal University,Tianshui 741001,China
d School of Sciences,Xi'an Technological University,Xi'an 710021,China
Keywords:Hexagonal BC3 Electric field Density functional theory CO2 capture and separation
ABSTRACT In order to reduce the greenhouse effect caused by the rapid increase of CO2 concentration in the atmosphere,it is necessary to develop more efficient,controllable,and highly sensitive adsorbing materials.In this study,the adsorption behavior of CO2 on BC3 nanosheets under an external electric field was explored based on density functional theory (DFT).It was found that CO2 experienced a transition from physisorption to chemisorption in the electric field range of 0.0060-0.0065 a.u..In addition,the adsorption/desorption of CO2 is reversible and can be precisely controlled by switching on/off at the electric field of 0.0065 a.u..The selective adsorption of CO2/H2/CH4 by BC3 can also be used to realize gas separation and purification under different electric fields.This study highlighted the potential application of BC3 nanosheets as a high-performance,controllable material for CO2 capture,regeneration,and separation in an electric field.
CO2capture and storage has attracted extensive research due to the greenhouse effect caused by its concentration rapid increase,which caused environmental deterioration and global warming.It is necessary to consider the nature of the adsorbent materials containing the high adsorption capacity,sensitive selectivity [1],low energy consumption during adsorption/desorption process,more reusable times [2,3]and structural stability [4].At present,the popular CO2adsorbents are the metal-organic frameworks(MOFS) [5,6],covalent organic frameworks (COFS) [7],2D solid materials including graphene [8],graphene-like materials [9],porous carbon materials and polymers [10-13].
In the past few years,many researches have been done on the use of external electric fields to promote CO2adsorption and achieve CO2separation.Guo et al.found that the hexagonal BN nanosheets have high-efficiency adsorption and large capacity for CO2under an applied electric field,which is effectively separate CO2from the CO2/H2/N2/CH4/CO/H2O mixture [14].The work of Adnan et al.shows that under an external electric field of 0.013 a.u.,CO2achieves the transition from physisorption to chemisorption,while the desorption of CO2is a spontaneous exothermic process with 0.06 eV after the removal of electric field [13].Qiao et al.reports that CO2capture/regeneration on MoS2monolayers can be controlled by external electric fields,and the adsorbed CO2can be released without any energy barrier once the electric field is turned off [15].
Recently,BC3monolayer with high crystalline quality has been successfully created by carbon-substituted technique in a boron hexagonal comb [16].BC3has excellent mechanical properties[17,18]and thermal stability [19].It has excellent performance in catalytic reactions [20],and the potential application for gas sensors [21,22].
The first principle is the ab initio calculation,which does not need any parameters.It only needs to start from the most basic physical principle to directly solve the Schrodinger equation with some approximated algorithm,according to the interactions between nucleus and electron and its basic motion rules.The first principle is widely used to calculate the catalysis process of single atom supported or bimetallic catalysts [23-25],chemical reactions caused by external electrical fields [26,27],surface adsorption and activity[28],organics degradation simulation[29],analysis of photocatalytic mechanism [30,31],optical properties prediction [32]and so on [33-45].
In this research,we systematically explored whether BC3has the potential to control capture and release of CO2under an external electric field.First,the adsorption of CO2/H2/CH4on BC3surface was studied,then the transformation of CO2adsorption behavior under gradient electric field and the thermodynamic process of CO2adsorption/desorption on the surface of BC3were further explored.Finally,the application of different electric field values to separate CO2/CH4/H2mixed gas was evaluated.
