Photocatalytic degradation of organic pollutants by MOFs based materials:A review
2021-12-29TianliangXiaYingchaoLinWeizunLiMeitingJu
Tianliang Xia,Yingchao Lin,Weizun Li,Meiting Ju
National &Local Joint Engineering Research Center on Biomass Resource Utilization,College of Environmental Science and Engineering,Nankai University,Tianjin 300350,China
Keywords:Metal-organic frameworks Synthesis methods of MOFs MOFs-based photocatalysts Photocatalytic degradation Organic pollutants
ABSTRACT Metal-organic frameworks(MOFs)are currently popular porous materials with research and application value in various fields.Aiming at the application of MOFs in photocatalysis,this paper mainly reviews the main synthesis methods of MOFs and the latest research progress of MOFs-based photocatalysts to degrade organic pollutants in water,such as organic dyes,pharmaceuticals and personal care products,and other organic pollutants.The main characteristics of different synthesis methods of MOFs,the main design strategies of MOFs-based photocatalysts,and the excellent performance of photocatalytic degradation of organic pollutants are summarized.At the end of this paper,the practical application of MOFs,the current limitations of MOFs,the synthesis methods of MOFs,and the future development trend of MOFs photocatalysts are explained.
1.Introduction
With the continuous advancement of the industrialization process,environmental problems and energy issues have become two major obstacles that restrict the sustainable development of human society.The rapid development of the economy and technology has put tremendous pressure on the ecological environment,and at the same time,the huge demand for energy from human society is also difficult to meet.Taking water pollution as an example,the current discharge of industrial wastewater is huge,which not only affects the ecological environment and threatens the health of residents,but also has a significant restrictive effect on the development of the industry itself[1].The pollutants contained in industrial wastewater are various.There are massive organic pollutants including organic dyes,pharmaceutical and personal care products,and some other organic pollutants[2-4].These substances are highly toxic and difficult to be degraded by conventional methods,which pose a great threat to the sustainable development of the ecological environment and human health.In this situation,looking for low energy consumption and environmentally friendly water pollution treatment methods has become a worldwide focus of attention.
On the other hand,the pressure brought by environmental issues has also promoted the development of environmental technology.With the continuous progress and innovation of environmental protection related technologies,advanced oxidation process (AOPs) has stood out in environmental governance technologies and has high efficiency and excellent performance[5].This is because of its widely used active substances.For example,•OH has a strong ability to degrade most organic pollutants.Most organic substances can be directly mineralized to produce CO2,H2O,and other inorganic substances [6].
Among AOPs technologies,photocatalysis technology is also of great significance in energy saving because it uses clean and abundant solar energy to catalyze chemical reactions.This makes photocatalysis technology an efficient,versatile,and economical water treatment method.
Since Fujishima and Honda et al.reported in 1972 that titanium dioxide single crystals can catalyze the decomposition of water to generate hydrogen under ultraviolet light,photocatalytic technology has developed rapidly [7].In recent years,photocatalytic oxidation technology has rapidly developed into an important part of the water treatment process,and many semiconductor photocatalysts have appeared for the treatmentof water-phase pollutants.Common semiconductor photocatalysts include TiO2[8,9],ZnO[10],Fe2O3[11],MoS2[12],CdS[13],g-C3N4[14]and so on.
However,these catalysts still have obvious limitations in actual large-scale use.This is because the efficiency of photocatalysis is directly affected by the activity of photocatalyst materials.In the past research summary,these defects mainly include:the rapid recombination of photogenerated electron-hole leads to low photocatalytic degradation of pollutants;the narrow spectral response range of the catalyst results in low solar energy utilization efficiency;few surface active sites on the catalyst[15].The combined influence of these factors has led to difficulties in the practical application of some photocatalysts.Therefore,photocatalytic technology needs to find,design,and modify efficient photocatalytic materials.
In the 1990s,chemist Yaghi synthesized a framework structure material constructed of metal and organic ligands,MOF-5,and first proposed the concept of metal-organic frameworks (MOFs) [16].Since then,this new type of material has widely appeared in the field of researchers'vision.The metal-organic framework material is a kind of porous material with intramolecular pores constructed by coordination by forming nodes of metal ions or a small number of metal ion clusters and using multiple organic ligands as the framework support [17].
With the in-depth study of MOFs by scientists,MOFs have application value in various fields such as chemistry,chemical industry,and materials,including battery energy storage,gas storage separation,catalytic reactions,adsorption materials,luminescence engineering and drug carriers [18-23].After MOF-5 was found to be able to decompose phenol under ultraviolet light irradiation,MOFs have also begun to receive attention in the photocatalytic degradation of water pollutants,especially difficultto-degrade organics [24].
As an emerging photocatalytic material,MOFs have an following advantages over traditional photocatalytic semiconductors:Firstly,the porosity of MOFs and its open frame structure facilitate the diffusion of degraded substances to the active sites of the catalyst[25].Secondly,MOFs have the extremely high specific surface area and adjustable porosity.Such properties are suitable for constructing composite catalytic materials with other active substances.Finally,the spectral response range of MOFs can be well regulated by introducing functional groups [26].Under the influence of these factors,the MOFs group has become more and more important in the field of photocatalysis,especially in the field of degradation of organic pollution.
Because most of the traditional MOFs have the properties of photocatalytic semiconductors,research on the photocatalytic degradation of organic pollutants by traditional MOFs has been relatively common so far.Commonly used MOFs,including MOFs,Zeolitic Imidazolate Frameworks(ZIFs),and the University of Oslo(UiOs),have researched the degradation of organic pollutants.Therefore,the current research direction focuses on the synthesis of new MOFs to improve the structure of materials,or to improve the photocatalytic reaction conditions of MOFs to improve the optical properties of pure MOFs.Also,simply using traditional MOFs as photocatalysts has limitations similar to traditional photocatalytic materials.The two most critical points are that the insufficient utilization of visible light and the rapid recombination of photo-generated carriers affect the photocatalytic activity of MOFs.In addition to simply using MOFs as catalysts,composite catalysts are also commonly used methods.By selecting suitable active materials and using certain methods to composite,such as the formation of heterojunction structures,the limitations can be compensated for each other through synergy and the catalytic advantage maximize.
This paper will mainly review the main synthesis methods of MOFs or their modified derivatives;the latest research results of this type of photocatalyst in the treatment of organic pollutants in water and summarize the current problems and the future development direction of this field.These latest research results include the degradation of organic pollutants such as organic dyes,pharmaceuticals and personal care products,as well as phenolic pollutants and pesticides,as shown in Fig.1.At the same time,according to the introduction just now the current development trend of MOFs photocatalysts,the follow-up research results in this paper mainly focus on new synthetic MOFs and composite derivatives of MOFs and other substances.
2.Main synthesis methods of MOFs
At present,the synthesis methods of MOFs are diversified,such as solvothermal method,microwave heating method,ultrasonic synthesis method,electrochemical method,and mechanochemical method.But there are many differences between different methods.These differences are not only reflected in the reaction cycle,but also in the economics of preparation and even the difference in yield.Even MOFs with the same metal and organic ligands as raw materials will differ in crystal structure with different synthesis methods.The following will introduce the common synthesis methods of MOFs and the characteristics and specific examples of the corresponding methods,as shown in Table 1.
