Identification and Expression of PPAR in Sinonovacula constricta and Their Potential Regulatory Effects on Δ6 Fad Transcription
2021-12-22RANZhaoshouKONGFeiLIAOKaiXUJilinLIUXingwangSHIPengZHANGMengqiWUKaibinandYANXiaojun
RAN Zhaoshou, KONG Fei, LIAO Kai, XU Jilin, 3), *, LIU Xingwang, 4), 5), 6),SHI Peng, ZHANG Mengqi, WU Kaibin, and YAN Xiaojun
Identification and Expression of PPAR inand Their Potential Regulatory Effects onTranscription
RAN Zhaoshou1), 2), KONG Fei1), LIAO Kai1), XU Jilin1), 3), *, LIU Xingwang1), 2), 4), 5), 6),SHI Peng1), ZHANG Mengqi1), WU Kaibin1), and YAN Xiaojun2), *
1),,,315211,2),,315211,3),,363503,4),315211,5),315211,6),,315211,
Peroxisome proliferator activated receptors (PPAR) are kinds of key transcriptional factors in regulating LC-PUFA biosynthesis.Until now,little is known about PPAR in marine molluscs as well as in other invertebrates.is the first marine mollusc that is proved to possess the complete LC-PUFA biosynthetic pathway, and it can be a perfect representative to clarify this situation. In this study, the molecular properties ofPPAR were characterized, and corresponding potential regulatory roles in() transcription were estimated by dual luciferase assay. Results showed that twohomologs (PPAR_a and _b) were identified inThey both contain typical features of vertebrate PPAR, suggesting highly conserved functional regions in PPAR. By phylogenetic comparison, they are clearly different with vertebrate PPAR and can be divided into two distinctive sub-groups.Moreover, they show a high expression level in gill, labial palps, mantle and intestine. Their down-regulated expressions in trochophore larva and veliger larva might be attributed to food-deprivation. Additionally, the transcriptional activity ofpromoter was significantly activated by both PPAR_a and _b, indicating thatPPAR might play a role in the regulation of LC-PUFA biosynthesis. To our knowledge, this is the first systematic analysis of PPAR in a marine mollusc.The results will provide a valuable reference for further researches on the function of marine molluscan PPAR and their underlying mechanisms in regulating LC-PUFA biosynthesis.
; peroxisome proliferator activated receptors;; long chain-polyunsaturated fatty acids
1 Introduction
Long chain-polyunsaturated fatty acids (LC-PUFA, C≥20, carbon bonds ≥3), especially arachidonic acid (20:4n-6, ARA), eicosapentaenoic acid (20:5n-3, EPA) and docosa- hexaenoic acid (22:6n-3, DHA) are essential nutrients for human beings because of their low self-supply (Swanson, 2012). Sufficient dietary intake of LC-PUFA has enor- mous benefits for human health by facilitating cell membrane formation (McMurchie, 1988), neurological develop- ment (Lauritzen, 2001), immune response (Fritsche, 2006) and cardiovascular disease treatment (Russo, 2009).Marine molluscs are usually characterized by high levels of LC- PUFA, representing as an excellent dietary source of sus- tainable natural LC-PUFA for people (Joseph, 1982). No- tably, evidences showed that LC-PUFA also appeared to be necessary for the normal developments of marine mol- luscs, especially at early-life stages (Navarro and Villanu- eva, 2003; Monroig, 2017; Ran, 2020b).
In order to efficiently utilize this unique LC-PUFA re- source of marine molluscs and clarify the essential LC- PUFA for their developments, in recent years, the mole- cular mechanisms of LC-PUFA biosynthesis have been well investigated in several representative species by characte- rizing key genes encoding enzymes of fatty acyl desaturase (Fad) and elongase of very long chain fatty acid (Elovl). Specifically, genes encoding Elovl5 with high preference for elongation of C18–20 fatty acids (FA) have been cha- racterized from the cephalopods(Mon- roig, 2012a) and(Monroig, 2016), and the bivalves(Liu, 2013) and(Zhang, 2018). Genes en- coding Elovl4 with high elongation activity toward C≥22 FA have been identified in(Liu, 2014b) and(Monroig, 2017). Besides, genes encod- ing ∆5 Fad with desaturation activity toward 20:3n-6 and 20:4n-3 have been determined in(Monroig, 2012b),(Liu, 2014a),(Mon- roig, 2016) and the gastropod(Li, 2013). And a gene encoding ∆8 Fad with de- saturation activity toward 20:2n-6 and 20:3n-3 has been identified in(Liu, 2014b). However, to date, the regulatory pathways of LC-PUFA biosynthesis in ma- rine molluscs are still very poorly understood (Ran, 2020a).
Peroxisome proliferator activated receptors (PPAR) are ligand activated transcription factors belonging to the nu- clear receptor superfamily and play a critical role in the regulation of lipid homeostasis in organisms (la Cour Poul- sen, 2012). PPAR typically contain a highly con- served DNA-binding domain (DBD) harboring two zinc finger (Zn-Fing) structures that bind the peroxisome pro- liferator response element (PPRE) within the promoter of target genes to active their transcriptions, and a ligand bind- ing domain (LBD) harboring a ligand binding pocket that senses ligands (la Cour Poulsen, 2012). In mammals,have diverged by gene duplication into three sub- types of alpha (), beta/delta (/) and gamma (). There are threevariants of, namely1,2 and3 (Fajas, 1998). PPARis a critical inducer of FA oxi- dation; PPARis mainly associated with the regulation of lipoprotein transport and activation of lipid oxidation, and PPARplays a major role in the regulation of adipocyte differentiation and lipogenesis (la Cour Poulsen, 2012; Echeverría, 2016). Though many studies have been conducted on PPAR in vertebrates, which include mammals(la Cour Poulsen, 2012) and teleosts (Ibabe, 2002;Leaver, 2005; Tsai, 2008; You, 2017; Zhu, 2018), little knowledge of PPAR is available in in- vertebrates including marine molluscs (Kaur, 2015; Vogeler, 2017; Capitão, 2020).
