堆肥种子发芽指数测定方法与敏感性种子筛选
2021-12-28王国英孔艺霖沈玉君李国学
王国英,袁 京,孔艺霖,沈玉君,杨 燕,李国学
·农业生物环境与能源工程·
堆肥种子发芽指数测定方法与敏感性种子筛选
王国英1,袁 京1,孔艺霖1,沈玉君2,杨 燕1,李国学1※
(1. 中国农业大学资源与环境学院农田土壤污染防控与修复北京市重点实验室,北京 100193;2. 农业农村部规划设计研究院,北京 100125)
种子发芽指数(Germination Index,GI)作为评价堆肥腐熟度的权威指标,被广泛应用于评价堆肥产品植物毒性等方面,但对于GI测定方法和供试种子类型仍缺少统一标准,导致不同堆肥研究的GI结果缺乏可比性。为此,该研究通过设置不同毒性梯度的堆肥试验、选取不同类型种子,详细介绍了GI测定方法,规定根长为0和1 mm的计量标准以及根长起始测量位置,并系统分析了表征堆肥腐熟度指标与种子发芽之间响应关系。结果表明:最终产品GI以纯猪粪(26.54%~80.49%)、纯羊粪(16.71%~92.98%)、猪粪+秸秆(28.28%~110.74%)、羊粪+秸秆(43.38%~119.69%)的顺序递增。综合分析不同堆肥处理理化指标(温度、氧气、pH值、碳氮比)和植物毒性因子(电导率、铵根、有机酸和有机质)与不同种子GI(黄瓜、萝卜、白菜、油菜)相关性关系,发现萝卜种子响应度最高(=0.96),在综合表征堆肥腐熟度和植物毒性上最具科学性和代表性。以上结果可为种子发芽指数测定、有机肥标准制定以及堆肥农田安全施用提供参考。
堆肥;种子;植物毒性;发芽指数;测定
0 引 言
堆肥技术作为有机固体废弃物处理处置有效方法,可实现畜禽粪便等有机废弃物肥料化利用,是衔接种养循环模式的关键技术[1-2]。将堆肥产品作为有机肥施用于农田,可改善长期大量使用化肥导致的土壤板结、盐渍化、肥力下降等问题,是实现农业绿色发展的重要举措[3-4]。但堆肥产品是否可以作为有机肥施用于农田,需要对其腐熟度进行评价。加州堆肥质量委员会(CCQC)2001年提出腐熟度指堆肥过程稳定化的程度,同年英国堆肥协会(UKCA)提到腐熟度还需考虑堆肥对种子发芽或植物生长产生抑制作用大小。而种子发芽指数(Germination Index,GI)是综合评价堆肥腐熟度生物学指标,于1981年由Zucconi等[5]提出,并认为GI<50%表示堆肥不腐熟,GI≥80%则表示堆肥完全腐熟,且对植物完全没有毒害作用。迄今为止,GI始终是评价堆肥腐熟度最有效的指标,且被大量应用于堆肥腐熟特性研究当中[6]。2019年实施的农业行业标准《畜禽粪便堆肥技术规范NY/T 3442-2019》首次规定堆肥产物GI≥70%安全施用标准。2021年6月1日农业农村部新修订的《有机肥料NY 525-2021》中,增加了GI的术语和定义。由此可见GI对于评价畜禽粪便有机肥的质量和农田安全应用等方面的重要性。
但是关于GI测算还未有标准方法和标准种子,且以往关于堆肥中种子发芽研究结果差异较大。例如同样将餐厨垃圾或畜禽粪便的堆肥进行GI测定,不同种子差别可达20%~217%[7-12],因此不同研究学者之间的GI结果通常没有可比性。依据GI测定和堆肥过程,可将影响GI因素分为两部分,一是指标测算过程与方法,二是堆肥中植物毒性物质胁迫作用。其中测算过程受到诸多因素影响,例如发芽如何界定、芽与根如何计量、根长起始位置等问题,均没有统一规范和标准,即使是同样的堆肥样品,测算人员不同,GI结果也会有较大差异。其次,不同种子对环境胁迫敏感性差异较大,敏感性强导致发芽率较低,使腐熟度较好的堆肥也无法满足GI≥70%标准;反之,敏感性差时发芽率较高,种子不受环境胁迫而使GI较大。研究中多使用黄瓜、萝卜、白菜等敏感性适宜的种子作为发芽试验对象[6,8,13]。再次,种子粒径大小对试验过程影响重要,粒径较大,所需培养液和培养皿较大,增加试验难度;粒径较小,消耗大量时间数种子,对试验人员技能提出更高要求。基于以上原因,不同学者所得GI结果差异较大(50%~200%)[7,12,14],甚至有学者提出将GI>110%作为堆肥腐熟标准[15]。由此可见,不同研究中GI之间可比性较差,缺乏广泛适用性。
堆肥过程中,往往伴随着复杂的物质转化过程,有机质含量及其在矿化和腐殖化作用下,产生的小分子有机酸、盐分离子和NH4+等是抑制GI的主要物质[16-19]。小分子有机酸主要通过酸化作用影响土壤微生物代谢生长,盐分浓度过高抑制植物组织生长和分化,NH4+会导致植物根系氨中毒[11]。有机质是微生物好氧代谢重要能源物质,直接影响堆肥腐熟程度,进而对种子发芽产生影响。且Wang等[20]研究有机质对羊粪堆肥GI影响中发现,添加不同类型碳源对GI关键影响因子不同。此外,温度、pH值、氧气等基本理化指标,通过影响堆肥体系环境状态,调节微生物代谢过程及其代谢产物,对GI有间接影响作用。因此,植物毒性物质和理化指标与堆肥GI之间存在显著的响应关系。此外,不同类型畜禽粪便性质不同,例如猪粪含水率较高、碳氮比(C/N)较低,而羊粪含水率较低且C/N较高,单独堆肥往往植物毒性差异较大[21]。与此同时,外援添加碳源辅料是改善堆肥环境、调节堆肥腐熟度最简单、直接和有效的途径,可以潜在地分解、消除或固定植物毒性物质[22-23]。目前较为常用的碳源辅料主要为秸秆、菌糠、园林剪枝等高木质纤维素含量的农业废弃物[24]。其中秸秆具有增加堆体含碳量、改善堆体孔隙度、扩大微生物附着位点等优良特性,对降低堆肥系统植物毒性、固定养分含量、提高腐熟度显著效果[25-26]。
目前关于堆肥GI测算方法与标准种子选取还未有报道。因此,本研究采用植物毒性差异较大的猪粪和羊粪作为堆肥原料,通过添加/不添加秸秆设置4个不同堆肥处理。基于已有的研究,按照大、中、小粒径选取黄瓜、萝卜、白菜、油菜4类种子,对GI测算过程进行详细描述,并将不同种子GI结果与堆肥过程中的植物毒性因子进行相关性分析,进而分析不同种子与影响种子发芽毒性因子总效应间响应关系,确定评价堆肥腐熟度最佳种子。以期对堆肥GI指标测定方法进行完善,本研究结果对于堆肥腐熟度评价和有机肥料农用安全评价具有重要的指导意义。
1 材料与方法
1.1 堆肥试验
1.1.1 试验原料与方法
为得到不同腐熟度和植物毒性的堆肥产品,按照畜禽饮食方式、消化结构以及粪便特点,选择猪粪和羊粪作为堆肥原料,玉米秸秆为辅料。新鲜猪粪取自中国农科院畜牧,羊粪取自奥鑫牧业有限公司(北京市顺义区),羊粪采用硬化地板的人工干清粪收集方式;玉米秸秆取自中国农业大学上庄试验站,经风干粉碎处理为2~3 cm小段。原料基本理化性质见表1。
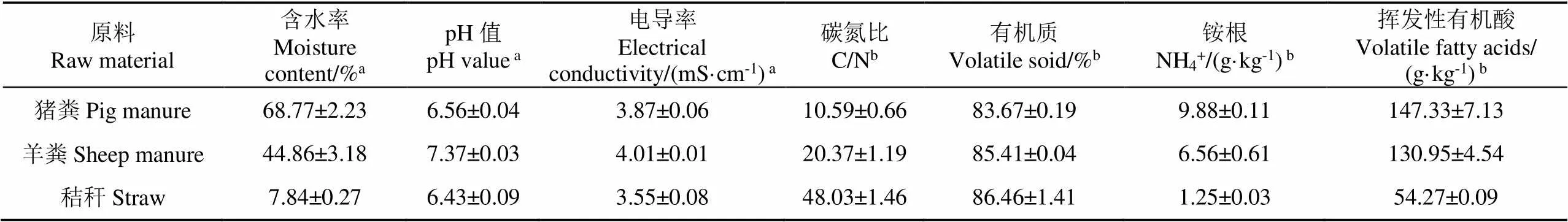
表1 堆肥原料基本理化性质
注:a基于湿基;b基于干基。
Note:awet weight basis;bdry weight basis.
