Identification and Characterization of Gene SpDMRT99B and Its Sex-Biased Expression Profile in the Mud Crab, Scylla paramamosain
2021-12-22ZHANGYinFANGShaobinLINFeiLIShengkangZHENGHuaipingZHANGYuelingIKHWANUDDINMhdandMAHongyu
ZHANG Yin, FANG Shaobin, LIN Fei, LI Shengkang, ZHENG Huaiping,ZHANG Yueling, IKHWANUDDIN Mhd, and MA Hongyu, 3), *
Identification and Characterization of Geneand Its Sex-Biased Expression Profile in the Mud Crab,
ZHANG Yin1), 2), FANG Shaobin1), 2), LIN Fei1), 2), LI Shengkang1), 2), ZHENG Huaiping1), 2),ZHANG Yueling1), 2), IKHWANUDDIN Mhd2), 3), and MA Hongyu1), 2), 3), *
1),,,515063,2),,515063,3),,
The() gene family is conserved from invertebrates to humans. The functions of DMRT are mainly involved in sex development and the formation of many tissues and organs. In this study, a DM (Doublesex/Mab-3)-domain gene was identified in the mud crab, and was namedbecause of its many similarities to arthropodand phylogenetically close relationship with arthropod DMRT99B. The cDNA ofgene is 1249bp in length, encoding 224 amino acids.From 254bp to 928bp there isa conserved DM domain.No transmembrane do- main was identified. Through multiple amino acid alignment and phylogenetic tree analysis, the closest gene toisDMRT99B, followed byandDMRT99B. The expressions of the gene were characterized in different tissues of female and male crabs during early development period of crab individuals, as well as in different development periods of gonads. The results showed thatgene is significantly highly expressed in testis than in ovary and other tissues. The expression level ofin testis at different stages is significantly higher than that in ovary,and it is particularly highly expressed in immature testis. In early developmental stages of larvae, the expressions ofre- main at a low level and reach a peak at zoea stage I when thebody segments shape up. It is speculated thatgene might be involved in the gonadal development process and somitogenesis of.
; DM domain gene;; sex determination/differentiation; early development stage
1 Introduction
Although major differences in the genetic control of sex- ual development occur among animal lineages, the() genesappear to function in all animals as tissue-specific transcrip- tion factors related to sex determination and many other developmental processes (Bellefroid., 2013). Many studies have reported thatgenes are highly conserved in the DM domain with a zinc finger structure bind-ing to specific genomic elements to regulate gene transcrip- tion and play a similar role in animal sex differentiation (Zhang and Zarkower, 2017; Galindo-Torres., 2018). In recent years, the functions ofgenes have been characterized in many animal groups and known to be in- volved in sex determination and/or sex differentiation in nematodes(Raymond., 1998), vertebrates (Kopp, 2012) and insects (Miller., 2003). In vertebrates, orthologs ofgenes, including human,, and, have been identified and they function during se- xual development (Raymond., 1999). Thegenefamily may represent the most conservative genes involved in sex determination and differentiation in the tree of ani- mal life (Rideout., 2010; Murphy., 2015). Other genes of the DM domain gene family are also expressed in other tissues in addition to gonads, such as,,and., 2003; Veith., 2006),ler., 2004),., 2004) and., 2000). In,, which is closely related to, affects mushroom body size in the adult brain (Zwarts., 2015) and is highly expressed in the midline cells of the central nervous system inlarvae (Fontana and Crews, 2012). In addition,gene may be respon- sible for establishing or maintaining the identity of octopaminergic neurons (Henry., 2012).
The DM-domain genes may play a related role in sex determination in crustaceans (Kato., 2008; Zhang and Qiu, 2010; Yu., 2014). However, the study ofgene in crustaceans is relatively limited (Yu., 2015, 2017; Yang., 2018). The first crustacean DM-domain gene was identified in the Chinese mitten crabwith a testis-specific expression pattern (Zhang and Qiu, 2010). Othergenes were subsequently report- ed in various crustacean species. In, the functionalgene,, presents a sexually dimor- phic expression pattern and is responsible for male-speci- fic traits (Kato., 2011; Toyota., 2013). In the Eas- tern spiny lobster,, the first hetero- gametic sex-linkedgene,, was identified(Chandler., 2017). Twogenes,and, were also identified in the giant freshwater prawn(Yu., 2014; Zhong., 2019), which is closely related to the oriental river prawn(Wang., 2019). Member of thegene family has also been identifiedin the Chinese shrimp(Li., 2018). Until now, there is no information on molecular characteristics and function ofgenes in mud crab.
is an important economic mariculture species and popular seafood in the South-East Asian coun- tries (Le Vay., 2007). The mud crab aquaculture has been conducted for more than 100 years in China and over the past decades throughout the Asia (Williams and Primavera, 2001) and occupied an increasingly momentous role in Chinese crab species farming industry (Li., 2018). The life cycle development consists of four main periods,including embryonic period; a zoea period with 5 different stages; a megalopa period; and crablet after the fixation and metamorphosis of megalopa, whose gonadic development is yet to be studied.males grow faster and reach larger size than females at harvest season. The mechanisms underlying the sexual phenoty- pic differentiation and dimorphic development are still un- clear.Although there have been some studies on the sex differentiation and determination related genes including,and(Jiang, 2020; Lin, 2020; Wang, 2020), whether thefamily genes contribute to the sex development and other functions of the mud crab have not been studied. This is the first study to reportgene in. The cloning of thisgene may provide a useful tool for the studies of male sex determination/differentiation in the crab.