The razor clamis an econo- mically important bivalve species native to the western Pa- cific coast. In 2016, its output exceeded 823000 tons, va- lued at US$ 1.3 billion (FAO, 2018). Specially, this biva- lve is rich in LC-PUFA, with DHA accounting for about 10% of the total FA (Ran, 2017). Moreover,is the first marine mollusc demonstrated to possess all Fad and Elovl activities required for LC-PUFA biosyn- thesisthe Sprecher pathway, including two(a and b) and one(Ran, 2018), oneandthree transcripts of(Elovl4_a, Elovl4_b and Elovl_c) (Ran, 2019b). Notably,is the firstreported in marine molluscs to date (Ran, 2018). Δ6 Fad not only catalyzes the first desaturation steptoward 18:2n-6 and 18:3n-3, but also catalyzes the key step in DHA biosynthesisSprecher pathway by conversion of 24:5n-3 to produce 24:6n-3 (Voss, 1991).Usually, it is considered as the rate-limiting enzyme in LC-PUFA biosynthesis.Accordingly, studies on the transcriptional re- gulation ofhave been one of the main topics in the regulation of LC-PUFA biosynthesis in organisms. As an important regulatory factor ontranscription,func- tion of PPAR has been well studied in vertebrates (Mat- suzaka, 2002; Tang, 2003; Dong, 2017; Li, 2019; Zhu., 2020);however, little know- ledge of its function is known in marine molluscs.
In the present study, the molecular properties ofinwas studied, including sequence alignment,phylogenetic comparison, and expression patterns in dif- ferent tissues and developmental stages (temporal expres- sion). Additionally, the potential regulatory roles ofPPAR intranscription was tested by dual luciferase assay. To our knowledge, this is the first syste- matic study of PPAR in a marine mollusc.The results will lay the foundation for further researches on the function of marine molluscan PPAR and their underlying mechanism in regulating LC-PUFA biosynthesis.
2 Materials and Methods
2.1 Cloning of S. constrictaPPAR
Tissues of foot muscle, gill and gonad were dissected from fresh adult, which were homogenized and used for RNA extraction with a MiniBEST Universal RNA Extraction Kit (Takara, Japan). Then 1μg RNA was further reverse-transcribed into template complementary DNA (cDNA) with a PrimeScriptTMRT-PCR Kit (Takara). Notably, the quality and quantity of RNA were determin- ed by running on a 1% agarose gel and a NanoDrop®ND- 1000 (NanoDrop, USA), respectively.
Forcloning, two genes with high ho- mology to vertebrate, namely PPAR_a and _b, were obtained by searching their genomic data (Ran, 2019a).To determine the veracity of the open reading frame (ORF) sequences, two pairs of gene-specific primers (Table 1) were designed using Prime 5 software and used for polymerase chain reaction (PCR) with LA Taq®Hot Star Version (Takara). Subsequently, the amplified products were purified by running on a 1.5% agarose gel, then inserted into pMDTM18-T Vector, and further transformed intoDH5com- petent cells. The recombinant single colonies successfully grown in LB plates containing ampicillin (50μgmL−1) were selected, incubated and sequenced (BGI, China).
2.2 Sequence and Phylogenetic Analyses
Using deduced amino acids (AA) of PPAR from,,,and, multiple sequence alignments were per- formed with ClustalX 2.1 (Larkin, 2007). Meanwhile, based on the deduced AA of PPAR fromand representative mammals, teleosts and invertebrates, toge- ther with representatives of other vertebrate nuclear recep-tor, a phylogenetic tree was constructed with MEGA 7 (Ku-mar, 2016) using maximum-likelihood approach.The confidence in the resulting phylogenetic tree branch topo- logy was measured by bootstrapping through 1000 itera- tions. Thehomologs of marine molluscs (,,and) were first obtained by retrieving their genomic data through functional annotation. Thenthe predicted peptide sequences were reconfirmed by the conserved regions of a typical DNA binding domain containing two Zn-Fing structures and a ligand binding domain using online software ExPASy-PROSITE(https:// prosite.expasy.org/). Finally, they were further confirmed by blasting against the NCBI database.
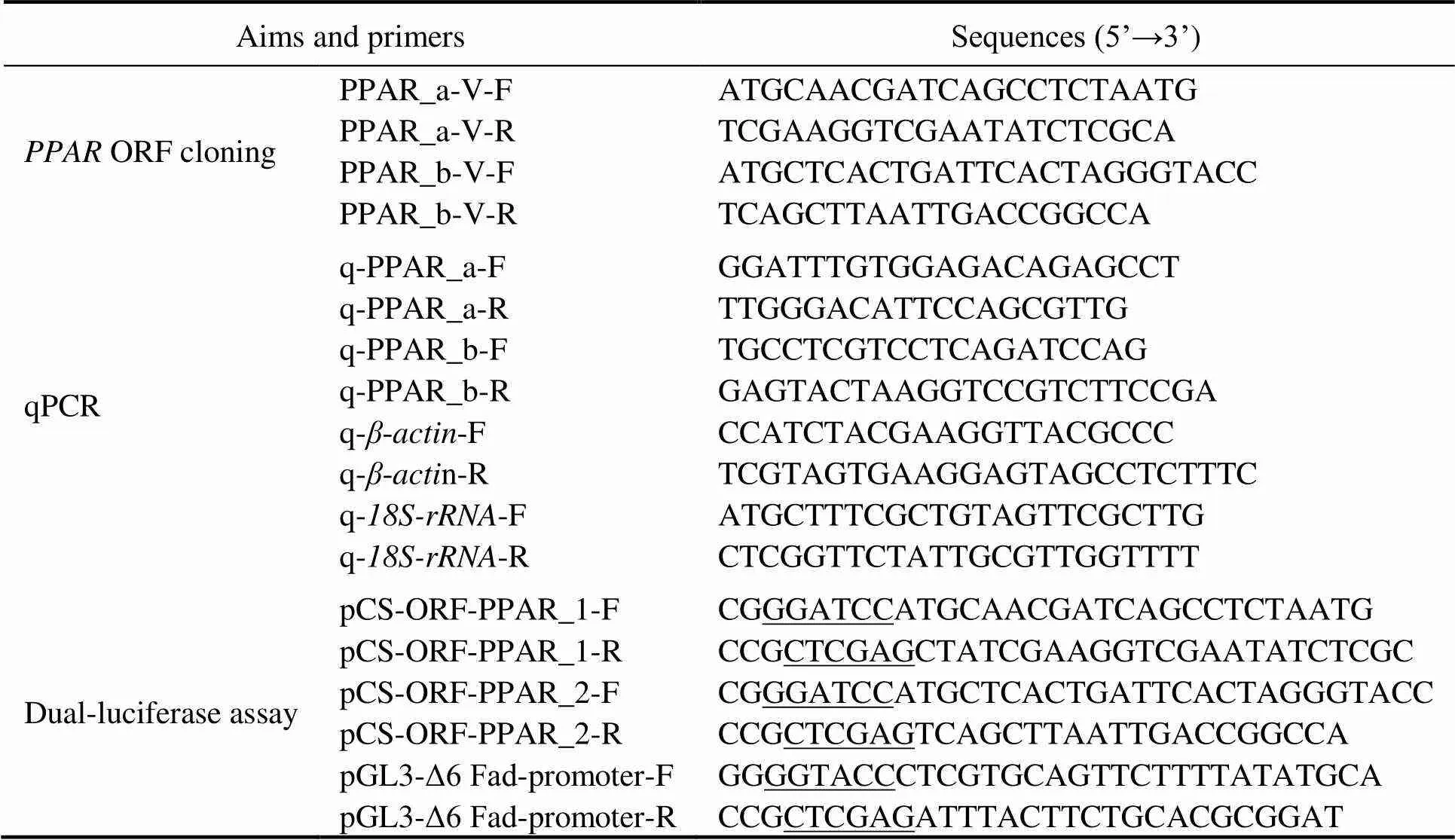
Table 1 Primers used for S. constricta PPAR ORF cloning, qPCR and dual-luciferase assay
Note: Restriction sites ofHI,I andIare underlined.