All of the spin-polarized DFTcalculations were performed using the Dmol3[46]software.Generalized gradient approximation(GGA) formulated by Perdew,Burke,and Ernzerhof (PBE) are carried out to describe the exchange-correlation energy functional[47],and meanwhile we used all-electron core treatment for the electron ion-core interactions [48],and a double numerical basis set containing d polarization function,such as double numeric with polarization(DNP)to increase accuracy.The spin polarization is turned on all the way,and Grimme method was applied to describe the van der Waals interactions [49].The charge distributions and the charge transfer between BC3monolayer and molecules were analyzed by using Hirshfeld method.For geometry optimization and electronic self-consistency,the convergence tolerance is set to 1×10-6a.u.The convergence threshold parameters of geometric optimization are specified as 1×10-5Ha,2×10-3Ha/Å and 5×10-3Å for energy,maximum force and displacement,respectively.In the process of structural optimization,the coordinates of the internal atoms were relaxed with the fixed lattice parameters.
The Brillouin k-point grid was sampled by the 6×6 × 1 Monkhorst packt grid [50].The BC3base model used 2×2 supercell cotaining 32 atoms with a 20 Å vacuum layer to weaken interaction between layers.The optimized lattice constant of the BC3structure is 10.35 Å,which is well consistent with the theoretica1 result of 10.33 Å [51].Then the adsorption energy was calculated by this formula (Eq.1):

Where EAllis the total energy of the absorbed combination,Esuband Egasrepresent the energy of separate BC3substrate and gas molecules,respectively.It is worth noting that the E-field perpendiculars to the BC3surface and points downward.
As shown in Fig.S1a(Supporting information),BC3nanosheets has a planar hexagonal honeycomb structure,each structural unit includes four C atoms and two B atoms.Each B atom was combined with three C atoms via sp2hybridization.The lengths of B--C and C--C are 1.565 and 1.422 Å,respectively,which was well consistent with the early literature [52].As depicted in the Fig.S1a,six adsorption sites are considered for molecular adsorption,including the top sites of B(T1)and C(T2)atoms,the bridge sites of C--C(B1)and B--C bond(B2),and the central position of C4B2six-membered ring (H1) and the C six-membered ring (H2).
The structural stability of BC3nanosheets under the application of an E-field can be roughly evaluated by the polymerization energy and molecular dynamics simulation.The formula for polymerization energy was defined as Eq.2:
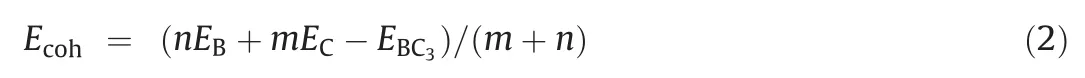
Where EB,EC,EBC3represents the energy of B and C atoms and BC3nanosheets,respectively,n and m are the number of C and B atoms[53].As shown in Fig.S1b(Supporting information),as the E-field strength increased from 0 to 0.008 a.u.,the average binding energy decreased from 7.52 eV to 7.40 eV.Compared with that of no Efield,the average binding energy between BC3atoms changes little,indicating that the structure is stable.For the optimized structure under the electric field the microcanonical ensemble(NVE)ensemble was adopted,at 300 K,molecular dynamics simulation was done.As shown in Fig.S1c (Supporting information),the energy change of the structure is very small in the 2 ps interval of 1 fs,which also confirmed the stability of the BC3structure under the applied E-field.
We studied three molecules (CO2,CH4,H2) adsorption on BC3substrate,the most stable adsorption structure and relative adsorption parameters were depicted in Fig.S2 and Table S1(Supporting information),respectively.As shown in Fig.S2,CO2tends to adsorb on H1 site of C4B2six-membered ring,however,CH4and H2tend to adsorb on T2 and T1 sites,respectively.No chemical bond is formed between the molecules and the substrate,where the CO2,CH4,H2are suspended above the surface.The minimum distance(Dm)between molecules and BC3substrate are 3.271,3.609 and 3.158 Å for CH4,CO2and H2,respectively,and the corresponding adsorption energy is very small with the value of-0.11,-0.09 and -0.07 eV,respectively.From the analysis of Hirshfeld-type charge transfer,the amount of charge transfer between gas molecules and BC3is also very small (0.004 e,-0.025 e and 0.012 e).