2 Materials and Methods
2.1 Crab and Tissue Sampling
The crabs were purchased from local fishermen in Shan- tou City, China. They were culturedin our laboratory and Raoping West Coast Biotechnology Co., Ltd., Chaozhou, Guangdong Province, China during the breeding season.Crabs were anesthetized with ice before tissues extraction. Five female (body weight 280g±10g) and male crabs (body weight 250g±10g), respectively, were used for gonadal RNA extraction for rapid amplification of cDNA ends (RACE) PCR. Different tissues including ganglion, stomach, heart, intestine, gill, muscle, hepatopancreas and go- nads were collected from five males and females, respectively, for the detection of spatial expression ofge-ne. Different gonadal samples were collected from five fe- male and five male crabs at each developmental stage, re- spectively. The identification of the gonads at different de- velopmental stages was according to the methods of Wu(2020). The different developmental stage including embryo, zoea I-V, megalopa and crablet I-II.
2.2 Cloning of DMRT Gene
Total RNA was isolated from the testis (40–60mg) of matureusing RNA isoPlus (TaKaRa, Ja- pan). The quantity and quality of isolated RNAs were de- termined by electrophoresis and spectrophotometry (Nano- drop 2000, Thermo Scientific, USA). Only samples with OD260/280 ration ranging from 1.8 to 2.0 were used for cDNA synthesis. Reverse transcription was conducted us- ing PrimeScript®RT reagent Kit with gDNA Eraser (TaKaRa, Japan).The 3’ and 5’ ends of cDNAs were cloned using the RACE-PCR with the SMART RACE Kit (Clon- tech, USA). The universal amplified primers of crab DMRTwas designed using Primer Premier 5 software. The primers listed in Table 1 are used to amplify the partial sequences. Programs for PCR were as follows: One denaturation cycle at 94℃ for 5min; 30 cycles of amplification and each cycle includes 30s at 95℃, 30s at 57℃ and 1min at 72℃;and a final extension at 72℃for 8min. The PCR products described above were all purified from 2.0% agarose by SanPrep Column DNA Gel Extraction Kit (Sangon, China) and loaded into the pEASY-T1 cloning vector (Transgen biotech, China). Then the positive transformants were se- lected and sequenced (Sangon, China). Genomic DNAs ex- tracted from male and female crabs were used as template to clone the DNA sequence of thegene.
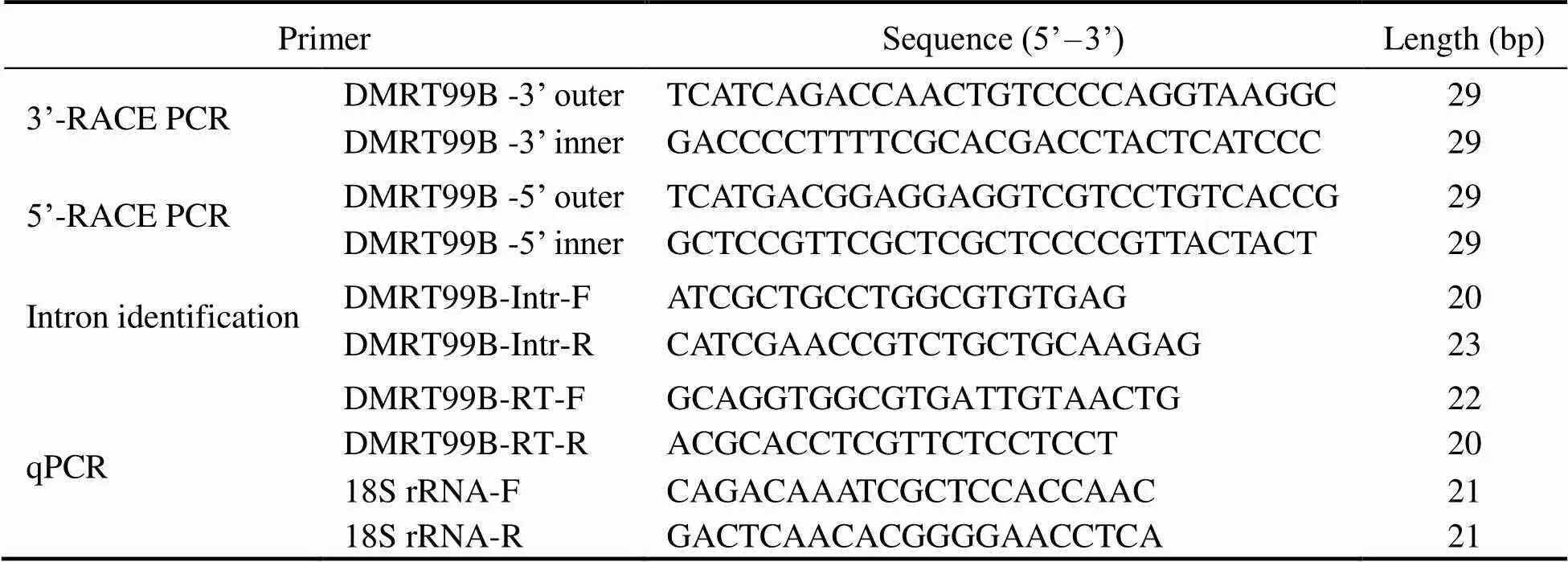
Table 1 Nucleotide sequences of the primers for cloning and qPCR of DMRT99B gene
Note: F and R represent the forward and reverse direction of the primers, respectively.