2.3 Collection of Tissues and Individuals at Different Developmental Stages
To investigate the expression patterns ofin different tissues, adult specimens (55.23±3.31mm, shell length) were purchased from a local seafood market of Lulin in Ningbo city of China. They were acclimated in the laboratory for 3 days without food supply to minimize the influence of intestine diets. Afterwards, the tissues in- cluding gill, labial palps, mantle, foot muscle, siphon, in- testine and gonad were dissected. Each tissue was isolated from six individuals and was pooled together as one sam- ple. Each sample was then divided in triplicate for further analyses.
To reveal the roles of PPAR inat different developmental stages, samples of zygotes, trochophore lar- va, veliger larva, umbo larva, creeping larva, single pipe larva and juvenile clam were collected from a bivalve hat- chery of Fujian Baozhi Aquatic Science and Technology Co., Ltd., in Zhangzhou city of China. The method of breed- ingis the same with our previous work (Ran, 2020b).Multiple individuals at different develop- mental stages were sampled from three independent rear- ing ponds.
2.4 Quantitative Real-Time PCR (qPCR)
RNA extraction was conducted with TRIzol Reagent (Ta- kara). Then 1μg RNA was reverse-transcribed into cDNA with PrimeScriptTMRT Master Mix (Perfect Real Time, Ta- kara). With qPCR primers in Table 1, the qPCR was per- formed on a quantitative thermal cycler (Mastercycler ep realplex, Eppendorf, Germany) with SYBR®TMII (Takara). The running program of qPCR was com- posed of an initial denaturation step at 95℃ for 30s, then 35 cycles of 95℃ for 5s, 55℃ for 15s and 72℃ for 20s, followed by a melting curve from 58℃ to 95℃ with an increment of 1.85℃min−1. Relative expressions ofwere calculated by 2−∆∆CTmethod (Livak and Schmittgen, 2001), normalized by the reference genes of-and(Table 1), and presented as the geometric mean of qPCR results derived from the expres- sion of the two reference genes. The relative expressions ofin different tissues and developmental stages were calculated in relation to that of gonad and trochophore larva respectively, which exhibited the lowest expression level.
2.5 Preparation of Recombinant Expression Vector and Report Vector
To test the potential regulatory roles ofPPAR intranscription, recombinant pCS2+ expression vec- tor andpGL3-basic report vector were constructed. In brief, firstly, ORFs ofPPAR_a and _b were cloned using primers harboring restriction sites ofH I andI (Table 1), respectively.promo- ter (2000bp upstream the translation initiation codon) (Gen- Bank accession number MK584921) (Ran, 2020a) was cloned using primers harboringI andI (Ta- ble 1). All PCR were conducted with Mighty AmpTMDNAPolymerase version 3 (Takara). Secondly, the resulting PCRproducts were purified, digested with corresponding re- striction endonucleases (New England BioLabs, USA), and inserted into similarly digested empty pCS2+ or pGL3 ba- sic vector, respectively. Thirdly, the recombinant plasmids of pCS-PPAR_a, pCS-PPAR_b and pGL3-Δ6 Fad-2000 were transformed intoDH5competent cells and confirmed by sequencing (BGI). Finally,DH5con- taining recombinant plasmids with correctsequence were used to isolate the corresponding recombinantplasmids with Endo-free Plasmid Mini Kit I (Omega, USA).
2.6 Dual-Luciferase Assay
A humidified incubator (ESCO, Singapore) was used to incubate the human embryonic kidney (HEK 293 T) cells at 37℃ with 5% CO2. The HEK 293 T cells were suppliedwith 100μL high glucose Dulbecco’s modified Eagle’s me-dium (DMEM) (Transgen Biotech, China) containing 10% fetal bovine serum (Transgen Biotech) per well in 96-well cell culture plates. Each experiment was in triplicate. Whenthe cells reached 90% confluence, the cultural medium was replaced with Opti-MEM I Reduced Serum Medium (Gibco, USA). Immediately, plasmids including 150ng of expression plasmid, 50ng of report plasmid, and 5ng ofRenilla luciferase reporter plasmid pRL-CMV (internal re- ference) (Promega, USA) in 10μL Opti-MEM I Reduced Serum Medium were co-transfected into cells using lipo- fectamine®2000 Reagent (Invitrogen, USA). At 6h after transfection, the cultural medium was replaced with 75μL fresh DMEM for further incubation. At 24h after transfection,luciferase activities were measured with Dual-Glo®Luciferase Assay System E2920 (Promega) using the va- rioskan flash 3001 (Thermo, USA). In detail, 75μL Dual- Glo luciferase Reagent (a mixture obtained by transfer- ring the Dual-Glo luciferase Buffer to one bottle of Dual- Glo luciferase substrate) was added to each plate well, gent- ly mixed and waited at least 10min to allow for cell lysis to occur, then measured the firefly luminescence. After which, 75μL Dual-Glo stop & Glo buffer (a mixture ob- tained by diluting Dual-Glo stop & Glo substrate 1:100 intoDual-Glo stop & Glo buffer) was added to plate wells, gently mixed and waited at least 10min, then measuredluminescence. The relative luciferase activity was expressed as the readout ratio of firefly luciferase/Renilla luciferase, and normalized by the corresponding result of control group.
2.7 Statistical Analyses
Statistical analyses of relative expressions ofand relative luciferase activities were performed with one-way ANOVA tests and pairwise multiple compa-risons by Tukey’s honestly significant difference test (SPSS 22.0, USA). All values were presented as means±stan- dard deviations, and<0.05 was deemed to be statistical- ly significant.