As we all know,the strong interactions of the adsorbates combined with surface contribute to the evident results of gas detection selectivity [54]and sensitivity.In order to enhance the CO2sensitivity,we apply a forward E-field perpendicular to the Z axis [55]on BC3surface [52].The corresponding adsorption configurations were presented in Fig.1.According to the difference charge density,the adsorption effect gradually increases along with the increasing of the electric field strength.As shown in Fig.1c,the angle of ∠O-C-O decreased sharply from 180°to 158.6°along with the electric field increased from 0 to 0.006 a.u.,and the adsorption distance reduced from 3.10 Å to 2.94 Å.The adsorption energy adds up to -0.43 eV,and the charge transfer amount increases to 0.2 e,the charge density expands a lot resulting a stronger chemical adsorption.When the electric field value further increased to 0.008 a.u.,the distance between CO2an-d BC3suddenly decreased to 1.75 Å,the angle of ∠O-C-O further decreased to 127.3°,and the amount of charge transfer increased to 0.9 e.Seen from the Fig.1d,the charge density between CO2and BC3increased dramatically,resulting that the interaction between the CO2molecular and the BC3surface has been greatly improved.These evidences indicate that under an applied electric field of 0.008 a.u.,CO2is chemically adsorbed on the surface of BC3.It is clearly that CO2is ‘fixed’ to BC3surface via employing an electric field.The results indicate the interaction between CO2and BC3surface is enhanced,which cause the stronger gas monitoring sensitivity.
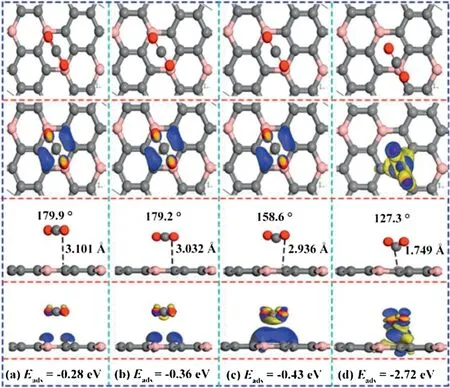
Fig.1.Top and side views of the most favorable CO2 adsorption configurations,corresponding charge density difference in an external E-field of(a)0.002 a.u.,(b)0.004 a.u.,(c) 0.006 a.u.,(d) 0.008 a.u..The blue (yellow) color represents accumulation(diminishing)of electrons.The values of isosurface of(a-c)are 0.003 e/Å3,and (d) is 0.02 e/Å3.
In order to explain the turning point of CO2adsorption affected by the electric field,three values of electric field (0.0065,0007,0.0075 a.u.) were performed to explore the corresponding adsorption structure,adsorption energy and the differential charge density (as shown in Fig.2).As shown in Fig.S3a (Supporting information),the adsorption distance suddenly decreased from 1.97 Å under the electric field value of 0.0065 a.u.,which was 33%shorter than that of values at 0.006 a.u.(2.94 Å).It is noted that the adsorption energy was increased to -0.84 eV,which is almost diploid compared with that of -0.43 eV.In addition,the angle of∠O-C-O is reduced to 137.5°,due to the strong interaction of electrons accumulate between CO2and the substrate,which indicates that can be regarded as strong chemisorption.When the E-field value increased to 0.0075 a.u.,the adsorption distance was further shortened to 1.772 Å,the angle of ∠O-C-O was further reduced to 128.2°,and the adsorption energy was increased to -2.25 eV,which indicates that the interaction between CO2and BC3substrate is further enhanced.The applied E-field will promote the polarization of the charged particles[56,57],which will lead to the enhanced adsorption of CO2on the surface of BC3[55].As shown in Figs.S3a-c(Supporting information),the critical point of CO2from physical adsorption to chemical adsorption on the BC3substrate is between 0.006-0.0065 a.u..As shown in Fig.S4,the minimum Efield value (0.0065 a.u.) required for the conversion of CO2from physical adsorption to chemical adsorption is much smaller than that of Penta-graphene [4],and is also smaller than the E-field value required in the previous literature to capture CO2materials,such as C2N [55],h-BN [27],C3N [58],P-doped C60[59],P-doped graphene[60].It is a pity that the value is just slightly larger than that of MoS2[29]and PC5[24].Such as it is,BC3captures CO2is more economical and efficient.