2.3 Structure Prediction, Sequence Alignment and Phylogeny
For deduced amino acid analysis, the calculated mole- cular weight and theoretical isoelectric point were obtain- ed by ProtParam tool (http://web.expasy.org/protparam/). Secondary and tertiary structure predictions were perform- ed using putative amino acid sequences. We submitted all 224 amino acids of the SpDMRT99B as inputs. The secondary structures and tertiary structure were predicted us- ing online resource SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html) and SMART (http://smart.embl-heidelberg.de/), respectively. The mul-tiple alignments of amino acid sequences were performed using BioEdit software.
The evolutionary history was inferred by using the Ma- ximum Likelihood method based on the JTT matrix-basedmodel (Jones., 1992). The bootstrap consensus tree in- ferred from 1000 replicates is taken to represent the evolutionary history of the taxa analyzed (Felsenstein, 1985). Branches corresponding to partitions reproduced in less than 25% bootstrap replicates are collapsed. The percen- tage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches (Felsenstein, 1985). Initial tree (s) for the heuristic search were obtained automatically by applying Neighbor-Joining and BioNJ algorithms to a ma- trix of pairwise distances estimated using a JTT model, and then selecting the topology with superior log likelihood va-lue. The analysis involved 31 amino acid sequences (Table2). All positions with less than 50% site coverage were eli- minated. There are a total of 472 positions in the final da- taset. Evolutionary analyses were conducted in MEGA7.0 (Kumar., 2015).

Table 2 Amino acid sequences used in the phylogenetic analysis
2.4 Quantitive Real Time PCR (qPCR)
Total RNA was extracted using RNAiso Plus (Takara Co.,Ltd., Japan). RNA samples were treated by RQ1 RNase-Free DNase prior to qPCR (Takara Co., Ltd., Japan) to avoid genomic DNA contamination. cDNA was generated from 500ng DNase-treated RNA using Talent qPCR Pre- mix (SYBR Green) kit (TIANGEN Biotech Co., Ltd., Bei- jing) following the manufacturer’s instructions. Primers (Table 2) for qPCR were designed using the Primer 6.0 Software. qPCR was conducted in a Mini Option real-time detector (Roche LightCycle@480). The qPCR re-action solution consisted of 10μL Talent qPCR Premix (2×), 0.6μL PCR forward primer (10μmolL−l), 0.6μL PCR reverse primer (10μmolL−l), 2.0μL RT reaction so- lution (cDNA 20ng), and 6.8μL RNase free water. The reaction conditions were 95℃ for 3min followed by 40 cycles of 95℃ for 5s, 60℃ for 10s and 72℃ for 15s. The florescent flux was then recorded, and the reaction con- tinued at 72℃ for 6s and 95℃ for 5s for melting curve. All amplicons were initially separated by agarose gel elec- trophoresis to ensure their correct sizes. The gene expres- sion levels were normalized towards the reference genes (18S rRNA). Optimized comparative Ct (2−ΔΔCt) value me- thod (Livak and Schmittgen, 2001) was applied to calcu- late gene expression levels.