3 Results
3.1 Sequences and Phylogenetics of S. constrictaPPAR
The ORF length ofPPAR_a was 1575bp encoding a protein of 524 AA, while the ORF length ofPPAR_b was 984bp encoding a protein of 327 AA. The detailed sequence information had been depo- sited in the GenBank database with accession number of MW048582 and MW048583, respectively. Both of them possessed typical features of vertebrate PPAR, including a DNA binding domain and a ligand binding domain (high- lighted by vertical lines with arrow in Fig.1). Specifically, the DNA binding domain was characterized by two Zn- Fing structures (highlighted by dotted lines) with eight con- served cysteine residues (highlighted by symbol of *) and six conserved residues of CEGCKG (highlighted by a so- lid frame) in Fig.1. Meanwhile, the AA identity betweenPPAR_a and _b was very low of 27.13% (Ta- ble 2). AndPPAR_a exhibited a relative high- er similarity (29.57%–35.53%) with vertebrate PPAR than that of PPAR_b (26.67%–28.12%) (Table 2).
A total of seven predictedwere retrieved from the four marine molluscs, and they all contained the fea- tures of typical vertebrate PPAR as described forPPAR above (data not shown), which were further used to construct the phylogenetic tree (Fig.2). Result show-ed that the tree is dominantly classified into three big clades, including vertebrate PPAR, invertebrate PPAR and repre- sentatives of other vertebrate nuclear receptors (Fig.2). Thevertebrate PPAR,andare generally clustered into threegroups.They all belong to a big clade, which is differentwith PPAR of invertebrate. Those PPAR forming a huge group were further obviously separated from other verte- brate nuclear receptors (Fig.2). Notably,PPAR_a and some predicted marine molluscan PPAR were group- ed together with previously reportedPPAR2 andPPAR2 (Kaur, 2015), whilePPAR_b and some other predicted ma- rine molluscan PPAR were grouped together withPPAR1 andPPAR1 (Kaur, 2015), and they were clustered into two distinctive sub-groups respec- tively (Fig.2).
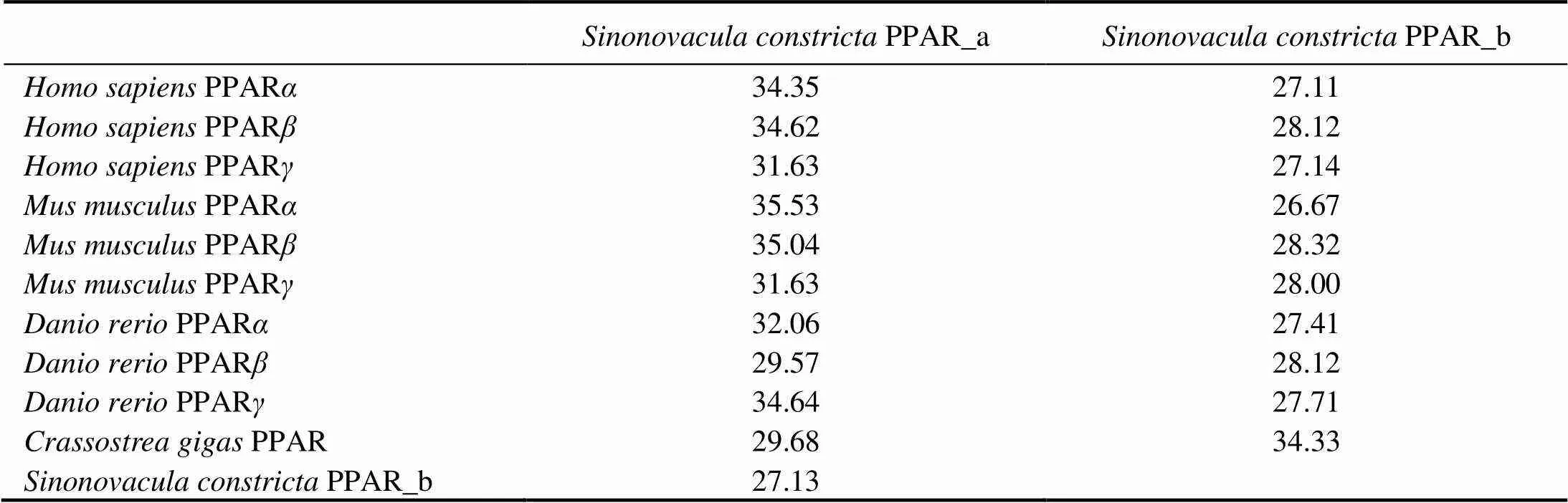
Table 2 Amino acid identity (%) between PPAR from H. sapiens, M. musculus, D. rerio, C. gigas and S. constricta

Fig.1 Peptide sequence alignment of PPAR from S. constricta (bold fonts) and representative mammals, teleosts and one marine mollusc.Identicalresidues are shaded with the same color in general.The DNA binding domain and ligand binding domain are indicated by the vertical lines with arrow respectively, and the zinc finger (Zn_Fing) structures are indicated by the dotted lines, which are predicted by online software ExPASy-PROSITE (https://prosite.expasy.org/). The eight conserved cysteine residues are indicated with symbol of ‘*’. The frames represent two typical features of vertebrate PPAR (Dreyer et al., 1992), including the conserved six residues and three residues space separated by two cysteines in the second Zn-Fing structure. The peptide sequences of PPAR include H. sapiens PPARα (AAB32649.1), PPARβ(AAX14041.1) and PPARγ (AAA80314.2); M. musculus (Mm) PPARα (CAA53042.1), PPARβ (NP_035275.1) andPPARγ (BAM95277.1); D. rerio PPARα (ABI30003.1), PPARβ (NP_571543.1) and PPARγ (ABI30002.1); and one PPAR reported previously in a marine mollusc Crassostrea gigas (ARM6537.1). The abbreviations of Hs, Mm, Dr, Cg and Sc represent H. sapiens, M. musculus, D. rerio, C. gigas and S. constricta, respectively.