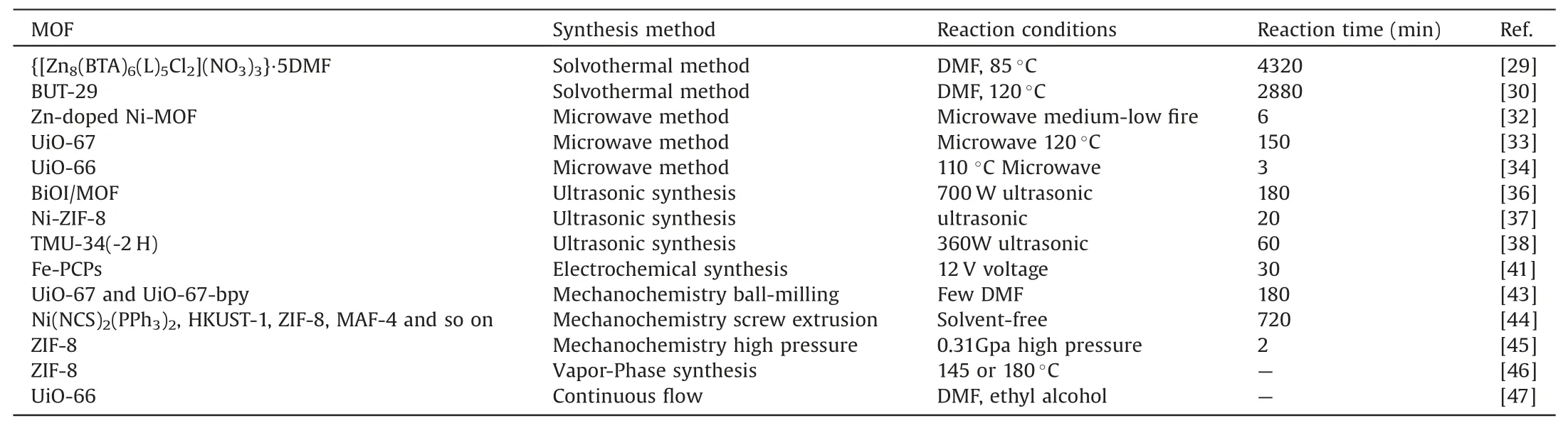
Table 1Summary of the main synthesis methods of MOFs.
2.1.Solvothermal method
The solvothermal method is one of the most common methods for the synthesis of MOFs.The solvothermal method is to dissolve metal salts and other substances in organic solvents containing selected organic ligands.Most of the reactions are carried out in the liquid phase,and finally,crystals are precipitated through selfassembly between organic ligands and metal salts,and finally obtained target MOFs [27].The MOFs synthesized by the solvothermal method has good dispersibility and complete crystal structure.At the same time,the conditions required for the synthesis reaction are easier to meet in the laboratory,and it is easy to prepare in large quantities.However,due to the long reaction time and the need for more organic solvents,such as N,Ndimethylformamide (DMF),methanol and acetone,the energy consumption and cost of the reaction are higher.Besides,the use ofa large amount of organic waste liquid causes greater environmental pollution [28].
Fig.1.Application of MOFs-based photocatalyst for degradation of organic pollutants.
He et al.used Zn(NO3)2•6H2O as the metal ion source and the angular ligand 1,3-bis(4-carboxyphenyl)imidazolium chloride(H2LCl),and the auxiliary ligand 1H-benzotriazolate (HBTA) in DMF Reacted in a solvent at 85°C for three days.Finally,{Zn4}cluster MOF {[Zn8(BTA)6(L)5Cl2](NO3)3}•5DMF,which is NUM-4,has excellent adsorption properties.At the same time,by dissolving NUM-4 in DMF,N,N-dimethylacetamide(DMA),CH2Cl2,and other solvents,the frame structure can be stably maintained,which proves that the NUM-4 prepared by this method has good solvent resistance [29].
Yang et al.synthesized a new MOF with In(NO3)2•5H2O as the metal ion and H4PBPTTBA as the organic ligand.In the presence of the competitive reagent HBF4,react in DMF solvent at 120°C for 48 h.Finally,the orthorhombic crystals of the new anion MOF,BUT-29,[In(PBPTTBA)][(CH3)2NH2]were obtained.This material has strong anionic dye removal ability and reusable properties[30].
2.2.Microwave heating method
The microwave method is already one of the commonly used methods for synthesizing nanomaterials.The synthesis reaction of MOFs can also be carried out by the microwave method.Due to the characteristics of microwave heating,the molecules inside the heated object generate heat through high-frequency motion.In this way,the object is heated uniformly without the need for heat conduction.The temperature and pressure of the reaction process can be precisely controlled,which has a significant improvement in saving reaction time and reducing energy consumption [31].Compared with traditional solvothermal synthesis,the microwave method greatly shortens the reaction time required for the synthesis of MOFs and generally can obtain MOFs crystals within one hour.However,due to the small volume of the microwave reactor,it is more suitable for small batches of MOFs synthesis.If the preparation scale is large,this method is not suitable.
For example,Chen et al.used NiCl2•6H2O and Zn(Ac)2•2H2O as the metal source,and 1,4-benzenedicarboxylic acid (PTA) as the organic ligand,dissolved in DMF solvent to carry out the synthesis reaction of MOFs.Zn-doped Ni-based MOFs can be obtained by heating the reaction for 6 min under the microwave,and the synthesis time is much shorter than that of solvothermal synthesis.At the same time,the comparison of MOFs materials was carried out by using the same raw materials and solvothermal synthesis,which proved the smaller size of the MOFs synthesized by microwave and the good application prospects of microwave synthesized MOFs in battery capacitors [32].
Similar experiments have also appeared in Zr-based MOFs.Vakili et al.used the microwave to synthesize UiO-67 and compared it with the conventional solvothermal method.There are still obvious advantages in the reaction speed of microwave heating,and the synthesis rate of UiO-67 by microwave method is more than ten times that of the solvothermal method [33].The synthesis reaction of UiO-66 can generate MOFs crystals even under 90 W,110°C microwave irradiation for 3 min,and the yield of UiO-66 can reach 90% [34].
2.3.Ultrasonic synthesis method
Like microwave radiation to promote the reaction,ultrasound is also feasible as an energy source for the MOFs synthesis reaction.The energy of high-frequency ultrasound induces the generation,growth,and rupture of bubbles in the liquid phase,which can make the local instantaneous temperature and pressure of the reactant rise sharply [35].Through such a process,sound energy can be transferred to the chemical synthesis process.This method is simple and low-cost,and the synthesis rate is relatively fast,but it is not as easy to control as solvothermal methods,and it is easy to have by-products that affect the final yield.