按照添加和不添加秸秆(添加量为15%,湿基)共设计4个处理,分别为猪粪(PM)、猪粪+秸秆(PM+CS)、羊粪(SM)、羊粪+秸秆(SM+CS)。初始条件均设置为含水率65%,连续通风且通风速率为0.27 L/(kg·min),物料质量30 kg,进行为期49 d堆肥试验。翻堆取样时间为第0、3、7、14、21、28、35、42和49 d,每次翻堆前后记录堆体质量。取样采用标准的五点取样法,将堆体充分混匀后堆成一个正圆锥体,在圆椎体上、中、下部选择与中心等距离的3~5个点进行取样(少量多次),充分混合后作为最终样品。控制样品总量为300 g,其中100 g风干之后,过0.2 mm筛,用于有机质(Volatile Solid,VS)和元素(C、N)测定;100 g鲜样暂存于4 ℃,用于含水率、pH值、电导率(Electrical Conductivity,EC)、有机酸(Volatile Fatty Acids,VFAs)和铵根(NH4+)含量测定;剩余部分主要用于GI测定。
1.1.2 试验装置与样品测定
堆肥试验在60 L好氧发酵反应器(图1)中进行,装置采用双层不锈钢圆柱形(内径为36 cm,高度为60 cm)罐体,具有良好的温度性和密闭性。罐体内底部设有多孔不锈钢筛板,通过进气管与空气泵相连,实现均匀曝气。空气泵通过自动通风控制系统,可实现对通风速率和通风频率的调控。从堆体顶部将带有自动传感器的温度探头插至罐体中间,可实现温度自动实时传输和记录。罐体外底部设置渗滤液收集口,上部设置气体采集口,同时实现多余尾气排出。
堆体温度通过热敏电阻法连续监测,含水率采用烘干法(105 ℃)测定,氧气(O2)含量采用便携式沼气分析仪(Biogas 5000,德国)测定,C/N通过元素分析仪(Vario Micro Cube,德国)所测定的TC与TN比值得出,VS含量采用马弗炉灼烧法(550 ℃)测定。样品水浸提液(即培养液制备方法见1.2.2)用于pH值、EC(多波长分析仪DZS-706-A,中国)以及GI测定(详见1.2),KCl浸提之后于流动分析仪(Auto Analyzer 3,德国)测定NH4+含量。
1.2 种子发芽试验
1.2.1 供试种子
本研究依据文献查阅、现行标准(NY 525-2021)以及种子自身特点(易发芽、较敏感、廉价易得等),按照粒径大、中、小分别选取黄瓜(12 mm)、萝卜(4 mm)、白菜和油菜(1~2 mm)4类种子进行堆肥样品种子发芽试验,种子均购买自中国科学研究院(入袋发芽率≥80%)。试验前需进行种子发芽率的测定,检验其是否满足80%以上标准;试验中需挑选饱满均匀的种子进行培养,保证对照组中不同类型种子发芽率间差异≤2%(一般为100%),以消除不同种子本身发芽率对研究结果所带来的影响。
1.2.2试验方法
1)培养液制备:
①将10 g堆肥样品(鲜样)与100 mL去离子水混合(按照样品质量与液体体积之比为1∶10进行),可用150 mL锥形瓶盛放,保证固体样品全部被去离子水浸没,对于堆肥中秸秆等粒径较大物料可适当剪碎后浸提;
②将①中所得混合物在25 ℃条件下,于180 r/min恒温摇床震荡30 min(启动摇床后,需待转速达到设定值之后,试验人员方可离开),注意固定锥形瓶,防止物料倾倒、洒出和飞溅,影响试验结果;
③小心取出②中混合物,静置5~10 min后,将混合物于无污染的中速定量滤纸(30~50m)过滤,期间可依据过滤速度更换滤纸,收集滤液于干燥洁净的容器中,即为种子发芽试验培养液。
2)培养过程:
①准确吸取5 mL培养液(对照为去离子水),于垫有一张滤纸的干燥无污染培养皿中(直径为9 cm)。滤纸需按照培养皿底面积大小适当裁剪以达到完全契合,使得滤纸完全被培养液浸湿(无气泡);
②挑选10粒均匀饱满的供试种子,均匀放置于培养皿中,保证每颗种子都处于培养液中,同时附着于滤纸之上不至漂浮,将培养皿于25 ℃恒温条件下黑暗培养48 h,每个处理设置4个生物学重复。
3)结果测量:使用游标卡尺(单位:mm)等工具对种子根长进行无损测定。
①发芽:种子是否发芽以种皮是否破裂为依据,种皮破裂即为发芽;
②根长:从长根毛的地方开始测量,主要分为3种情况(图2):a)种皮破裂但没有长芽,或者刚刚出芽且出芽很短,此时根长均计为0;b)种子发芽,芽较长但无根毛长出,此时根长计为1 mm;c)种子发芽,芽很长且长出根毛,以长根毛处为起始位置测量根长。
4)指标计算:
该计算方法与许多研究相似[20],首先,分别统计处理和对照中发芽种子的个数,并按照公式(1)求得相对发芽率;其次,分别测量并计算处理和对照中种子根长之和,并按照公式(2)求得相对根长;最后,将(1)与(2)结果按照公式(3)计算,所得结果即为该处理的GI结果。
1.3 数据分析方法
本研究所有数据均由Microsoft Excel 2016进行数据统计分析,由Origin 2021(Origin Lab,USA)绘图。用SPSS软件(Statistical Product and Service Solutions 25.0 Windows,USA)进行不同指标之间Pearson相关性分析,用SAS软件(Statistical Analysis System 9.4,USA)进行处理组间显著性差异分析(<0.05)。
2 结果与讨论
2.1 种子发芽指数
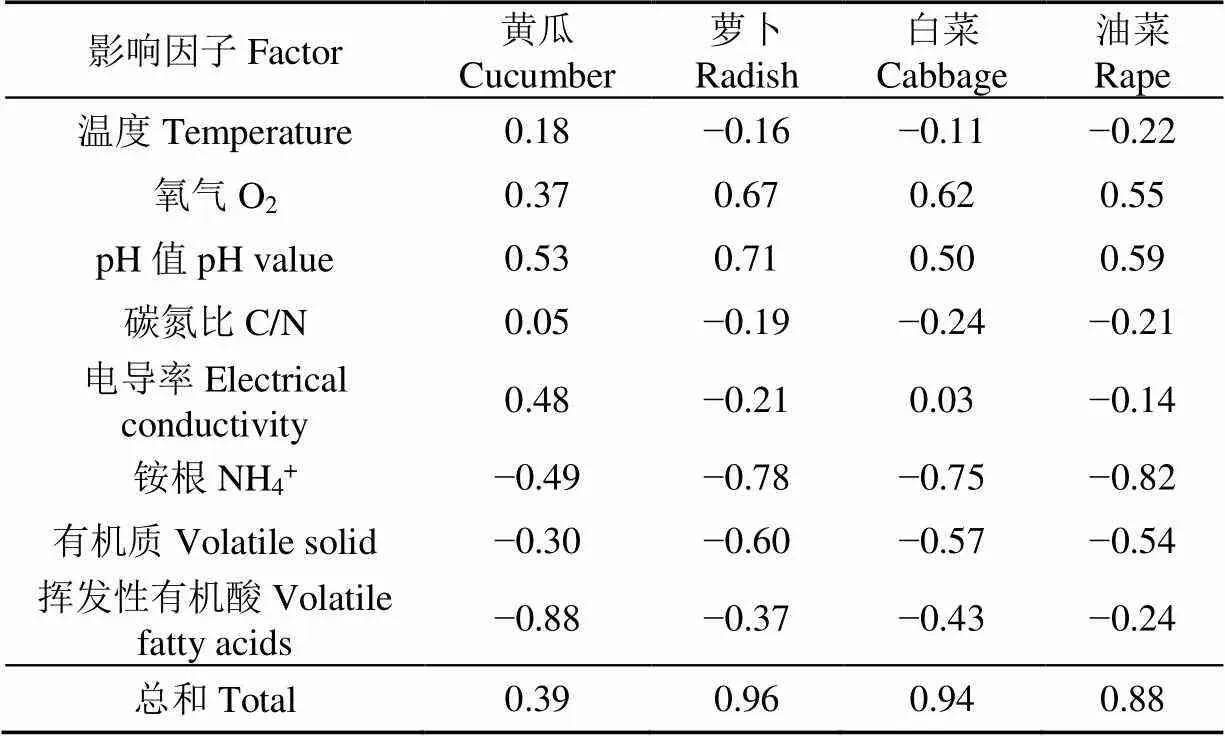
不同处理堆肥采用不同类型种子所得最终GI值,基本按照(除黄瓜外):猪粪(26.54%~80.49%)、羊粪(16.71%~92.98%)、猪粪+秸秆(28.28%~110.74%)、羊粪+秸秆(43.38%~119.69%)的顺序递增。由于不同类型种子对环境敏感性不同(图3),整体而言,GI按照萝卜(≤130%)>黄瓜(≤100%)>油菜(≤70%)>白菜(≤45%)的顺序递减。Li等[27-28]同样采用萝卜种子测定猪粪-秸秆和厨余-污泥联合好氧堆肥腐熟情况时,表示GI同样为先快速上升之后稳定于100%~120%;Yuan等[29]研究结果显示,黄瓜作为发芽试验的种子时,GI可达100%以上且差异不显著。李红霞等[30]研究羊粪好氧堆肥过程中,采用油菜作为GI试验对象,结果均在70%~90%之间。而本研究中小白菜种子GI结果与以往研究有所不同,例如Sun等[31]检测禽粪便堆肥浸提液在小白菜种子中GI时,结果其发芽率达120%以上,这可能是由于初始物料中添加的菌糠、碳源物质及活性酶等物质改变堆肥系统中微生物群落结构,改善堆体腐熟情况降低堆肥产品植物毒性而使得GI提高。