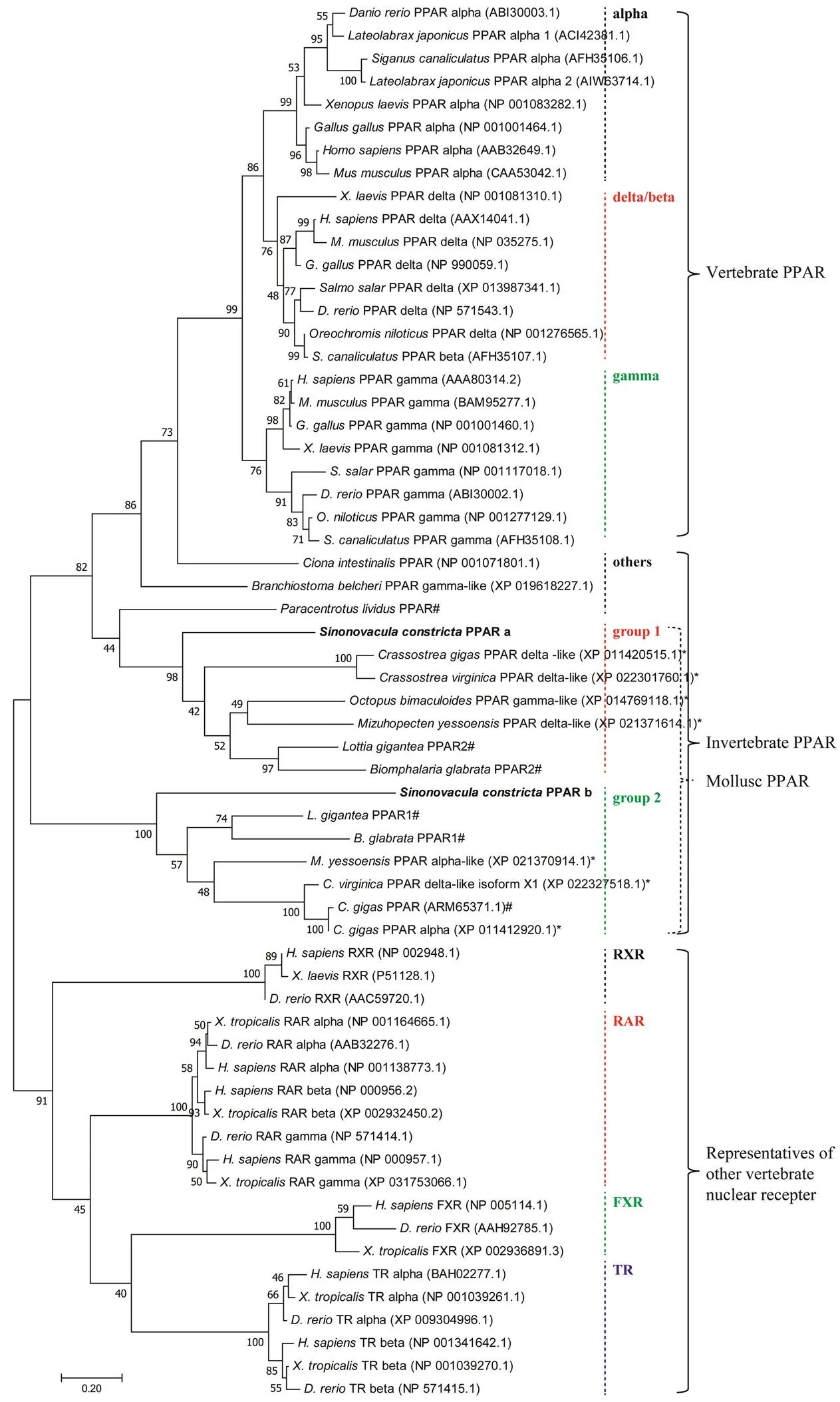
Fig.2 Phylogenetic tree comparing the deduced amino acids sequences of PPAR from S. constricta (bold fonts) and representative mammals, teleosts and molluscs, together with some other vertebrate nuclear receptors (RXR, RAR, FXR, TR). The tree was constructed using the maximum-likelihood approach with MEGA 7. The horizontal branch length is proportional to amino acids substitution rate per site. The numbers represent the frequencies with which the tree topology presented was replicated after 1000 iterations. The symbol (*) indicates the predicted PPAR genes derived from genomes of C. gigas (genome ID: 10758), C. virginica (genome ID: 398), M. yessoensis (genome ID: 12193) and O. bimaculoides (genome ID: 41501). The symbol (#) indicates the previously reported invertebrate PPAR, including Lottia gigantea and Biomphalaria glabrata (Kaur et al., 2015), Crassostrea gigas (Vogeler et al., 2017) and Paracentrotus lividus (Capitão et al., 2020). Meanwhile, some other vertebrate nuclear receptors (RXR, RAR, FXR, TR) have also been added as outgroups.
3.2 Expression Patterns of S. constrictaPPAR in Tissues and at Different Developmental Stages
In general,had a wide distribution in tissues detected (Fig.3A). Particularly,PPAR_awas expressed highest in gill (<0.01), followed by la-bial palps and mantle (<0.05), and lowest in gonad. WhilePPAR_b exhibited relatively high expressions in gill and labial palps, and had lowest expression in go- nad (Fig.3A). In addition,PPAR_a and _b ex- hibited similar expression patterns in individuals at diffe- rent developmental stages (Fig.3B). Specifically, the expres- sions of these two genes were high in zygotes, then drop-ped sharply, and then gradually recovered to a high le- vel and maintained during the following developmental stages (Fig.3B).

Fig.3 Expression patterns of S. constrictaPPAR in different tissues (A) and developmental stages (B). Relative expres- sion ofS. constrictaPPARare examined by qPCR and nor- malized by β-actin and 18S rRNA, and presented as the geo- metric mean of qRT-PCR results derived from the expres- sion of the two reference genes. Relative expression of S. constrictaPPAR in tissues and developmental stages are in relation to the normalized expression data in gonad and trochophore larva, respectively. The values (mean±SD, n=6) sharing a common letter within the same color (black or grey) are not significantly different (P≥0.05).
3.3 Transactivation Activity of S. constricta∆6 Fad Promoter by PPAR
When co-transfection with the recombinant pGL3 basic report plasmid containing 2000bp ofpromoter and the recombinant pCS2+ expression plasmid containingPPAR_a or _b in HEK 293 T cells, the relative luciferase activities were significantly increased by 2.19 folds and 1.83 folds respectively (<0.01) com- pared with that of the control groups (Fig.4). In addition,PPAR_a induced a significant higher promo- ter-driven luciferase intensity (<0.05) than that of PPAR_b (Fig.4).