Lee et al.used 700 W,20 kHz,and 30%amplitude high-intensity ultrasound to irradiate a mixture of 1,3,5-benzenetricarboxylic acid and Cu(NO3)2•3H2O dissolved in a mixed solvent.After 3 h of reaction time,octahedral MOFs crystals were obtained [36].Ma et al.used a mixture of Ni(NO3)2•6H2O and Zn(NO3)2•6H2O to obtain Ni-doped ZIF-8 under ultrasonic treatment for 20 min.The experiment also proved that the ZIFs material prepared by the ultrasonic method has smaller and more uniform crystal characteristics through the means of characterization [37].Razavi et al.synthesized functionalized MOFs TMU-34(-2 H) using an ultrasound-assisted method.And by trying different ultrasonic action time,it is confirmed that the uniformity of TMU-34(-2 H) under 360 W ultrasonic action for 60 min is the best [38].
2.4.Electrochemical method
The method of electrochemical synthesis of MOFs is similar in principle to electrolytic cells.The difference with the traditional method is that the metal source is not a metal salt substance but is replaced by a metal anode.The metal ions and organic ligands produced after anodic dissolution are coordinated and assembled in a conductive medium to generate MOFs crystals [39].The fast reaction speed of electrochemical synthesis of MOFs can be continuously prepared compared to the intermittent and slow synthesis speed of the traditional solvothermal methods.The electrochemical synthesis of MOFs was first proposed by BASF.Theexperiment is to use Cu and 1,3,5-benzenetricarboxylic acid as raw materials for the synthesis of MOFs and continuously dissolve copper to form HKUST-1 through electrochemical methods [40].
Zhang et al.used a quick and simple electrochemical method under a voltage of 12 V to synthesize and prepare iron-based MOFs after adding organic ligands to the scrap iron through physical treatment such as grinding and other physical treatments [41].
2.5.Mechanochemical method
The mechanochemical method refers to the use of mechanical energy to promote the synthesis of MOFs and proceed through the application of mechanical force to inorganic and organic solid materials for grinding and extrusion,and finally obtain MOFs crystals [42].A major feature of this method is that the reaction process generally does not need to use or only a small number of organic solvents.The reason for this type of general synthesis method is to reduce the need for a large number of solvents in the synthesis of MOFs.Different from the liquid phase MOFs synthesis reaction in the presence of solvents,this type of method does not provide external mechanical energy,and solid materials such as metal sources and organic ligands are simply physical mixing,and chemical reactions will not proceed spontaneously.Therefore,multiple energy inputs such as ball milling,grinding,extrusion,high-pressure compression,are required.Because only a small amount of solvent is needed,this type of method is conducive to the industrialization and commercialization of large-scale preparation of MOFs,which is suitable for large-scale continuous production.
Ali-Moussa et al.under the condition of few DMF solvents,the mixtures of different organic ligands and Zr metal sources were ground using a conventional ball mill to obtain UiO-67 and UiO-67-bpy with good purity.In subsequent experiments,the UiO-67-bpy prepared by the ball milling method was modified with CuBr2,and the obtained complex can effectively oxidize olefins to epoxides,which is equivalent to UiO-67-bpy prepared by conventional methods.This has proved that MOFs prepared by ball milling can still be modified,providing the possibility of high-purity functionalization [43].
Crawford et al.used a screw extruder to continuously extrude and synthesize a variety of MOFs.Including Ni(salen),Ni(NCS)2(PPh3)2,HKUST-1,ZIF-8,MAF-4 and Al(fumarate)(OH),all achieve solvent-free or solvent-less extrusion synthesis.In addition,it provides a continuous and uniform synthesis of ZIF-8 using a screw extruder,which is an order of magnitude higher than the space-time output of other synthesis methods [44].
Paseta et al.used another mechanochemical method to prepare MOFs material,high-pressure compression.Without using a solvent,shake the mixture e of ZnO and 2-methylimidazole by hand and place it in a metal cylinder.After starting the reactor,the mixture is applied with high pressure of 0.31 Gpa,and the ZIF-8 product can be obtained in 2 min,which provides a possible way for the rapid industrial production of MOFs [45].
2.6.Other synthesis methods
In addition to the methods described above,there are also some unconventional methods for the synthesis of MOFs,such as Vaporphase synthesis and continuous flow strategies.These unconventional methods are not used frequently in the laboratory,and are generally for special purposes and other reasons,while the optimization of traditional methods or the development of new methods.
Tanaka et al.synthesized ZIF-8 membranes using vapor phase transport(VPT).The experiment used three different types of ZnO precursors as the metal source,and they were respectively heated with a small amount of steam source and heated at a temperature of 145°C or 180°C for some time,and finally,the ZIF-8 material was obtained.It provides development space and prospects for the continuous large-scale green production of MOFs [46].
Rubiomartinez and others designed the UiO-66 continuous batch production reaction system based on the continuous flow strategy.The raw and auxiliary materials such as ZrCl4are put into the spiral reactor for heating and mixing and then flow through the backpressure regulator into the sealed container.After washing and soaking with DMF and ethanol,UiO-66 powder can be finally obtained.The overall yield can reach 67%.Moreover,the entire reaction system can accurately control various reaction factors[47].
3.Application of MOFs-based photocatalyst to degrade organic pollutants in water
3.1.Application of MOFs-based photocatalyst for the degradation of organic dyes
In recent years,the development of the dye industry has brought vest commercial synthetic dyes.These dyes are widely used in printing and dyeing,medicine,and cosmetics industries.A large amount of dye wastewater comes with it.According to statistics,it is only the textile printing and dyeing industry.The world produces about 100 tons of dye wastewater a year[48].The composition of dye wastewater is extremely complex,and most of them contain refractory organic matter,high chroma,and strong continuous pollution.Furthermore,because of the obvious color changes during the degradation of dyes,there are many research advances on the degradation of organic dyes by MOFs-based photocatalysts.Common dyes,such as rhodamine B (RhB),methylene blue,methyl orange,are often used as target pollutants for the photocatalytic degradation of organic dyes by MOFs,as shown in Table 2.
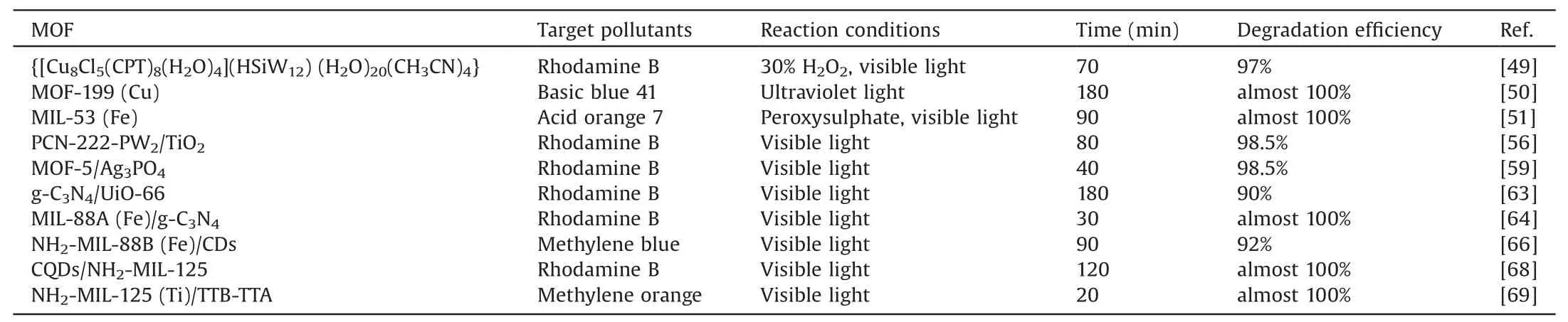
Table 2Summary of the latest progress in the degradation of organic dyes by MOFs-based photocatalysts.