其中,黄瓜种子GI均较高,在第7 d均达到50%以上,且后续堆肥腐熟期等过程并没有对GI产生显著影响(提高2.11%~26.83%)。对于腐熟度较低的纯粪便处理(PM和SM)也表现出较高的GI,且与腐熟度较高处理(PM+CS和SM+CS)近似。萝卜种子GI范围较大,腐熟度较低的纯粪便处理与腐熟度较高的辅料添加处理表现出极大差异性(<0.01)。GI在14~28 d升高速度最快,28 d以后基本不变,表明不同堆肥阶段对植物毒性消减情况不同。随着堆肥进行,GI基本按照PM 温度在一定程度上可以反映堆肥进程,同时是微生物活跃程度的体现[35]。添加辅料的处理(PM+CS和SM+CS)(图4a)有显著的高温期,与以往Estrella-Gonzalez等[14]研究堆肥温度过程一致,且温度显著高于纯粪便的处理(PM和SM)(<0.01)。秸秆可以升高堆体温度至74.3~75.6 ℃,且延长高温期10~20 d。纯粪便处理温度显著降低(20~45℃)(<0.01),主要由于原料致密、碳源缺乏、氧气不足等原因所致。O2含量由于微生物好氧消耗而降低(图4b),在辅料添加处理中,O2含量均在第8 d降至最低(13%~14%),至第14 d恢复至环境后,波动减少。而纯粪便处理,O2含量最低可至1%~2%,已经不再适合于微生物呼吸,O2主要消耗于堆肥前14 d和后14 d。研究表明,堆肥O2含量为10%~18%时,微生物活性最佳[36-37],纯粪便处理中O2含量不足是微生物活性受限的重要原因。O2与温度呈显著负相关关系(<0,<0.05),且在之前研究中也有类似的结果[28],且与温度一致,O2含量在纯粪便处理与秸秆添加处理中差异显著(<0.01)。 pH值是影响堆肥过程中微生物生长代谢的重要因素之一,可反映堆肥进程和物料腐熟度[38]。由于初始原料中(图4c),微厌氧作用导致有机酸、H+、SO42-等产生而使得PM及PM+CS处理呈现弱酸性,而SM本身颗粒结构、含水率较低等导致其无酸化发酵现象[39]。在好氧通风条件下,辅料添加处理(PM与SM)在初始2~3 d内pH值迅速升高至8.23与8.29,至第14 d降至7.84与7.67,微弱升高后保持于7.7~7.8直至堆肥结束。Zhang等[40]研究堆肥中pH变化过程同样发现,秸秆等碳源辅料对于提高畜禽粪便堆肥系统pH值具有重要作用。而纯粪便处理均在堆肥前7 d迅速升高(PM+CS为7.40,SM+CS为7.57),随后波动于7.5上下。所有处理均满足5.5~8.5有机肥安全标准[41],且秸秆添加与不添加处理之间差异极显著(<0.01),纯粪便处理之间差异显著(<0.05)。 C/N是反应有机质降解程度、堆肥进程和腐熟度的重要指标[35],有机质矿化、腐殖化是堆肥反应中重要生化过程,伴随着CO2、CH4、NH3、N2O等排放,使得堆肥中C、N有机质在组成、形态及含量等发生重大改变[42]。微生物好氧活动最适C/N为20~25[43],由于氮源消耗速度快、堆体水分蒸发等原因,导致堆体C/N均呈现下降变化趋势(图4d)。但对于秸秆添加处理,C/N下降程度(PM+CS为40.83%、SM+CS为37.57%)显著高于纯粪便处理(PM为32.65%、SM为20.26%),这与前述温度、氧气、pH值等条件有关。而C/N与初始物料密切相关,Liu等[24]将秸秆添加至畜禽粪便中得到不同初始C/N堆体(27.0~37.1),经过堆肥之后其比值下降24.2%~51.7%,包含本研究所得结果。另外,PM处理组较SM处理组VS下降程度更大,这主要与羊粪中纤维素、木质素等难降解有机质含量较高有关。Muscolo等[44]研究表明,堆肥结束与初始C/N之比<0.7,则表明堆肥腐熟度较好,按照PM、PM+CS、SM、SM+CS分别为0.67、0.59、0.80、0.62,表明不同堆肥处理腐熟度不同。 EC是衡量堆肥中可溶性盐含量的重要指标,在一定程度上反应堆肥物料对植物生长产生抑制作用强度或植物毒性大小[45],当EC>4 mS/cm时,堆肥会对植物生长产生抑制作用[46]。堆肥前期(图5a),有机质矿化分解作用产生盐分离子导致EC升高,随后腐殖化作用使得离子被固定聚合EC降低。SM处理中EC较高主要与羊粪本身特性有关(表1),不同处理EC变化趋势基本一致,按照SM>PM>SM+CS>PM+CS顺序递减且差异极显著(<0.01)。最终添加秸秆的处理均符合安全施用标准(3.57和3.97 mS/cm),而纯粪便的处理均未能达标(4.36和4.92 mS/cm),表明其中植物毒性物质含量较高。Wang等[13]将添加/不添加辅料于鸡粪-烟末好氧堆肥体系中进行对比研究,发现不添加的处理GI最终没有降至标准以下,且毒性较高。 NH4+主要由于原料粪便中微生物分解和积累作用而在堆肥初期处于较高水平,随着高温对硝化细菌活性抑制、NH3大量排放致使NH4+急剧下降[47-48]。这与以往研究结果相似,最高为初始阶段(约10 g/kg),且在前7 d降低速度最快[14]。秸秆添加处理(PM和SM),NH4+在降温期继续下降(图5b),直至腐熟期基本稳定并趋于0,而纯粪便处理(PM+CS和SM+CS)NH4+再次升高,可能是翻堆供氧使微生物再次活跃,以及有机酸等活化作用,而使得堆体中NH4+增加,但随着NH3释放,NH4+再次降低。腐熟期NH4+进一步下降,可能与反硝化作用产生的NO3-有关[49]。不同处理之间NH4+含量差异显著(<0.01),并按照PM>SM>PM+CS>SM+CS顺序递减,表明SM处理组对种子抑制作用可能较小。 VFAs易产生于堆肥高温期(82.11~147.33 g/kg)(图5c),此时微生物好氧活动剧烈、易导致堆体局部厌氧,有机质降解不完全而产生,但随着堆肥进行,堆体氧气环境改善、有机质分解作用减弱,VFAs含量逐渐降低(最终为25.36~62.55 g/kg)。小分子有机酸中的甲酸、乙酸、丙酸等在Wang等[20]研究中进行详细报道,且发现VFAs主要产生于高温期且集中于堆肥前3 d,是堆肥植物毒性的重要物质之一。添加秸秆处理(PM+CS和SM+CS)相对于纯粪便处理(PM和SM),初始VFAs被稀释而分别降低23.03%和37.30%,经过49 d好氧堆肥过程,稀释作用效果被扩增至39.10%和45.46%。表明辅料添加对于堆肥植物毒性降低和腐熟度提高的重要作用,在Wang等[13]鸡粪-菌糠共堆肥研究中也得出相似结论。另外,SM处理组VFAs均小于PM处理组,表明羊粪植物毒性较猪粪更低。 VS作为能源物质直接与微生物生长代谢相关,但对于植物来讲,有机质降解消耗氧气使得作物根系生长呼吸有限制作用[50]。堆肥初期(图5d),秸秆增加堆体VS含量约2.72%~3.14%,至堆肥结束时,VS降解幅度可增加2.39%~4.34%。SM处理组中VS含量整体较高,但PM处理组VS降低10.89%~16.43%,显著高于SM处理组(3.10%~10.16%),且VS降解程度按照PM+CS>PM>SM+CS>SM的顺序递减。这与以往研究相似,堆肥可使VS降低10%左右,且有机质含量不同会影响其降解程度,进而对堆肥结果腐熟效果和植物毒性情况造成一定影响[28]。由此表明,虽然羊粪中有机质含量较丰富,但其中主要为难降解有机质,而猪粪中有机质量降解量较多,其降解过程产物对种子会产生抑制作用,表明猪粪植物毒性较高。 为筛选出可综合评价堆肥植物毒性的种子类型,将本研究所选4类种子(黄瓜、萝卜、白菜、油菜)所得GI与表征堆肥腐熟和植物毒性情况8项指标(温度、pH、C/N、EC、NH4+、VS、VFAs)进行相关性分析(表2)。结果表明,二者之间响应关系按照:萝卜>油菜>白菜>黄瓜顺序递减,表明萝卜是综合表征堆肥腐熟度和植物毒性,最具科学性和代表性的种子类型。堆肥过程不同影响指标对种子GI相关性总和,按照NH4+>pH>O2>VS>VFAs>EC>C/N>温度的顺序递减,该结果与对萝卜种子GI影响顺序完全一致,且萝卜种子与总和相关性最强(=0.96),表明萝卜种子对8项指标有充分解释性,可作为表征堆肥腐熟度和植物毒性最佳种子类型。