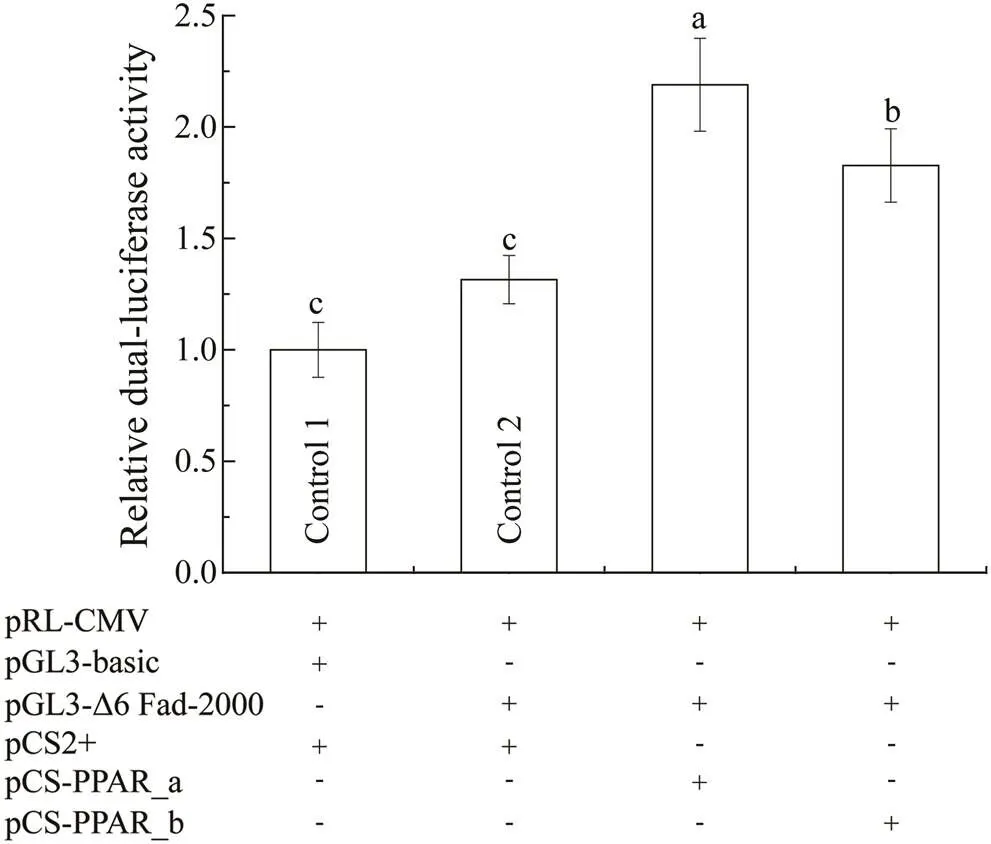
Fig.4 Transcriptional activity of S. constricta Δ6 Fad pro- moter regulated by PPAR. pRL-CMV, the Renilla lucife- rase expression plasmid (internal reference); pGL3-ba- sic and pGL3-Δ6 Fad-2000, the empty firefly luciferase re- porter plasmid and the recombinant pGL3-basic plasmid inserted with 2000bp upstream of S. constrictaΔ6 Fad pro- moter; pCS2+ and pCS-PPAR_a or _b, the empty expres- sion plasmid and the recombinant pCS2+ plasmid inserted with the ORF sequence of S. constricta PPAR_a or _b; ‘+’ and ‘-’, the plasmid was transfected into HEK 293 T cells or not. Relative luciferase activity was expressed as means±SD (n=3), which was first calculated by the readout ratio of firefly luciferase activity/Renilla luciferase activity, and then normalized by the corresponding result of the control 1. Values sharing a common letter above the bar graph are not significantly different (P≥0.05).
4 Discussion
Two members ofwere cloned from, which is different with the evidence that threeiso- forms (,and) exist in vertebrates (Ibabe, 2002; Leaver, 2005; Tsai, 2008; la Cour Poulsen,2012; You, 2017; Zhu, 2018). Meanwhile, by comparing genomes of four species of marine molluscs,in- cluding,,and, it was found that three species have both of the twotranscripts whilehas onlyone(Fig.2). The results suggest thatmight have undergone gene duplication events during species evolu- tion. Previously, Vogeler(2017) found that there is only onehomolog in, and they assumed that there may not be another PPAR in this bivalve. The twopredicted inin this article will change this view. Importantly, the deduced AA ofPPAR_a and _b both contain typical features of vertebratePPAR, with a DNA-binding domain harboring two Zn-Fing structures and six conserved residues (CEGCKG),and a ligand-binding domain (la Cour Poulsen, 2012), indicating that the functional regions were highly conser- ved in PPAR.
Analyzing AA identity,low similarity of 26.67%–35.53% was found betweenPPAR and representative vertebrate PPAR. Consistently, the phylogenetic tree re- vealed that PPAR of molluscs were clearly different from vertebrate PPAR. The results indicated thatvariants significantly differentiated during evolution. In addition,PPAR_a and _b obviously clustered into two distinctive sub-groups, indicating that they might belong to different isoforms of vertebrate PPAR. Notably, there is an absolutely conserved sequence of CEGCKG in vertebrate PPAR (highlighted with a solid frame in Fig.1), which is critical for sequence-specific DNA binding to the PPRE of target genes (Dreyer, 1992; Tsai, 2008; You, 2017). This is the case with all predicted PPAR of marine molluscs though difference also exists. There are only three AA difference between the first two cysteines of the second Zn-Fing structure in vertebrate PPAR (high- lighted with a dotted frame in Fig.1), which is specific for vertebrate PPAR compared with other nuclear receptors (Dreyer, 1992). But this was not the case with ma- rine molluscan PPAR as there are five AA difference be- tween the two cysteines. Those findings might provide a new insight into the evolution of.
Given that the lipids are critical for normal physiologi- cal activities and PPAR are key modulators for lipid ho- meostasis in organisms (la Cour Poulsen, 2012), the expression patterns ofin different tis- sues and developmental stages were examined. In general, bothPPAR_a and _b exhibited relatively high- er expressions in gill, labial palps and mantle, followed by siphon and intestine, indicating that lipids might be essen- tial for their physiological functions by providing energy or functional components. Similar phenomenon had been found with, which showed high ex-pressions in labial palp, gill and intestine (Ran, 2018), suggesting a potential regulatory role betweenPPAR and. Notably, qPCR results derived from individuals at different developmental stages showed that the expressions of bothPPAR_a and _b de-creased sharply in trochophore larva and veliger larva, which might be attributed to food deprivation in trochophore lar- va that inhibitedexpression because thewas supplied as the first-feeding diet at the latestage of trochophore larva during sampling (Ran, 2020b).
The transcriptional regulation ofby PPAR had been intensively studied in vertebrates (Matsuzaka, 2002; Tang, 2003; Dong, 2017; Li, 2019; Zhu, 2020). However,it is still unclear in marine mol- luscs. Typically, DNA binding by PPAR firstly requires li- gand activation and heterodimerization with the retinoid X receptor (RXR), then the PPAR-RXR heterodimer fur- ther binds to the PPRE within the promoter of target gene. Exceptionally, evidences have shown that PPAR and RXRcan function independently to modulate gene expression (Tan, 2005). Meanwhile, it has been demonstrated in many fishes that PPAR can stimulate the expression of tar- get genes via direct binding their promoters (Dong, 2017; Li, 2019; Zhu, 2020). Consequently, to elucidate whethercan be regulated byPPAR, promoter activities were analyzed. Herein, the luci- ferase activity ofpromoter was sig- nificantly activated by bothPPAR_a and _b, indicating their potential regulatory roles in LC-PUFA bio- synthesis. Importantly, the luciferase intensity induced byPPAR_a was significantly higher than that of PPAR_b, implying a stronger regulatory role ofPPAR_a for LC-PUFA biosynthesis. Previously we have reported thattranscription was controlled by sterol regulatory element binding protein (SREBP) (Ran, 2020a). Thustranscription might be regulated by both SREBP and PPAR. Similar phenomenon had been observed in vertebrate(Matsuzaka, 2002; Dong, 2017). Though two potential PPRE were predicted inpromoter using online software JASPAR2020 (http://jaspar.genereg.net/) and LASAGNA-Search 2.0 (https://biogrid- lasagna.engr.uconn.edu/lasagna_search/) (data not shown), we failed to verify their binding to the purifiedPPAR protein expressed in(DE3) (result not shown). The reason might be that the purified PPAR pro- tein has lost their original activities, or it needs to be ac- tivated by appropriate ligand and heterodimerization with RXR. Therefore, further studies on the regulatory role ofPPAR inexpressionwere needed.