3 Results
3.1 Identification of DMRT
Based on the EST file of mud crab gonadal transcriptome sequencing (Yang., 2018), a pair of specific pri- mers were designed to verify and amplify the core region ofgene. The 5’ and 3’ ends ofwere amplified using RACE method. As a result, the full length ofcDNA was 1249bp. The analysis of the cDNA sequence showed that it has 5’ untranslate region (UTR) of 253bp; open reading frame (ORF) of 672bp containing an ATG start codon at 254bp and a TAA stop codon at 928bp; and 3’ UTR sequence of 321bp with poly (A) tail but without any polyadenylation signal AATAAA. The ORFofcodes for 224 amino acids (Fig.1) which con-tains a conserved doublesex DNA-binding motif (DM do- main) from 39–92aa. A putative nuclear localization sig- nal (NLS) KGHKR from 74–78aa is also detected, and it locates in the zinc module making of two intertwined Zn2+- binding sites (site I C61/C64/H76/C80 and site II H67/ C85/C87/C90) (Fig.2). The predicted molecular size and theoretical pI of mud crab DMRT are 24254.8 Da and 9.69,respectively. The amino acid contents of Ser, Ala, Leu and Arg are all above 8% of total amino acids. The sequence was deposited in the GenBank database with the acces- sion number MN395823.
The genomic DNA was used to determine the introns in genomicgene sequence using a pair of spe- cific primers at 5’ and 3’ ends of the cDNA sequence. The sequencing of the PCR products showed no difference be- tween the amplifications from genomic DNA and those from cDNA templates using the same primers. It indicatedthatgene has no intron. Additionally, the ge- nomic sequences of male and femalegene showed no sex dimorphism in the crab genome.

Fig.1 Full length of SpDMRT99B cDNA sequence. The sequences with gray background show the DM domain region. The amino acids in red box are putative nuclear localization signal.
3.2 Phylogeny of SpDMRT99B
According to the amino acid alignment, the DM domain inDMRT99B protein sequence werehighly conserved among DMRT99B and other DMRTsubfamilies with 100% sequence identity toDmrt99B and 99% identity toand(Fig.2). Thus, we named thegene identified in the present study. The DM domain also contains a conserved nuclear localization signal (NLS) and an ordered moiety consisting of invariant cysteine and histidine as shown in Fig.2. In SpDMRT99B, the two intertwined Zn2+-binding sites (site I and site II) were ob- served (Fig.3). Outside the DM domain, little sequence homology can be identified. Moreover, SpDMRT99B lacks the conserved DMA domain which is similar toandbut unlike theandDMRT99B (shown in Fig.4). It indicates that the DMRT99B in mud crab showed a high conservatism dur- ing the evolution.
To identify the phylogenetic affinities between SpDMRT- 99B and other members of DMRT family, the molecular phylogenetic analysis was performed in an unrooted phylogenetic tree constructed usingORF nucleotide sequences-deduced amino acid sequences (Table 2; Fig.5). SpDMRT99B isthe closest toDMRT99B, sharing a high level of similarity of 74%, followed byDMRT99B in the same branch with insect andDMRT99B. DMRT99B is closely related with mammal and vertebrate DMRT4, DMRT5.
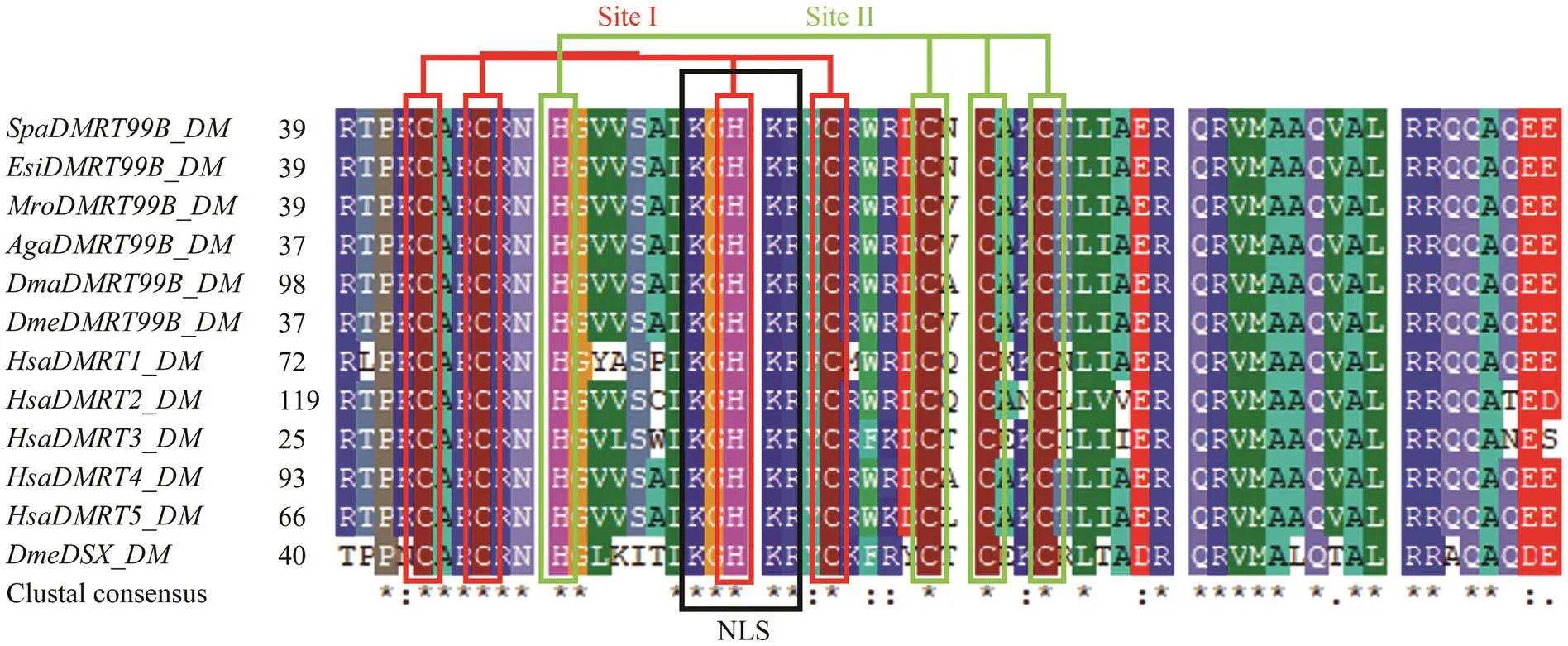
Fig.2 Amino acid sequence alignment of DM domain from SpDMRT99B and other species. Red and green rectangles indicate residues of the two intertwined zinc binding sites (site I and site II, respectively). Putative nuclear localization signals (NLSs) are underlined.