迄今为止,萝卜种子以其生长速度快、颗粒大小适宜、植物毒性敏感性强而在堆肥研究中被广泛应用[20-21]。 表2 植物毒性因子与不同种子GI之间相关性分析 1)测算种子发芽指数(Germination Index,GI)时,将破皮种子记为发芽,将破皮无发芽、刚刚发芽或者发芽很短种子记根长为0,将发芽较长但无根毛长出种子记根长为1 mm,将根毛生长处选定为根长测定起始位置,共设置4个重复。 2)不同处理堆肥GI,按照猪粪<羊粪<猪粪+秸秆<羊粪+秸秆的顺序递增,添加秸秆堆肥可显著提高堆体温度、进而提升堆肥腐熟度,改善堆体pH值,降低电导率、铵根、小分子有机酸、有机质等毒性物质含量,提高C和N降解程度。 3)萝卜种子与堆肥GI响应系数最大,与其他种子相比,最能充分解释腐熟度和植物毒性对其影响作用(=0.96),可用于表征堆肥过程植物毒性变化,作为种子发芽指数的普遍标准适用种子类型。 [1] Ge J, Huang G, Huang J, et al. Particle-scale modeling of methane emission during pig manure/wheat straw aerobic composting[J]. Environmental Science & Technology, 2016, 50(8): 4374-4383. [2] Liang J, Li X, Yu Z, et al. Amorphous MnO2modified biochar derived from aerobically composted swine manure for adsorption of Pb (II) and Cd (II)[J]. Acs Sustainable Chemistry & Engineering, 2017, 5(6): 5049-5058. [3] Feng X, Ling N, Chen H, et al. Soil ionomic and enzymatic responses and correlations to fertilizations amended with and without organic fertilizer in long-term experiments[J]. Scientific Reports, 2016, 6(1): 24559. [4] Adugna G. A review on impact of compost on soil properties, water use and crop productivity[J]. Agricultural Science Research Journal, 2018, 4(3): 93-104. [5] Zucconi F, Monaco A, Debertoldi M. Biological evaluation of compost maturity[J]. Biocycle, 1981, 22(4): 27-29. [6] Luo Y, Liang J, Zeng G, et al. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects[J]. Waste Management, 2018, 71: 109-114. [7] Agapios A, Andreas V, Marinos S, et al. Waste aroma profile in the framework of food waste management through household composting[J]. Journal of Cleaner Production, 2020, 257: 120340. [8] Yuan J, Yang Q Y, Zhang Z Y, et al. Use of additive and pretreatment to control odors in municipal kitchen waste during aerobic composting[J]. Journal of Environmental Sciences, 2015, 37: 83-90. [9] Ye Z M, Ding H, Yin Z L, et al. Evaluation of humic acid conversion during composting under amoxicillin stress: Emphasizes the driving role of core microbial communities[J]. Bioresource Technology, 2021, 337: 125483. [10] Feng Y, Wang G Z, Liu Y W, et al. The impacts of oxytetracycline on humification during manure composting can be alleviated by adjusting initial moisture contents as illustrated by NMR[J]. Journal of Integrative Agriculture, 2021, 20(8): 2277-2288. [11] Zhu N, Zhu Y Y, Li B Q, et al. Increased enzyme activities and fungal degraders by Gloeophyllum trabeum inoculation improve lignocellulose degradation efficiency during manure-straw composting[J]. Bioresource Technology, 2021, 337: 125427. [12] Abdellah Y A Y, Li T Z, Chen X, et al. Role of psychrotrophic fungal strains in accelerating and enhancing the maturity of pig manure composting under low-temperature conditions[J]. Bioresource Technology, 2021, 320: 124402. [13] Wang G, Kong Y, Liu Y, et al. Evolution of phytotoxicity during the active phase of co-composting of chicken manure, tobacco powder and mushroom substrate[J]. Waste Management, 2020, 114: 25-32. [14] Estrella-Gonzalez