In conclusion, the molecular characteristics of(_a and _b) are identified in this research. They are obviously different with vertebrate. Addi- tionally, the promoter activity ofwas significantly activated by the deduced protein of the newlycloned, indicating a potential regulatory role ofPPAR intranscription. However, based on the results of present study, it is still unclear which sub- type (,, or) of vertebrateis homologous to. Nevertheless, the results will provide a reference for further researches on the function of marine molluscan PPAR and their underlying mechanism in re- gulating LC-PUFA biosynthesis, ultimately facilitate to op- timize the LC-PUFA biosynthesis in marine molluscs.
Acknowledgements
This research was supported by the National Key Re- search and Development Program of China (No. 2019YF D0900400), the Zhejiang Major Science Project, China (No. 2019C02057), the Ningbo Science and Technology Re- search Projects, China (Nos. 202003N4124, 2019B10006),the China Agriculture Research System of MOF and MARA, and the Open Project Funding of ‘National and Local Joint Engineering Laboratory of Marine Biotechnology and Engi-neering, Key Laboratory of Applied Marine Biotechnology, Collaborative Innovation Center for Zhejiang Marine High- efficiency and Healthy Aquaculture, and Key Laboratory of Marine Biological Engineering, Zhejiang, China’.
Capitão, A., Lopes-Marques, M., Páscoa, I., Ruivo, R., Mendi- ratta, N., Fonseca, E.,., 2020. The echinodermata PPAR: Functional characterization and exploitation by the model li- pid homeostasis regulator tributyltin., 114467.
Dong, X. J., Tan, P., Cai, Z. N., Xu, H. L., Li, J. Q., Ren, W.,.,2017. Regulation of FADS2 transcription by SREBP-1 andPPAR-influences LC-PUFA biosynthesis in fish., 7: 40024.
Dreyer, C., Krey, G., Keller, H., Givel, F., Helftenbein, G., and Wahli, W., 1992. Control of the peroxisomal-oxidation path- way by a novel family of nuclear hormone receptors., 68: 879-887.
Echeverría, F., Ortiz, M., Valenzuela, R., and Videla, L. A., 2016. Long-chain polyunsaturated fatty acids regulation of PPARs, signaling: Relationship to tissue development and aging., 114: 28-34.
Fajas, L., Fruchart, J. C., and Auwerx, J., 1998. PPARγ3 mRNA: A distinct PPARmRNA subtype transcribed from an inde- pendent promoter., 438, 55-60.
FAO, 2018.. Food and Agriculture Organization of the United Nations, Rome, 30pp.
Fritsche, K., 2006. Fatty acids as modulators of the immune re- sponse., 26: 45-73.
Ibabe, A., Grabenbauer, M., Baumgart, E., Fahimi, D. H., and Ca- jaraville, M. P., 2002. Expression of peroxisome proliferator- activated receptors in zebrafish ()., 118: 231-239.
Joseph, J. D., 1982. Lipid composition of marine and estuarine invertebrates. Part II: Mollusca., 21: 109-153.
Kaur, S., Jobling, S., Jones, C. S., Noble, L. R., Routledge, E. J., and Lockyer, A. E., 2015. The nuclear receptors ofand: Implications for develop- ing new model organisms., 10 (4): e0121259.
Kumar, S., Stecher, G., and Tamura, K., 2016. MEGA7: Molecu- lar Evolutionary Genetics Analysis Version 7.0 for Bigger Da- tasets., 33: 1870-1874.
la Cour Poulsen, L., Siersbæk, M., and Mandrup, S., 2012. PPARs: Fatty acid sensors controlling metabolism., 23 (6): 631-639.
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., Mc- Gettigan, P. A., McWilliam, H.,., 2007. Clustal W and Clus- tal X version 2.0., 23: 2947-2948.
Lauritzen, L., 2001. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina., 40: 1-94.
Leaver, M. J., Boukouvala, E., Antonopoulou, E., Diez, A., Fa- vre-Krey, L., Ezaz, M. T.,., 2005. Three peroxisome pro- liferator-activated receptor isotypes from each of two species of marine fish., 146: 3150-3162.
Li, M. Z., Mai, K. S., He, G., Ai, Q. H., Zhang, W. B., Xu, W.,., 2013. Characterization of two Δ5 fatty acyl desaturases in abalone (Ino)., 416-417: 48-56.
Li, Y. Y., Yin, Z. Y., Dong, Y. W., Wang, S. Q., Monroig, Ó., To- cher, D. R.,., 2019. Pparγ is involved in the transcrip- tional regulation of liver LC-PUFA biosynthesis by targeting the Δ6Δ5 fatty acyl desaturase gene in the marine teleost., 21 (1): 19-29.
Liu, H. L., Guo, Z. C., Zheng, H. P., Wang, S. Q., Wang, Y. J., Liu, W. H.,., 2014a. Functional characterization of a Δ5- like fatty acyl desaturase and its expression during early em- bryogenesis in the noble scallop., 41: 7437-7445.
Liu, H. L., Zhang, H. K., Zheng, H. P., Wang, S. Q., Guo, Z. C., and Zhang, G. F., 2014b. PUFA biosynthesis pathway in ma- rine scallop., 62: 12384-12391.
Liu, H. L., Zheng, H. P., Wang, S. Q., Wang, Y. J., Li, S. K., Liu, W. H.,., 2013. Cloning and functional characterization of a polyunsaturated fatty acid elongase in a marine bivalve no- ble scallop., 416-417: 146-151.
Livak, K. J., and Schmittgen, T. D., 2001. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCTmethod., 25: 402-408.
Matsuzaka, T., Shimano, H., Yahagi, N., Amemiya-Kudo, M., Yo- shikawa, T., Hasty, A. H.,., 2002. Dual regulation of mouseΔ5- and Δ6-desaturase gene expression by SREBP-1 and PPAR., 43: 107-114.
McMurchie, E. J., 1988. Dietary lipids and the regulation of mem- brane fluidity and function., 3: 189-237.