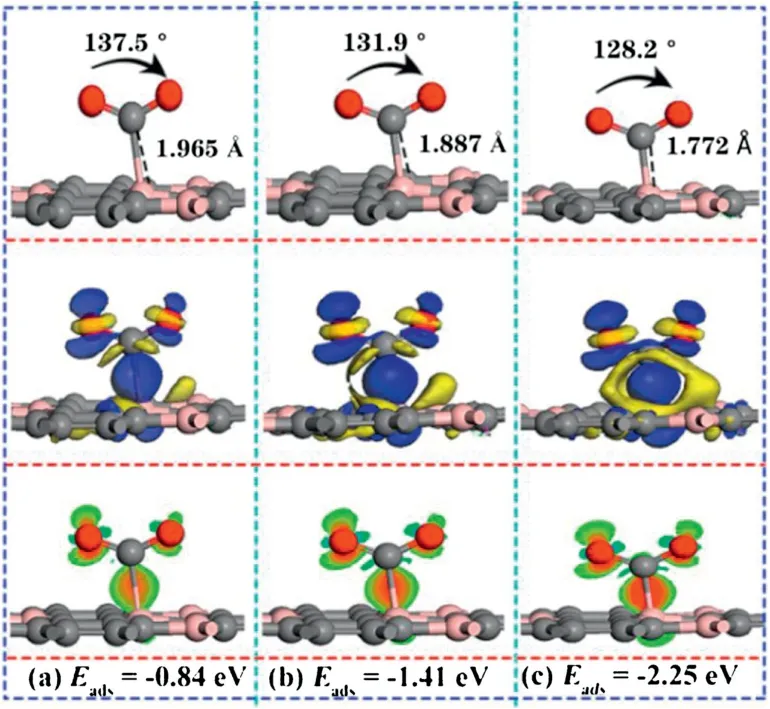
Fig.2.The side view of the most stable configurations and corresponding charge density difference in E-field of(a)0.0065 a.u.,(b)0.007 a.u.,(c)0.0075 a.u..The blue(yellow) color represents accumulation (diminishing) of electrons.Red and green represent increasing and decreasing electron density.The values of isosurface of(a),(b),(c) are 0.015 e/Å3,respectively.
In order to further study the interaction between CO2and BC3substrate under different E-field values,partial density of states(PDOS) was studied and presented in Fig.S5 (Supporting information).As shown in Fig.S5,between the E-field value of 0~0.004 a.u.,there is almost no overlap[48]between the orbits of CO2and B atoms,which indicates that the interaction between them is relatively weak physical adsorption.At this time,the E-field has little effect on the capture of CO2.However,when the E-field value increases to 0.006 a.u.,it can be seen that the overall PDOS has a significant right shift,and there is a clear overlapped orbitals of 2p(CO2)and 2p(B)between 3.0~5.0 eV,indicating the adsorption effect was enhanced,similarly as the previous results[52].Similarly,when the E-field values at 0.0065 and 0.007 a.u.,the orbital overlap formed by the 2p (CO2) and 2p (B) hybrid contributions can appear at -5.5,-1.2 and 5.0 eV,and the same results can be seen at 0.0075 and 0.008 a.u.It should be noted that the applied E-field can significantly enhance the ability of BC3to capture CO2.