M J, Suarez-Estrella F, Jurado M M, et al. Uncovering new indicators to predict stability, maturity and biodiversity of compost on an industrial scale[J]. Bioresource Technology, 2020, 313: 123557. [15] Ko H J, Kim K Y, Kim H T, et al. Evaluation of maturity parameters and heavy metal contents in composts made from animal manure[J]. Waste Management, 2008, 28(5): 813-820. [16] Gavilanes-Teran I, Jara-Samaniego J, Idrovo-Novillo J, et al. Windrow composting as horticultural waste management strategy-A case study in Ecuador[J]. Waste Management, 2016, 48: 127-134. [17] Bargmann I, Rillig M C, Buss W, et al. Hydrochar and biochar effects on germination of spring barley[J]. Journal of Agronomy and Crop Science, 2013, 199(5): 360-373. [18] Farhoudi R. Effect of seed size on salt tolerance at germination and seedling growth stages of wheat (L.)[J]. Research on Crops, 2011, 12(2): 308-311. [19] Chang R, Li Y, Chen Q, et al. Comparing the effects of three in situ methods on nitrogen loss control, temperature dynamics and maturity during composting of agricultural wastes with a stage of temperatures over 70 degrees C[J]. Journal of Environmental Management, 2019, 230: 119-127. [20] Wang G, Yang Y, Kong Y, et al. Key factors affecting seed germination in phytotoxicity tests during sheep manure composting with carbon additives[J]. Journal of Hazardous Materials, 2021, 421: 126809. [21] Wang G, Li G, Chang J, et al. Enrichment of antibiotic resistance genes after sheep manure aerobic heap composting[J]. Bioresource Technology, 2021, 323: 124620. [22] Zhang D, Luo W, Yuan J, et al. Effects of woody peat and superphosphate on compost maturity and gaseous emissions during pig manure composting[J]. Waste Management, 2017, 68: 56-63. [23] Yang F, Li G, Yang Q, et al. Effect of bulking agents on maturity and gaseous emissions during kitchen waste composting[J]. Chemosphere, 2013, 93(7): 1393-1399. [24] Liu T, Awasthi M K, Verma S, et al. Evaluation of cornstalk as bulking agent on greenhouse gases emission and bacterial community during further composting[J]. Bioresource Technology, 2021, 340: 125713. [25] Chang R, Li Y, Li N, et al. Effect of microbial transformation induced by metallic compound additives and temperature variations during composting on suppression of soil-borne pathogens[J]. Journal of Environmental Management, 2021, 279: 111816. [26] Kiatkamjon I, Sajid L, Cao Z, et al. Characterisation of biochar from maize residues produced in a self-purging pyrolysis reactor[J]. Bioresource Technology, 2018, 265: 224-235. [27] Li S, Li D, Li J, et al. Effect of spent mushroom substrate as a bulking agent on gaseous emissions and compost quality during pig manure composting[J]. Environmental Science and Pollution Research International, 2018, 25(13): 12398-12406. [28] Zhang D, Luo W, Li Y, et al. Performance of co-composting sewage sludge and organic fraction of municipal solid waste at different proportions[J]. Bioresource Technology, 2018, 250: 853-859. [29] Yuan J, Chadwick D, Zhang D F, et al. Effects of aeration rate on maturity and gaseous emissions during sewage sludge composting[J]. Waste Management, 2016, 56: 403-410. [30] 李红霞,蔡禄,季祥,等. 