First,introduce the research of pure MOFs on dye degradation.Chen et al.synthesized a new type of multi-metal acid group MOF with{Cu4Cl}7+and{Cu2(CO2)4}as the structure center by using the organic ligand HCPT with two functional groups of carboxylic acid and triazole,{[Cu8Cl5(CPT)8(H2O)4](HSiW12)(H2O)20(CH3CN)4}n.This MOF has a three-dimensional interpenetrating network structure based on four-connected {Cu2(CO2)4} and eight-connected {Cu4Cl}7+clusters.In the photocatalytic degradation experiment,the MOF can degrade 97% of the RhB under an environment that has 30%H2O2and visible light irradiation within 70 min.Moreover,the material is resistant to acids and alkalis.And it has more practical application value than traditional MOFs[49].
Mahmoodi et al.used a green method to synthesize Cu-based MOFs with nanoporous structure,MOF-199.And without using any oxidizing agents such as H2O2and potassium persulfate,only MOF-199 catalyzes the degradation of Basic Blue 41.The results show that under ultraviolet light irradiation,nanoporous MOF-199 can reach a degradation rate of 99%at a concentration of 0.04 g/L and has high photocatalytic activity.Besides,experiments have proved that MOF-199 mainly oxidizes basic blue 41 dye through the generated•OH [50].
In the method of improving the photocatalytic reaction conditions of MOFs,adding the external electron acceptor is a commonly used option.Gao et al.used traditional Fe-based MOFs,MIL-53 to study the degradation process of acid orange 7 in aqueous solutions.Because of the rapid recombination of electrons and holes,the effect of simply using MIL-53 for degradation is poor,and the degradation rate under visible light irradiation is only 24%.Therefore,the researchers added a certain amount of persulfate as the external electron acceptor,and the degradation efficiency of acid orange 7 in 90 min was close to 100%.Experiments haveproved that the external electron acceptor has a significant effect on improving the photocatalytic performance of MIL-53 and the application prospects of traditional MOFs in photocatalytic water treatment.Experiments also used characterization methods to prove that after the introduction of persulfate,the electron-hole of MIL-53 can be effectively separated and facilitate the generation of active free radicals of MIL-53,which is helpful for the oxidative degradation of acid orange 7 [51].
As explained above,it is still difficult to use MOFs alone to meet the current requirements for the photocatalytic degradation of organic pollutants.However,the use of composite catalysts of MOFs and other substances,such as the use of functionalized MOFs and other metallic or non-metallic materials to construct heterojunctions,can well make up for the defects of MOFs [52].
As the most mature photocatalytic semiconductor,TiO2is a classic choice for the preparation of MOFs-based catalysts combined with MOFs.Traditional MOFs,such as Cu-based MOF HKUST-1,Zn-based MOF ZIF-8,and Ti-based MOF MIL-125,have been treated with TiO2with composite catalysts to degrade RhB or methyl orange [53-55].
However,most of these composite catalysts cannot effectively degrade in visible light.Now researchers are also beginning to improve the visible light response of MOFs-based catalysts.Li et al.used the solvothermal method to combine TiO2and Bronsted acid H3PW12O40with Mn-based MOF PCN-222 to prepare a ternary MOFs-based composite catalyst PCN-222-PW2/TiO2.In the subsequent experiments on the degradation of RhB by visible light,the composite catalyst with a PCN-222 content of 5% showed the strongest photocatalytic performance,and the degradation rate reached 98.5%.Under the same reaction conditions,the composite catalyst is better than pure TiO2and PCN-222(its reaction speed is 10 times higher than that of a single catalyst).Through analysis,the composite material enhances the visible light response of MOFs,and the bandgap matching relationship effectively inhibits the recombination of photogenerated electrons and holes.Among them,h+,O2•-and•OH are the main active substances in the degradation of organic dyes by this material,which provide ideas for the catalytic application of MOFs under visible light [56].
In addition to TiO2,materials such as precious metals are also commonly used composite options.Related materials such as Pd and Ag have had examples of composite catalytic degradation of dyes with Zr-based MOF UiO-66 [57,58],and in current research,the formation of heterojunctions with Ag-coupled MOFs-based catalysts is more popular and effective.Recently,to improve the photocatalytic performance of MOF-5,Tong et al.tried to use Ag3PO4and MOF-5 to construct a heterojunction complex.The composite catalyst of MOF-5/Ag3PO4was successfully synthesized by Ag3PO4based on the in-situ deposition method.The experimental data show that the degradation effect of various heterojunctions on RhB is better than pure MOF-5 and pure Ag3PO4.Among them,the heterojunction with a mass ratio of 7% has the best effect.Under visible light irradiation,RhB can be catalytically degraded,and a degradation rate of 98.5% can be achieved in 40 min,which is much higher than 63% of Ag3PO4.According to subsequent capture experiments,it is concluded that O2•-and h+are the main factors in the degradation reaction [59].
In addition to the traditional composite of metal materials and MOFs,with the gradual rise of graphene,g-C3N4,and other carbonbased materials in the field of catalysis,researchers have begun to try the construction of composite materials of carbon-based materials and MOFs [60-62].These carbon-based materials can effectively promote the generation and separation of photogenerated electrons and holes and improve photocatalytic performance.
g-C3N4is a non-metal semiconductor with a two-dimensional planar conjugate structure that has emerged in recent years.It has a medium-width bandgap and can fully absorb and utilize light energy within a certain wavelength range.Zhang et al.used g-C3N4and Zr-based MOF UiO-66 to synthesize a UiO-66 nanohybrid wrapped in graphite phase carbon nitride through a solvothermal method.Among them,CNUO-1 with a carbon nitride weight content of 1 has the best photocatalytic performance.Under the irradiation of visible light,the RhB aqueous solution is degraded,and the degradation rate is 80% within 60 min,and 90% within 180 min,which is 6 times the speed of using graphite phase carbon nitride alone.It is proved that the addition of g-C3N4can enhance the separation and migration rate of light-induced charges,which leads to an increase in photocatalytic efficiency [63].
In addition to Zr-based MOF,Fe-based MOF has also been studied accordingly.Shao et al.used g-C3N4and Fe-based MOF MIL-88A to construct a heterojunction to perform photocatalytic degradation of organic pollutants.Since a direct Z-type heterojunction is formed between g-C3N4and MIL-88A,the bandgap of the composite material is reduced,which helps accelerate photogenerated charge separation.This makes MIL-88A/g-C3N4have a significantly enhanced photocatalytic activity compared to graphite phase carbon nitride.Among them,M88/g-C3N4-10,which has the best photocatalytic activity,has a degradation rate of nearly 100% for RhB under 30 min of visible light irradiation,and the degradation rate constant is 0.15985 min-1,which is about 4.7 times higher than g-C3N4under the same conditions.And the capture experiment proved that the main active substance of the reaction is the•OH [64].