In order to further study the mechanism of CO2adsorption on BC3under an external E-field,the crystal orbital hamiltonian population (COHP) was performed to analyze the different adsorption structures [61-64].As shown in Fig.3,the red area is the contribution of the anti-bonding state,and the cyan area is the bonding state.Fig.3a shows that in the absence of an external E-field,the molecular orbital between C-O is composed by the σs-pbonding orbital(at-6.8 eV)that formed by the interaction of Csand Opand the anti-bonding π*p-p(at 4.5 eV)formed by the interaction of Csand Op.In addition,integrated COHP(ICOHP)was calculated to analyze the energy integral up to the highest occupied bands,and the value of ICOHP quantitatively reflected the strength of bonding.As shown in Figs.3a-d,as the value of the E-field increases,ICOHP gradually decreases,which indicating the weaker of C--O bond in CO2.The reason is that with the increases of the E-field,the interaction between the C of CO2and B atom is enhanced,inducing the electron cloud more shared with the B atom and a reduction in the overlap of the electron cloud of the C-O,which is accordance with the weakened C--O interaction.Figs.S6a-d show that as the E-field strength increases,the ICOHP between C and B atom increases with the bonding state broaden,indicating that the interaction between C and B is strengthen.It should be highlighted that in the range of 0.0065-0.0075 a.u.,the bonding molecular orbital σp-pbetween C and B atoms is mainly composed by Cpand Bp.Interestingly,bonding molecular orbitals σp-pwhich located in lower energy contributed by Cpand Bpappeared near the Fermi level.
In order to further explore the possible mechanism of CO2adsorption and desorption on BC3substrate under the external E-field existence,the potential energy [65]surface was shown in Fig.4.The energy of the CO2adsorption system without an E-field is defined as 0.Clearly,the energy of the system gradually decreases along with E-field increases,indicating that the adsorption of CO2under the external E-field is a spontaneous exothermic process.In addition,when applying 0.006 a.u.E-field,the relative system energy decreases to-0.26 eV.It is worth noting that when the external E-field increases to 0.0065 a.u.,the relative energy difference immediately decreases to-0.69 eV,well consistent with the Eadsof -0.84 eV,resulting to strong chemical adsorption.Besides,we also systematically studied the capture and separation of CO2gas on the BC3substrate by switching the E-field.As shown in Fig.4b,under the external electric field of 0.0065 a.u.,CO2is chemically adsorbed on the surface of BC3.When the external electric field is turned off,the CO2adsorption distance is extended from 1.965 Å to 3.264 Å with the CO2angle shifted from 137.5°to 179.9°after restructuring optimization.The above results demonstrate that the removal of the electric field can promote the transition from chemical adsorption to physical adsorption,in this process the endothermic reaction relative energy value is 0.69 eV.Similarly,when the E-field is switched on,CO2changed from physical adsorption to chemical adsorption with a linear structure to a V-shaped structure,and the adsorption distance is shortened to 1.965 Å.This process is exothermic process with relative energy-4.06 eV without energy barrier.These results shows that the adsorption/desorption of CO2on the BC3substrate can be easily controlled by the opening/closing of the electric field.
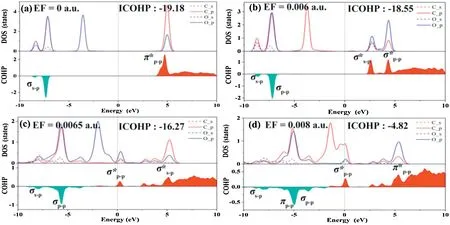
Fig.3.PDOS(partial density of states)and molecular orbital of CO2 molecule adsorbed on BC3 under different E-field values,above and below the 0 value of crystal orbital hamiltonian population (COHP) are the anti-bonding state and bonding state,respectively.Red stands for bonding contributions,while cyan stands for antibonding contributions.
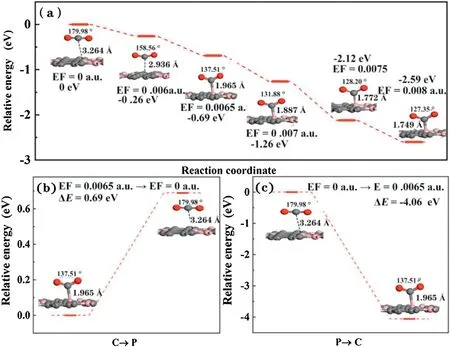
Fig.4.The energy profile for the chemisorption to physisorption of the adsorbed CO2 over BC3 due to removing or switching of E-field (EF=0.0065 a.u.).