羊粪好氧堆肥最佳工艺参数的优化研究[J]. 中国农机化学报,2019,40(6):215-220. Li Hongxia, Cai Lu, Ji Xiang, et al. Optimization of optimum process parameters for aerobic composting of sheep manure[J]. Journal of Chinese Agricultural Mechanization, 2019, 40(6): 215-220. (in Chinese with English abstract) [31] Sun C Y, Wei Y B, Kou J N, et al. Improve spent mushroom substrate decomposition, bacterial community and mature compost quality by adding cellulase during composting[J]. Journal of Cleaner Production, 2021, 299: 126928. [32] Liu Y, Ma R, Li D, et al. Effects of calcium magnesium phosphate fertilizer, biochar and spent mushroom substrate on compost maturity and gaseous emissions during pig manure composting[J]. Journal of Environmental Management, 2020, 267: 110649. [33] Zhang L, Sun X. Improving green waste composting by addition of sugarcane bagasse and exhausted grape marc[J]. Bioresource Technology, 2016, 218: 335-343. [34] Tiquia S M, Tam N F Y, Hodgkiss I J. Effects of composting on phytotoxicity of spent pig-manure sawdust litter[J]. Environmental Pollution, 1996, 93(3): 249-256. [35] Meng X, Liu B, Zhang H, et al. Co-composting of the biogas residues and spent mushroom substrate: Physicochemical properties and maturity assessment[J]. Bioresource Technology, 2019, 276: 281-287. [36] Magalhaes A M T, Shea P J, Jawson M D, et al. Practical simulation of composting in the laboratory[J]. Waste Management and Resource, 1993, 11(2): 143-154. [37] Miller F C. Composting As A Process Based on the Control of Ecologically Selective Factors[M]. New York: Soil Microbial Ecology: Applications in Agricultural and Environmental Management. 1992. [38] Voběrková S, Maxianová A, Schlosserová N, et al. Food waste composting-Is it really so simple as stated in scientific literature?-A case study[J]. Science of Total Environment, 2020, 723: 138202. [39] Bustamante M A, Ceglie F G, Aly A, et al. Tittarelli. Phosphorus availability from rock phosphate: combined effect of green waste composting and sulfur addition[J]. Journal of Environment Management, 2016, 182: 557-563. [40] Zhang Z, Liu D H, Qiao Y, et al. Mitigation of carbon and nitrogen losses during pig manure composting: A meta-analysis[J]. Science of the Total Environment, 2021, 783: 147103. [41] Ivankin A N, Pandya U, Saraf M. Intensification of Aerobic Processing of the Organic Wastes into Compost[M]. Cham: Springer International Publishing, 2014. [42] Haouas A, Modafar C E, Douira A, et al. Evaluation of the nutrients cycle, humification process, and agronomic efficiency of organic wastes composting enriched with phosphate sludge[J]. Journal of Cleaner Production, 2021, 302: 127051. [43] Bernal M P, Alburquerque J A, Moral R. Composting of animal manures and chemical criteria for compost maturity assessment. A review[J]. Bioresource Technology, 2009, 100(22): 5444-5453. [44] Muscolo