Carbon nanodots (CDs) are newly emerging light-absorbing nanomaterials,which have good light stability and water solubility[65].Shao et al.synthesized a composite of NH2-MIL-88B(Fe)and CDs by the solvothermal method.Through the photocatalytic degradation experiment of methylene blue,it is proved that the composite material has good photocatalytic performance and recycling performance.Under visible light irradiation for 90 min,the degradation rate of the dye reached 92%,which was 2.6 times that of NH2-MIL-88B(Fe) alone.This is because the CDs in thecomplex as a local electron acceptor can effectively enhance charge transfer and inhibit electron-hole recombination [66].
Carbon quantum dots(CQDs)are also a new type ofcarbon-based nanomaterials,which have good electrical conductivity and special properties forconverting luminescence[67].Based onthecharacteristicsofCQDs,Wangetal.chosefunctionalizedMOFNH2-MIL-125as the basis,designed and synthesized CQDs/NH2-MIL-125 by solvent deposition.The composite material with 1% CQDs has the best performance.Whether it is irradiated under the full-spectrum,near-infrared light,or visible light,the degradation efficiency of the composite material for RhB has been significantly improved,and RhB can be completely degraded within 120 min.Subsequent analysis shows that the good conductivity of CQDs improves the separation efficiency of photogenerated electrons and holes.Simultaneous conversion of luminescence properties,broadening the response spectrum of NH2-MIL-125[68].
In addition to these carbon-based materials mentioned above,the covalent organic framework has similar research results.He et al.used covalent organic frameworks (COFs) and MOFs to construct a Z-type heterojunction.By using highly stable TTB-TTA(a COF synthesized from 4,4′,4′′-(1,3,5-triazine2,4,6-triyl)tribenzaldehyde (TTB) and 4,4′,4′′-(1,3,5-triazine-2,4,6-triyl)trianiline(TTA)) to encapsulate functionalized NH2-MIL-125(Ti),a heterojunction hybrid material layer was prepared.The material has a large specific surface area,good crystallinity,and good response performance under visible light,which fully inherits the advantages of both COFs and MOFs.Experiments confirmed that due to the formation of a heterojunction structure,the photogenerated electrons in NH2-MIL-125(Ti) combine with the holes in TTB-TTA,thereby enhancing the extraction of charge carriers and the utilization of light excitation.The experiment uses methyl orange dye to test,the photodegradation kinetics of the composite heterojunction is 9 times that of only NH2-MIL-125(Ti),showing excellent photocatalytic performance [69].
3.2.Application of MOFs-based photocatalyst for degradation of pharmaceuticals and personal care products
Pharmaceuticals and personal care products(PPCPs)is a newly emerging water pollutant that has attracted much attention.Since Daughton and Ternes proposed related reports on the environmental problems of PPCPs in 1999,the pollution of PPCPs has been concerned by countries all over the world[70].Such products are mainly composed of commonly used drugs and personal cleaning and care daily necessities.Common PPCPs include antibiotics,analgesics,and fungicides,etc.With the development of society,the production and use of these drugs have increased year by year.And because PPCPs are relatively persistent in toxicity and bioaccumulation,they will enter the water environment in various ways after use,causing serious environmental problems.For example,it affects human health,induces bacterial gene mutations,and screens for antibacterial properties to produce drugresistant pathogens [71].Therefore,it is urgent to find a safe and effective means to degrade PPCPs.As an emerging material,MOFsbased photocatalysts have important research value for the completion of related drugs.The following will introduce the application status of different types of PPCPs degraded by MOFsbased photocatalysts,as shown in Table 3.
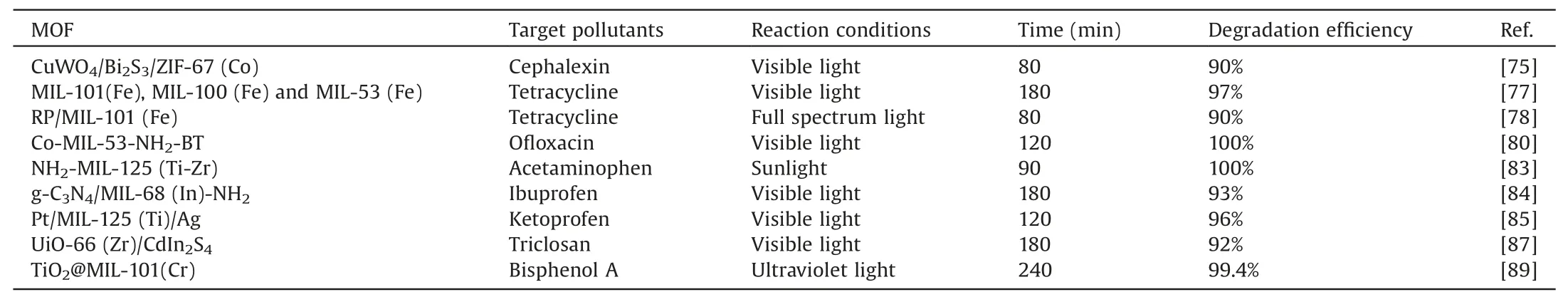
Table 3Summary of the latest progress in the degradation of PPCPs by MOFs-based photocatalysts.
3.2.1.Application of MOFs-based photocatalyst for degradation of antibiotics
Antibiotics are a common type of substance in PPCPs.According to reported statistics,the current annual use of antibiotics in the world reaches 100,000-200,000 tons [72].Therefore,to solve the environmental problems of antibiotics,MOFs-based photocatalysts have also undergone many studies on the degradation of antibiotics.Such as β-lactams,tetracyclines and quinolone antibiotics,there have been studies on the degradation of MOFs.
β-Lactam antibiotics are antibacterial drugs represented by penicillin and cephalosporin antibiotics.And these antibiotics are currently the most common [73,74].Among them,cephalosporin antibiotics are widely used because of their broad-spectrum antibacterial properties.However,the environmental problems caused by large-scale use have also forced people to find ways to efficiently degrade antibiotics.
Askari et al.constructed a CuWO4/Bi2S3/ZIF-67 ternary MOFsbased heterojunction catalyst based on a simple hydrothermal synthesis method based on ZIF-67.The degradation process of the photocatalyst for cephalexin and metronidazole was studied in the continuous flow mode,and the optimal operating parameters of the process were obtained through the central compliance design.Under optimal conditions and visible light irradiation,the degradation efficiencies of cephalexin and metronidazole PPCPs reached 90.1% and 95.6%,and the maximum total organic carbon removal rates reached 74% and 83.2%.Compared with the use of Bi2S3and the binary catalyst CuWO4/Bi2S3alone,the performance of the new three-way photocatalytic degradation with MOFs has been significantly improved.The former is 9 times and 4 times the reaction rate of the latter.This is due to the higher specific surface area and better photo-generated charge separation due to the double Z-type heterostructure [75].