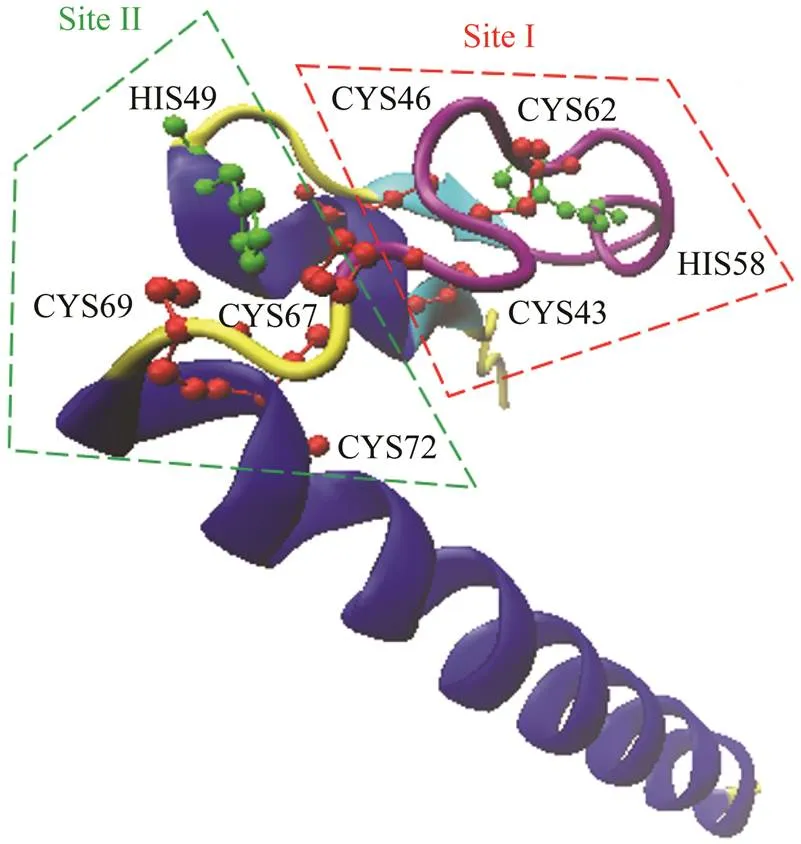
Fig.3 3D-structures of SpDMRT99B predicted by SMART. Red and green lines circle out the residues of the two intertwined zinc binding sites (site I and site II, respectively).

Fig.4 Schematic diagrams of DMRT proteins of different species. Size of each bar is drawn according to the position of amino acids in each protein.