A, Papalia T, Settineri G, et al. Are raw materials or composting conditions and time that most influence the maturity and/or quality of composts? Comparison of obtained composts on soil properties[J]. Journal of Cleaner Production, 2018, 195: 93-101. [45] Sayara T, Basheer-Salimia R, Hawamde F, et al. Recycling of organic wastes through composting: process performance and compost application in agriculture[J]. Agronomy, 2020, 10(1): 1838. [46] Karak T, Bhattacharyya P, Paul R K, et al. Evaluation of composts from agricultural wastes with fish pond sediment as bulking agent to improve compost quality[J]. Clean-Soil Air Water, 2013, 41(7): 711-723. [47] Li Y, Luo W, Li G, et al. Performance of phosphogypsum and calcium magnesium phosphate fertilizer for nitrogen conservation in pig manure composting[J]. Bioresource Technology, 2018, 250: 53-59. [48] Chen M, Huang Y, Wang C, et al. The conversion of organic nitrogen by functional bacteria determines the end-result of ammonia in compost[J]. Bioresource Technology, 2020, 299: 122599. [49] Guo H, Gu J, Wang X, et al. Microbial driven reduction of N2O and NH3emissions during composting: effects of bamboo charcoal and bamboo vinegar[J]. Journal of Hazardous Materials, 2020, 390: 121292. [50] Zhu H, Banuelos G. Influence of salinity and boron on germination, seedling growth and transplanting mortality of guayule: A combined growth chamber and greenhouse study[J]. Industrial Crops and Products, 2016, 92: 236-243. Determination of seed germination index and selection of sensitive seeds for phytotoxicity evaluation of composting Wang Guoying1, Yuan Jing1, Kong Yilin1, Shen Yujun2, Yang Yan1, Li Guoxue1※ (1.1001932100125) Composting has historically been carried out by farmers for manure management, which is a reliable way to recycle manure for use on croplands in sustainable agriculture. However, poor management of the composting process can result in un-mature compost product, which inhibits plant growth and deteriorates soil condition. The seed Germination Index (GI), an authoritative parameter to evaluate the maturity of compost, has been widely utilized in evaluating the phytotoxicity and other aspects of composting process. However, there are still lack of unified standards for the measurement method of GI and the representative type of experimental seed until now, which leads to the diverse results of GI (fluctuated from 20%-217%), and the newest standard of organic fertilizer (NY525–2021) requirements GI≥70% of organic fertilizer. Thus, it is meaningless to compare these values between different studies in related fields and urgently for public to provide a reference measurement process for GI during composting. Therefore, this study protocoled four treatments, composting chicken manure with or without auxiliary material (carbon additive) for different phytotoxicity compost, and selected four types of seeds for sensitive assays according to previous studies, mainly included cucumber, radish, Chinese cabbage and oil rape. Besides, in