In addition to β-lactam antibiotics,tetracycline antibiotics have a similar situation.As the second-largest class of antibiotics after β-lactams,the production and use of tetracycline antibiotics have accounted for 1/3 of all antibiotics [76].
Wang et al.used different Fe-based MOFs(Fe-MIL-101,Fe-MIL-100,and Fe-MIL-53) to study the process of photocatalytic degradation of tetracycline and compared the performance gap of Fe-based MOFs in this regard.The experimental results show that MIL-101 has the best photocatalytic degradation of tetracycline.At an initial tetracycline concentration of 50 mg/L and visible light irradiation for 3 h,96.6%of tetracycline was removed,which is 1.7 and 2.4 times that of MIL-100 and MIL-53 under the same conditions.Furthermore,capture experiments and ESR experiments show that O2•-,•OH,and h+are the main active substances inthis degradation process.This experiment proves the highefficiency degradation ability of MIL-101 for tetracycline,and at the same time provides certain MOFs-based catalyst design ideas for the subsequent photocatalytic degradation of tetracyclines or other refractory antibiotics [77].
Subsequently,Lei et al.used Fe-based MOF MIL-101 and nonmetallic red phosphorus (RP) to construct a composite catalyst to optimize the photocatalytic degradation of tetracycline by Febased MOFs.It uses a simple low-temperature solvothermal synthesis method to synthesize RP/MIL-101 heterojunction composite material using MIL-101 and red phosphorus.photocatalytic degradation effect of tetracycline.Under full-spectrum irradiation,the degradation efficiency of RP/MIL-101 catalysts with different red phosphorus mass fractions over 80 min for tetracycline is more than 85%,which is significantly higher than about 50%of MIL-101 alone.Among them,RP/MIL-101 with a mass fraction of 15% has the best effect,and the degradation efficiency of 80 min reaches 90.1%.The formation of a heterojunction with red phosphorus provides an improvement in the intensity of absorbed light and the reason for the improvement of the photocatalytic effect of inhibiting the recombination of photogenerated electron holes [78].
Also,ofloxacin is also an antibiotic widely used in medical,aquaculture,and animal husbandry industries.Ofloxacin is one of the most widely used quinolone antibiotics.However,due to the low body absorption and the prone to residues,ofloxacin enters the environment after production or use,causing environmental problems [79].Lv et al.used benzothiadiazole and Co to modify the Fe-based MOF NH2-MIL-53.Through the step-by-step assembly strategy,a new benzothiadiazole functionalized co-doped MOF-based photocatalyst,Co-MIL-53-NH2-BT with electronic defect units was synthesized by the solvothermal method.In the experiment of this photocatalyst to degrade ofloxacin,the degradation efficiency of ofloxacin reached 99.8% within 120 min of visible light irradiation.The reason for the obvious improvement in photocatalytic performance is that the benzothiadiazole electron-deficient group effectively promotes the separation and transfer of photogenerated electrons and holes.At the same time,TOC analysis shows that most of the pollutants have been degraded into CO2and H2O.Simultaneously used in six cycles After that,it still has a degradation rate of more than 90% and good stability[80].
3.2.2.MOFs-based photocatalyst to degrade non-steroidal antiinflammatory drugs
In addition to the commonly used antibiotics for PPCPs,nonsteroidal anti-inflammatory drugs (NSAIDs) are also antipyretic and analgesics widely used in people's daily life for fever and pain relief.For example,acetaminophen(paracetamol),ibuprofen,and ketoprofen are all similar drugs [81].Although these substances have low biological toxicity,their good stability allows them to continue to accumulate in the aquatic environment and organisms,causing serious consequences [82].
For the photocatalytic degradation of paracetamol,Gómez-Avilés et al.used a solvothermal method to replace part of the Ti atoms in the functionalized Ti group MOF NH2-MIL-125 with Zr atoms.Finally,a hybrid Ti-Zr MOF photocatalyst based on NH2-MIL-125 was synthesized.Among them,the sample doped with Zr with a molar percentage of 15% has the highest photocatalytic activity.Under the irradiation of simulated sunlight,acetaminophen can be completely degraded in 90 min.TOC analysis results show that the value is reduced by 65.3%,and the degree of mineralization is obvious.This is because Ti4+is replaced by Zr4+and has a higher average crystal size and expanded unit cell,showing a high surface area value and better porosity.The catalyst remains quite stable after three consecutive uses,with a degradation rate of paracetamol of more than 90% [83].
Cao et al.used the solvothermal method with the assistance of ultrasound to construct a heterojunction composite catalyst of g-C3N4and amino-functionalized In-based MOF MIL-68 for photocatalytic degradation of ibuprofen.The 10 wt% composite material with the highest photocatalytic activity,g-C3N4/MIL-68-NH2,has a degradation rate of 93% for ibuprofen without other oxidants and under 180 min of visible light irradiation.Besides,the TOC removal efficiency reaches 70%,and the reaction rate is 19.28 times that of g-C3N4,showing extremely high photocatalytic activity [84].
Similar pollutants such as Ketoprofen,Miao et al.added Pt and Ag precious metals through solvothermal and light deposition methods and finally constructed a surface plasmon resonance interface with Ti-based MOF MIL-125,Pt/MIL-125/Ag.And use this composite catalyst to degrade ketoprofen.Through the dual interface effect between MOF and precious metals,the visible light response of MIL-125 and the effective separation of photogenerated electron holes are greatly enhanced.Through the XPA-7 photocatalytic device,within 120 min of visible light irradiation,the degradation rate of ketoprofen is close to 95.5%,which increases the degradation rate of MIL-125 by about 20 times.
This proves that the design of the dual interface between semiconductor and precious metal is a feasible strategy for difficult-to-decompose organics [85].
3.2.3.Application of MOFs-based photocatalyst to degrade other PPCPs
In addition to drugs,some common chemicals in personal care products can also flow into the environment after use,causing environmental problems,such as the fungicide triclosan and chemical products Bisphenol A (BPA).Triclosan is a broadspectrum bactericide,almost all daily toiletries contain this substance [86].
To study the mineralization and degradation of triclosan under visible light,Bariki et al.used Zr-based MOF UiO-66 and CdIn2S4,which are heat-resistant,acid-resistant,and stable,to construct a coupled semiconductor heterojunction through a simple solvothermal method.After characterization,it was found that the composite material contains dispersed UiO-66 spheres anchored on CdIn2S4nanosheets.In the experiment of the visible light degradation of triclosan,the degradation rate of triclosan reached 92%within 180 min,and the degradation rate constant is about 12 times that of pure CdIn2S4,which reflects the excellent photocatalytic activity of the MOF-based photocatalyst after improving the photoelectric performance.Experiments have also confirmed that O2•-and•OH are the main active substances in the degradation reaction of triclosan [87].