Monroig, Ó., De Llanos, R., Varó, I., Hontoria, F., Tocher, D. R., Puig, S.,., 2017. Biosynthesis of polyunsaturated fatty acids in: Molecular cloning and functional characterisation of a stearoyl-CoA desaturase and an elonga- tion of very long-chain fatty acid 4 protein., 15: 82.
Monroig, Ó., Guinot, D., Hontoria, F., Tocher, D. R., and Navar- ro, J. C., 2012a. Biosynthesis of essential fatty acids in(Cuvier, 1797): Molecular cloning, functional charac- terisation and tissue distribution of a fatty acyl elongase., 360-361: 45-53.
Monroig, Ó., Hontoria, F., Varó, I., Tocher, D. R., and Navarro, J. C., 2016. Investigating the essential fatty acids in the common cuttlefish(Mollusca, Cephalopoda): Mo- lecular cloning and functional characterisation of fatty acyl de- saturase and elongase., 450: 38-47.
Monroig, Ó., Navarro, J. C., Dick, J. R., Alemany, F., and Tocher, D. R., 2012b. Identification of a Δ5-like fatty acyl desaturase fromthe cephalopod(Cuvier 1797) involved in thebiosynthesis of essential fatty acids., 14: 411-422.
Navarro, J. C., and Villanueva, R., 2003. The fatty acid composi- tion ofparalarvae reared with live and inert food: Deviation from their natural fatty acid profile., 219: 613-631.
Ran, Z. S., Kong, F., Xu, J. L., Liao, K., and Yan, X. J., 2020a. Transcriptional regulation mechanism of SREBP on Δ6 Fad in razor clam., 124: 881-889, DOI: https://doi.org/10.1017/S000711452 0002068.
Ran, Z. S., Kong, F., Xu, J. L., Liao, K., Xu, X. R., Shi, P.,., 2020b. Fad and Elovl expressions, fatty acid compositions, and feed effects of three representative microalgae in(Lamarck 1818) at early developmental stages., 521: 735101.
Ran, Z. S., Li, S., Zhang, R. T., Xu, J. L., Liao, K., Yu, X. J.,., 2017. Fatty acid and sterol changes in razor clam(Lamarck 1818) reared at different salinities., 473: 493-500.
Ran, Z. S., Li, Z. Z., Yan, X. J., Liao, K., Kong, F., Zhang, L.,., 2019a. Chromosome‐level genome assembly of the razor clam(Lamarck, 1818)., 19: 1647-1658.
Ran, Z. S., Xu, J. L., Liao, K., Li, S., Chen, S. B., and Yan, X. J., 2018. Biosynthesis of polyunsaturated fatty acids in the razor clam: Characterization of Δ5 and Δ6 fatty acid desaturases., 66: 4592-4601.
Ran, Z. S., Xu, J. L., Liao, K., Monroig, Ó., Navarro, J. C., Oboh, A.,., 2019b. Biosynthesis of long-chain polyunsaturated fatty acids in the razor clam: Charac- terization of four fatty acyl elongases and a novel desaturase capacity.–, 1864 (8): 1083-1090.
Russo, G. L., 2009. Dietary n-6 and n-3 polyunsaturated fattyacids: From biochemistry to clinical implications in cardiovas- cular prevention., 77: 937-946.
Swanson, D., Block, R., and Mousa, S. A., 2012. Omega-3 fatty acids EPA and DHA: Health benefits throughout life., 3: 1-7.
Tan, N. S., Michalik, L., Desvergne, B., and Wahli, W., 2005. Mul- tiple expression control mechanisms of peroxisome prolifera- tor-activated receptors and their target genes., 93 (2-5): 99-105.
Tang, C. R., Cho, H. P., Nakamura, M. T., and Clarke, S. D., 2003.Regulation of human Delta-6 desaturase gene transcription: Identification of a functional direct repeat-1 element., 44: 686-695.
Tsai, M. L., Chen, H. Y., Tseng, M. C., and Chang, R. C., 2008. Cloning of peroxisome proliferators activated receptors in the cobia () and their expression at diffe- rent life-cycle stages under cage aquaculture., 425 (1-2): 69-78.
Vogeler, S., Galloway, T. S., Isupov, M., and Bean, T. P., 2017. Cloning retinoid and peroxisome proliferator-activated nuclear receptors of the Pacific oyster and in silico binding to envi- ronmental chemicals., 12 (4): e0176024.
Voss, A., Reinhart, M., Sankarappa, S., and Sprecher, H., 1991. The metabolism of 7, 10, 13, 16, 19-docosapentaenoic acid to 4, 7, 10, 13, 16, 19-docosahexaenoic acid in rat liver is inde- pendent of a 4-desaturase., 266: 19995-20000.
You, C. H., Jiang, D. L., Zhang, Q. H., Xie, D. Z., Wang, S. Q., Dong, Y. W.,., 2017. Cloning and expression characteri- zation of peroxisome proliferator-activated receptors (PPARs) with their agonists, dietary lipids, and ambient salinity in rab- bitfish.–, 206: 54-64.
Zhang, H. K., Liu, H. L., Cheng, D., Liu, H. L., and Zheng, H. P., 2018. Molecular cloning and functional characterisation of a polyunsaturated fatty acid elongase in a marine bivalve., 6: 89-95.
Zhu, K. C., Song, L., Zhao, C. P., Guo, H. Y., Zhang, N., Guo, L.,., 2018. The Transcriptional factor PPARpositively regulates Elovl5 elongase in golden pompano(Linnaeus 1758)., 9: 1340.
Zhu, K. C., Zhang, N., Liu, B. S., Guo, L., Guo, H. Y., Jiang, S. G.,., 2020. Transcription factor pparαb activates fads2s to pro- mote LC-PUFA biosynthesis in the golden pompano(Linnaeus 1758)., 161: 605-616.
October 9, 2020;
December 8, 2020;
March 9, 2021
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2021
E-mail: xujilin@nbu.edu.cn
E-mail: yanxiaojun@nbu.edu.cn
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Meshless Method with Domain Decomposition for Submerged Porous Breakwaters in Waves
- Facial Features of an Air Gun Array Wavelet in the Time-Frequency Domain Based on Marine Vertical Cables
- Magma Evolution Processes in the Southern Okinawa Trough:Insights from Melt Inclusions
- Summery Intra-Tidal Variations of Suspended Sediment Transportation–Topographical Response and Dynamical Mechanism in the Aoshan Bay and Surrounding Area, Shandong Peninsula
- High-Resolution Geochemical Records in the Inner Shelf Mud Wedge of the East China Sea and Their Indication to the Holocene Monsoon Climatic Changes and Events
- Geological Guided Tomography Inversion Based on Fault Constraint and Its Application