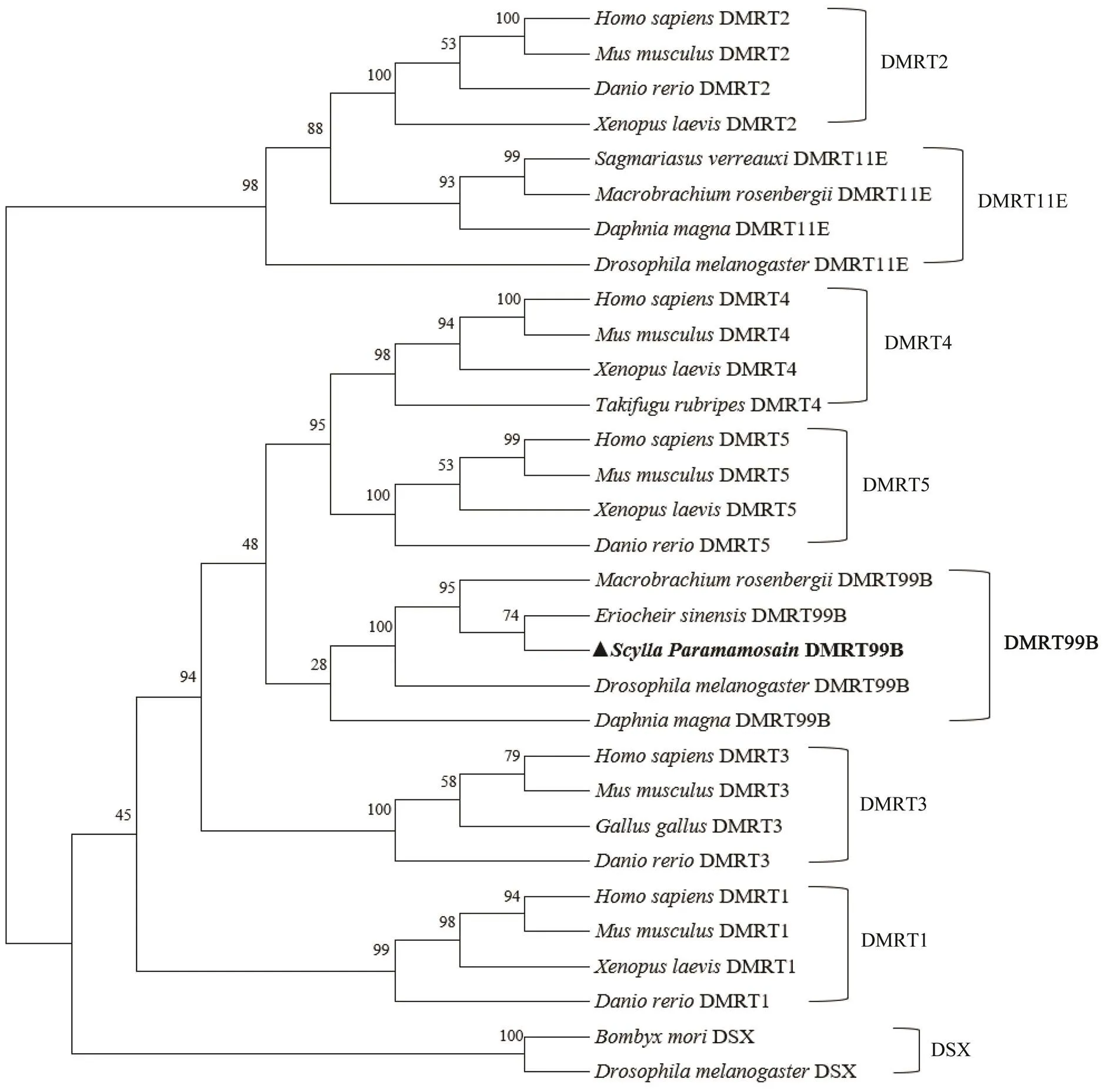
Fig.5 A comparative phylogenetic tree based on the amino acid sequences of SpDMRT99B and 30 other proteins.
3.3 Sex-Biased Expression Profile of SpDMRT99B
The expression levels were analyzed in adult mud crab tissues by qPCR, and results showed thatmRNA was distributed in all tissues. The expression ofgene shows different patterns in different sexes of crab.is expressed more highly in the male tissuesthan in the female, including ganglion, heart, intes- tine, muscle and hepatopancreas. However, the levels of ex- pression in the stomach and gill are the opposite. It is main- ly expressed in testis, significantly higher (over 10 times higher) than in ovary and other tissues (Fig.6). Consider- ing its dominant expression in testis, SpDMRT99B might play critical role in the male gonad development of mud crab. Thus, we detected the expressions ofat different developmental stages of gonads, namely ovary I–V and testis I–III (Fig.7). It showed that the expressions ofin testis were significantly higher than that in ovaryno matter at which stages, which is accordance with the expression detection in different tissues. Among the different developmental stages of testis, the peak oc- curs in the stage II of testis, and the expression is signifi- cantly higher in this stage than those in stagesI and III. Thusmight be related to the development of testis development. During different stages of early de- velopment in mud crab, the expressions ofgenediffer obviously. Thegene is significantly ex- pressed at the stage of zoea I mud crab (Fig.8). The expres- sions at other developmental stages change without sig- nificances.