BPA is one of the most widely used chemical products in the world.It is an indispensable additive in the production of epoxy resins and polycarbonate plastics [88].However,BPA is also a pollutant in PPCPs that will affect the ecological environment.
Tang et al.chose Cr-based MOFs MIL-101 and classic photocatalyst TiO2to build a composite material and tried to degrade BPA.Finally,the composite catalyst TiO2@MIL-101(Cr) was synthesized by the solvothermal method.Experimental characterization found that the introduction of TiO2improves the separation of photogenerated electrons and holes,reduces the band gap width,and successfully improves the photocatalytic activity.In the subsequent experiments on the degradation of BPA,the composite catalyst achieved a BPA degradation rate of 99.4% within 240 min in the ultraviolet radiation,which was significantly better than using the two catalysts alone.In the mechanism study,the detection of intermediate productsconfirmed that O2•-in the reaction process was the main active substance [89].
3.3.Application of MOFs-based photocatalytic degradation of other organic pollutants
In addition to the above two types of organic pollutants,in recent years,MOFs-based catalysts have been partially studied for photocatalytic degradation of residual organic pesticides in water or phenolic pollutants in chemical wastewater such as petrochemicals.These studies are not much more than the organic dyes and PPCPs reviewed in the previous paper,and the main content is concentrated on the common substances in pesticides atrazine and malathion,phenol pollutants,phenol,nitrophenol,etc.,as shown in Table 4.
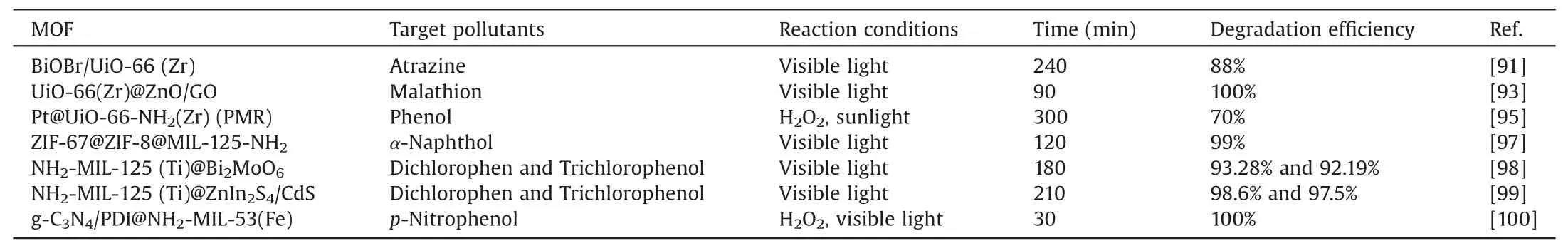
Table 4Summary of the latest progress in the degradation of other organic pollutants by MOFs-based photocatalysts.
3.3.1.Application of MOFs-based photocatalysts in the degradation of organic pesticides
Atrazine is a highly effective herbicide widely used in agricultural crop production.However,due to its difficult biodegradability,the atrazine remaining in the soil after use will enter the water system along with precipitation and runoff,causing environmental risks [90].Therefore,the photocatalytic degradation of atrazine is a better choice.Xue et al.used BiOBr and UiO-66 to construct a MOF-based composite photocatalyst by insitu growth method to degrade atrazine under visible light irradiation.By forming a heterojunction with BiOBr,the shortcomings of the easy recombination of UiO-66 photogenerated electron holes have been improved,and it can have a good response in visible light.In the 240 min degradation reaction,88%of the atrazine was successfully degraded,which was significantly improved compared to the 50% degradation rate of the monomer catalyst BiOBr.The researchers also verified the influence of typical environmental factors on the degradation reaction.The acidic conditions of low pH 3.1 are conducive to the degradation of atrazine.Except for pure water,all environmental water quality shows an inhibitory effect on the degradation reaction.The capture experiment proved that h+and O2•-are the active substances of BiOBr/UiO-66 in this reaction [91].
The situation of malathion is similar to the atrazine in this respect.As an organophosphorus pesticide widely used in agriculture,it has the negative problems of high toxicity,difficult degradation,and easy residue [92].Also based on UiO-66,Fakhri et al.used graphene oxide and metal oxide ZnO to construct a ternary MOFs-based heterojunction catalyst for photodegradation of malathion.Through the electron transport mechanism of the Z-type heterojunction and the excellent electron mobility of graphene oxide,the efficient separation of charge carriers of UiO-66 is realized.In addition,the composite catalyst shows a redshift at the absorption edge,which is a significant improvement in the visible light response of the pure UiO-66.The UiO-66@45 ZG group with the best effect in the experimental group achieved 100%complete degradation of malathion within 90 min.This work provides an effective strategy for the design of MOF-based photocatalysts [93].
3.3.2.Application of MOFs-based photocatalyst to degrade phenolic pollutants
The source of phenolic pollutants in the water environment is mainly human factors.Wastewater such as petrochemical and pharmaceutical industries often discharge such substances into aquatic systems.Because of their obvious toxicity to organisms,good stability,and slow biodegradation,they are often found in soil,water,and organisms [94].Common phenolic pollutants include phenol,naphthol,chlorophenol,and nitrophenol,etc.
Chen et al.added precious metal Pt to functionalized MOF UiO-66-NH2and finally prepared a composite MOF-based photocatalyst Pt@UiO-66-NH2by highly dispersing Pt on UiO-66.At the same time,in order to study the process of photocatalytic degradation of phenol,a photocatalytic membrane reactor(PMR) is made with α-Al2O3as the basic carrier and Pt@UiO-66-NH2on it was designed.In the TOC experiment,a composite catalyst with a Pt/Zr ratio of 0.5 mineralized 70%of phenol within 300 min of simulated sunlight.At the same time,the cycle test proved that the membrane reactor has good reusability,and the catalytic activity remains good after multiple cycles.It provides a design strategy and implementation basis for the large-scale MOFs-based photocatalytic membrane reactor to degrade waterphase phenol and other phenolic pollutants [95].
Naphthol is also a common phenolic pollutant,among which α-naphthol has a direct toxic effect on human blood circulation[96].Abdelhameed et al.designed a composite of MOF ZIF-67,ZIF-8,and MIL-125-NH2to obtain a multi-component nanocatalyst ZIF-67@ZIF-8@MIL-125-NH2.This catalyst is used to test the photocatalytic performance of the composite catalyst by photocatalytic degradation of α-naphthol.Experiments show that under the condition of visible light irradiation for 120 min,ZIF-67@ZIF-8@MIL-125-NH2degrades 98.9% of naphthol pollutants,and the degradation rate is significantly higher than that of ZIF-8@MIL-125-NH2and 42% and 50% of ZIF-67@MIL-125-NH2.At the same time,the ternary MOF material still has a naphthol degradation rate of 94% after being used 5 times and has the potential to become a photocatalytic treatment agent for industrial phenolcontaining wastewater [97].