CH4and H2are high-quality energy gases and important raw materials for the manufacture and synthesis of many chemical products.However,in the actual production and processing of CH4and H2,the presence of CO2impurities will inevitably be introduced.Therefore,the separation of CO2from the CO2/H2/CH4mixed gas is of great research significance for improving the purity of CH4and H2.In order to evaluate the separation effect of the electric field on the CO2/CH4/H2mixed gas,we studied the adsorption behavior of BC3to CO2/CH4/H2under the E-Field of 0.004 and 0.0065 a.u..In the external E-Field of 0.004 a.u.,the most stable adsorption structure is shown in Fig.S7 (Supporting information).The adsorption distances of CO2/H2/CH4on the BC3substrate are 3.032,2.118,and 2.952 Å,and the corresponding Eadsare -0.35,-0.43 and -0.38 eV,respectively.No obvious electron distribution was observed between the CO2/CH4gas molecules and the substrate.According to the difference in adsorption energy,it can be concluded that H2can be efficiently separated from the CO2/H2/CH4mixed gas under the 0.004 a.u..In addition,the adsorption behaviors of CO2/H2/CH4gas under an electric field of 0.0065 a.u.has been studied,which is shown in Fig.S8(Supporting information).Differ from the physical adsorption of CO2/H2/CH4under no electric field (Fig.S2.),the Eadsand Dmof CO2change significantly (-0.11 to -0.84 eV,3.371 to 1.965 Å).There is no electron density distribution between the H2/CH4and the BC3substrate,but there is a distinct electron density overlaps between the CO2and the substrate,as shown in Fig.S8.Obviously,the adsorption properties of CO2and H2/CH4are different,under this circumstance the former is chemical adsorption and can be captured by BC3,but in fact the latter is physical adsorption.
We used the spin-polarized DFT calculation method to study the adsorption/desorption behavior of CO2gas with an external Z-axis vertical positive electric field.An external electric field vertical to the BC3surface can significantly enhance the interaction between CO2and the substrate.In particular,in the range of 0.006-0.0065 a.u.electric field value,CO2shifted from BC3physisorption on C4B2vacancy with the rapid decrease of Eadsby 663%(-0.11 to-0.84 eV).Desorption of chemisorbed CO2occurred from the BC3surface without passing any energy barrier and released an energy of 0.69 eV when the E-Field is switched off.Utilizing the difference in the adsorption properties of CO2/CH4/H2on BC3under the EField value of 0.004 and 0.0065 a.u.,H2and CO2gas can be separated from the CO2/H2/CH4gas mixture.These results confirmed that BC3is a potential material for capturing CO2under external electric field.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (No.21603109),the Henan Joint Fund of the National Natural Science Foundation of China(No.U1404216),the Scientific Research Program Funded by Shaanxi Provincial Education Department (No.20JK0676).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2021.03.038.
杂志排行
Chinese Chemical Letters的其它文章
- Decatungstate as a direct hydrogen atom transfer photocatalyst for synthesis of trifluromethylthioesters from aldehydes ★
- Synthesis of[6-6-6]ABE tricyclic ring analogues of methyllycaconitine
- Host-guest inclusion for enhancing anticancer activity of pemetrexed against lung carcinoma and decreasing cytotoxicity to normal cells
- pH-Responsive amorphous room-temperature phosphorescence polymer featuring delayed fluorescence based on fluorescein
- Boronic acid-containing carbon dots array for sensitive identification of glycoproteins and cancer cells
- Ultrasmall green-emitting carbon nanodots with 80%photoluminescence quantum yield for lysosome imaging