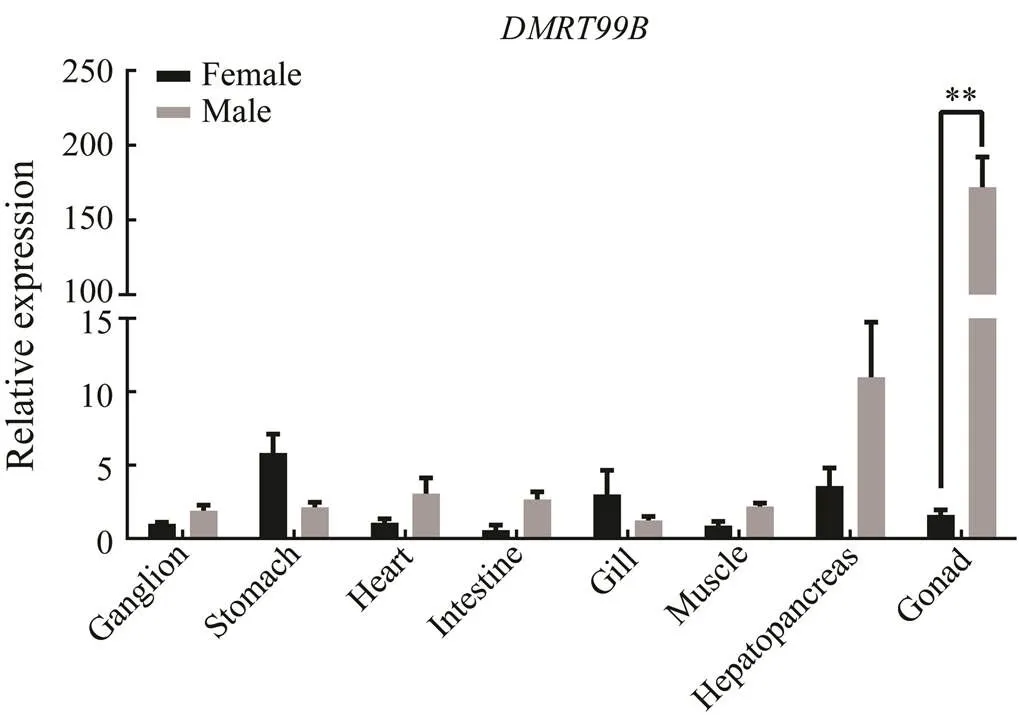
Fig.6 Quantitative real-time PCR validation of SpDMRT99Bexpressions in different tissues.
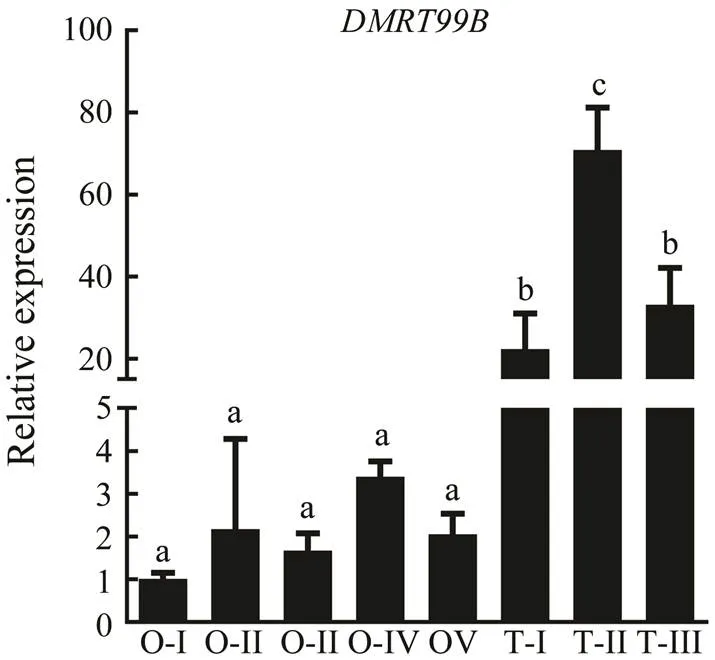
Fig.7 Quantitative real-time PCR validation of SpDMRT99B expressions at different developmental stages of gonads. O-I, ovary stage I; O-II, ovary stage II; O-III, ovary stage III; O-IV, ovary stage IV; O-V, ovary stage V; T-I, testis stage I; T-II, testis stage II; T-III, testis stage III.

Fig.8 Quantitative real-time PCR validation of SpDMRT99B expressions at the early developmental stages of Scylla paramamosain. E, embryonic stage; Z-I, zoea stage I; Z-II, zoea stage II; Z-III, zoea stage III; Z-IV, zoea stage IV; Z-V, zoea stage V; M, megalopa; C-I, crablet stage I; C-II, crablet stage II.