Chlorophenol is a pollutant widely used in industrial and agricultural discharge water,and it is also a phenolic pollutant that needs to be treated preferentially.For the photocatalytic degradation of dichlorophenol and trichlorophenol,Zhang et al.synthesized a mesoporous core-shell heterojunction catalyst A with surface defects based on functionalized Ti-based MOF NH2-MIL-125 and Bi2MoO6.The proper pores of the composite catalyst and the structure of MOFs provide sufficient active surface sites.At the same time,the core-shell heterojunction improves the charge separation effect of MIL-125.This makes the catalyst's degradation effect on chlorophenol under visible light greatly improved.In the 180 min visible light irradiation photocatalytic experiment,thebest effect is that the photocatalytic degradation efficiency of dichlorophenol and trichlorophenol is 93.28%and 92.19%,respectively,and the corresponding rate constant is higher than that of the original NH2-MIL-125(Ti) The speed is about 8 times and 17 times higher [98].
Subsequently,Zhang et al.chose ZnIn2S4,CdS and functionalized Ti-based MOF NH2-MIL-125 to construct a defect-rich and electron-rich hierarchical tandem core-shell heterojunction catalyst,NH2-MIL-125(Ti)@ZnIn2S4/CdS by the solvothermal method.And for the photocatalytic degradation of dichlorophenol and trichlorophenol,the photocatalytic degradation efficiency of the catalyst is high,98.6%,and 97.5%,respectively.This novel design of Ti-MOF-based core-shell heterojunction may provide new strategies and insights for the design of high-performance heterojunction catalysts for the degradation of phenolic pollutants [99].
Like other phenolic pollutants,nitrophenol also has the above properties.Therefore,some researchers have used nitrophenol as the target of photocatalytic degradation.Li et al.chose the commonly used method of constructing heterojunctions based on MOFs to study the photocatalytic degradation of nitrophenol.By the solvothermal method,the surface of aminated Fe-based MOF NH2-MIL-53 was grown on g-C3N4doped with pyromellitimide(g-C3N4/PDI),and a new type of MOFs-based heterostructure,g-C3N4/PDI@MOF was obtained.The experimental process uses the catalyst to decompose p-nitrophenol in the presence of H2O2and under the irradiation of visible light.The experimental results once again prove that the construction of an energy-level matching heterojunction has a significant effect on improving the photogenerated electron transfer of MOFs.CPM-2 which has the best experimental effect can reach a 100% degradation rate of p-nitrophenol within 30 min.It reflects the potential of nonmetallic semiconductors and MOFs to construct heterojunctions for the degradation of phenolic pollutants [100].
4.Conclusion
Due to the open porous structure and excellent specific surface area of MOFs,the guest molecules and active sites are easily contacted when MOFs are used for catalysis,which reduces the migration distance of photogenerated carriers;in addition,the porosity is easy to adjust and the framework characteristics of metal nodes and organic ligands allow MOFs to be widely combined with other active materials to construct more efficient composite photocatalysts.These advantages make MOFs a hot spot for researchers in the field of photocatalysis.In short,this work is summarized as the main synthesis methods of the current MOFs and the latest progress in the application of MOFs-based photocatalysts to the catalytic degradation of common aqueous organic pollutants are discussed.
At present,the synthesis method of MOFs-based catalysts in the laboratoryisstillmainlysolvothermal.Asimpleprocess,controllable crystal structure,and good crystallinity are the reasons why this method is widely favored by laboratory personnel.Microwave,ultrasound,and electrochemistry are used for some special situations,such as MOFs needs to be obtained in a short time.However,the current difficulty lies in the synthesis methods of MOFs,such as solvothermal methods,which usually require a large amount of organic solvent,which is costly and not environmentally friendly.This is a severe test for the practical application and commercial production of MOFs.Under the influence of today's green chemistry and sustainable development concepts,the synthesis methods of MOFs will be more solvent-free or solventless,low-energy-consumption,and fast and controllable in the future.For example,mechanochemical synthesis methods may perform well in the production of commercial MOFs in the future.
In terms of photocatalytic degradation of water-phase organic pollutants,as summarized in this review,a variety of MOFs-based photocatalysts have been widely used in the photocatalytic degradation of organic dyes,PPCPs,and other refractory organics.Among them,except for a small number of researchers who are committed to synthesizing new MOFs,most of the research is based on MOFs to select other active materials to form composite materials,such as constructing heterojunctions.This is because,compared with the former,the design principle of the latter is simple and effective,and has wide applicability.
By retaining the advantages of MOFs'ultra-high specific surface area and adjustable porosity,it makes up for the disadvantages of poor visible light response and low separation of photogenerated electrons and holes.Thus,MOFs-based composite catalyst with high photocatalytic activity has become the common catalyst to degrade organic pollutants in the water phase.As for the photocatalyst of pure MOFs,the future development trend is to meet the response range of visible light and solve the complex problem of photogenerated electrons.Therefore,it is necessary to try more organic ligands to meet the requirements of photocatalytic activity.And designing and synthesizing new MOFs with photocatalytic properties has become the key to research.That is because there are just a few types of pure MOFs materials that can be used as photocatalysts for now.
However,in these experimental studies,there are still some issues worthy of further study.First,although there are many types of MOFs,the types of MOFs used in photocatalysis are currently relatively single,and newly synthesized MOFs with photocatalytic effects are rare.Secondly,the goal of current research is to truly use MOFs-based photocatalysts to degrade organic pollutants in the water phase.But the experiment is still based on laboratory environment simulation.
Whether the photocatalytic performance of MOFs in the real environment is consistent with that in the laboratory requires more in-depth research.The development trend of MOFs-based photocatalysts is still in the direction of designing high-performance photocatalysts.At the same time,reducing the use of precious metals and combining non-metallic materials to build heterojunctions,such as carbon-based materials,maybe a good choice in the future.
However,despite some limitations,MOFs-based materials are currently showing a rapid rise in research in many fields,especially in photocatalytic engineering,which still reflects the development space and potential of MOFs-based photocatalysts.Under continuous exploration and research,it is believed that MOFs-based photocatalysts can provide new ideas and ways for the degradation of water-phase organic pollutants.And MOFsbased photocatalysts can contribute to the realization of sustainable development.
Declaration of competing interest
All the authors declare that they have no financial and personal relationships with other people or organizations that can inappropriately influence this work.And there is no professional or other personal interest of any nature or kind in any product,service and company that could be construed as influencing the position presented in the manuscript entitled.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(No.21878163)and Tianjin Municipal Science and Technology project (No.18PTZWHZ00150).
杂志排行
Chinese Chemical Letters的其它文章
- Decatungstate as a direct hydrogen atom transfer photocatalyst for synthesis of trifluromethylthioesters from aldehydes ★
- Synthesis of[6-6-6]ABE tricyclic ring analogues of methyllycaconitine
- Host-guest inclusion for enhancing anticancer activity of pemetrexed against lung carcinoma and decreasing cytotoxicity to normal cells
- pH-Responsive amorphous room-temperature phosphorescence polymer featuring delayed fluorescence based on fluorescein
- Boronic acid-containing carbon dots array for sensitive identification of glycoproteins and cancer cells
- Ultrasmall green-emitting carbon nanodots with 80%photoluminescence quantum yield for lysosome imaging