the process of germination experiments and indexes calculation, detailly instruction for the whole process of GI determination and calculation was documented, which stipulated the definition of radical length for 0, 1 mm and the start position for measure (with diagram). Furthermore, systematically detecting the response relationships between influenced parameters and GI of different composting treatments. The results showed that, according to the GI of final compost, the maturity increased in the order of pure pig manure treatment (26.54%-80.49%), pure sheep manure treatment (16.71%-92.98%), pig manure and straw treatment (28.28%-110.74%), sheep manure and straw treatment (43.38%-119.69%). Carbon additive of straw could significantly improve temperature of composting systems and further maturity of the final products. Increasing pH and decreasing phytotoxicity of electronical conductivity, low molecular organic acid and organic matter contents at the same time. Seeds’ GI decreased with the order of radish (≤130%), cucumber (≤100%), oil rape (≤70%), Chinese cabbage (≤45%). The sensitives were reflected with correlation analysis, which between physical and chemical indicators (temperature, oxygen, pH, carbon and nitrogen ratio) and biological toxicity factors (electronical conductivity, ammonium nitrogen, organic acid and organic matter) and GI with different seeds (cucumber, radish, cabbage, rape) of four composting processes, and fond the factors influenced GI with the sequence of NH4+, pH value, O2, VS, VFAs, EC, C/N, temperature, which was in accordance with the affected sequence of radish seed. Besides, radish seed showed the highest responsivity (3.69,=0.96) of GI for influenced factors, which was the most scientific and representative type of seeds for comprehensively reflecting maturity and phytotoxicity of compost of discrepancy qualities compost. Furthermore, radish has been widely studied for the reasons of fasting germination speed, moderating molecular size, obtaining easily and lowering price. These results can provide scientific references for the determination and comparation of GI, the establishment of organic fertilizer standards and the safe application of compost in farmlands. composting; seed; phytotoxicity; germination index; determination 王国英,袁京,孔艺霖,等. 堆肥种子发芽指数测定方法与敏感性种子筛选[J]. 农业工程学报,2021,37(19):220-227.doi:10.11975/j.issn.1002-6819.2021.19.025 http://www.tcsae.org Wang Guoying, Yuan Jing, Kong Yilin, et al. Determination of seed germination index and selection of sensitive seeds for phytotoxicity evaluation of composting[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2021, 37(19): 220-227. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2021.19.025 http://www.tcsae.org 2021-06-10 2021-08-16 财政部和农业农村部国家绒毛用羊现代农业产业技术体系(CARS-39-19) 王国英,博士生,研究方向为固体废弃物资源化利用。Email:wangguoying@cau.edu.cn 李国学,教授,博士生导师,研究方向为固体废弃物资源化利用。Email:ligx@cau.edu.cn 10.11975/j.issn.1002-6819.2021.19.025 X713; X502 A 1002-6819(2021)-19-0220-082.2 基本理化指标
2.3 植物毒性物质
2.4 敏感性种子筛选
3 结 论