4 Discussion
Despitegenes have been cloned in several in- vertebrates, limited genomic information made it difficult to annotate and determinegenes in different in- vertebrate groups (Bellefroid., 2013; Wexler., 2014). In the current study, we report the molecular characterization ofgene, which is the first complete sequence of a DM factor identified in the. The full-length cDNA ofgene is 1249bp, coding 224 amino acids with 24kDa predicted mole- cular mass, which is similar with thein the length of the cDNA and protein (Zhang and Qiu, 2010). The deduced serine- and proline-rich amino acid sequence presents a well-conserved DM domain characteristic among all DMRT subfamilies. The DM domain has 100% high identity with that ofandDMRT99B. The DM motif is a cysteine-rich DNA- binding domain that contains two intertwined Zn2+-bind- ing sites (site I, C61/C64/H76/C80 and site II, H67/C85/ C87/C90) which are necessary for DNA binding (Zhu., 2000), and a putative NLS consisting of KGHKR (Fig.3). Unlike the insect andDMRT99Bs, SpDMR99B lacks a conserved DMA domain, which is similar withandDMRT99B (Zhang and Qiu,2010; Yu., 2014). The presence of DMA which is spe- cific to DMRT99B and DMRT3-5 (Fig.4) is patrimonial. The loss of DMA domains here may contribute to the evolution of novel roles, as DMRT1 lacks DMA domain func- tioning in mammalian sexual differentiation (Raymond., 2000; Brunner., 2001; Volff., 2003). Phylogenetic analysis indicates that theDMRT99B protein clustered with the DMRT99B sequences of other species, which implied that SpDMRT99B might have si- milar function with DMRT99B in other species.
is also predominantly expressed in testis, approximately a hundred times higher than that in ovary. In,is also prominent in the testis,and much lower in the ovary (Yu., 2014). During the different developmental stages of the gonads,was significantly highly expressed in testis stage II which is in an immature status (Fig.6).genes are also expressed in immature testes inand(Zhang and Qiu, 2010; Yu., 2014). It is reported thatmight play an essential role in tes- ticular development and differentiation (Zhang and Qiu, 2010; Yu., 2014). The primary function ofge- nes in the gonad is to promote male-specific and repress female-specific differentiation (Kopp, 2012). In general, mostgenes are expressed in males and function in male sexual differentiation. However, inand,genes are expressed in a fe- male-specific manner (Kato., 2008; Kasahara., 2018), which might be unique to Branchiopoda and Insecta species (Kasahara., 2018). Tissue- and sex-specificgenes expressions in different portal gonads in chor- data, arthropoda, and molluska suggests that they already played a role in testicular development in a common bilateral ancestor. In contrast, the inconsistent functions ofgenes in some species may reflect independent co- option (Kopp, 2012). In non-gonadal tissues,genes appear to have the ability to modulate a wide range of de- velopmental processes (Hong., 2007), to which only limited attention has been paid to date. The time-depen- dent expression patterns ofgenes inthe period of the embryo, larval, post-larval and adult stages might indicate that these proteins may be involved in so- matic genesis rather than in reproductive development (Yu., 2014). Furthermore,might conservatively function for somitogenesis both in zebrafish and mouse (Meng., 1999; Sato., 2010). It suggested thatgenes play regulatory roles in both sexual and so- matic development (Abayed., 2019). Here, the expression ofduring the early developmen reaches peak at zoea I stage which is equipped with segments compared to the embryonic stage, whichsuggests that themight also play roles in somitoge- nesis in mud crab larvae.
In absence of sufficient elucidations in arthropods, the functions of DMRT99B can be indicated by their vertebrate homologs, namely DMRT4/5s (Fig.5). Dmrt4 and Dmrt5 inand Dmrt5 in zebrafish are involved in neurogenesis (Yoshizawa., 2011; Parlier., 2013), and so as DMRT99B in(Kasahara., 2018). However, the Dmrt4 mutant in mouse has normal olfactory function (Balciuniene, 2006). Except for gonad development and neurogenesis, Dmrt5 has important roles in various processes including embryonic development andsomite formation (Urquhart., 2016; Muralidharan., 2017; De Clercq., 2018). Furthermore, tissue dis- tributions of Dmrt4 and Dmrt5 in adults are divergent in different species (Guan., 2000; Kondo., 2002;Ottolenghi., 2002; Balciuniene., 2006; Veith.,2006). All functions of DMRT4 and DMRT5 in vertebratesmay reflect their intermediate or multi-functional statusduring evolution of the DMRT family, so does SpDMRT- 99B in this study. Thewas reported in the embryonic development and sexual differentiation of(Yu., 2014). In silkworm, it plays an important role in behavior-related neurogenesis (Kasahara., 2018). In addition, due to the technical difficulties in manipulating mud crab embryos, the effects ofgene on early embryonic development or larvae cannot be studied by up-regulating or down-regulating methods. While more researchesare needed on studying the function of these molecules in crustaceans, current expression pattern evidences suggest that thegene has a multifunctional role.
In the present study, we identified the DM-domain gene in the mud crab and detected its expression characteriza- tions. The expression ofpresents sexually di- morphism and male-bias pattern. The peak expression in zoea I stage might indicate the involvement ofin somitogenesis. However, many efforts should be con- ducted on revealing the functions ofgenes in mud crab possibly by means of up-regulating or down-regula- ting technique in the future. Our present findings enhance our understanding of DM domain genes in sexual dimor- phism in crustaceans and lay the foundation for the fur- ther study ingenes in mud crab.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 31772837), the National Key Research & Development Program of China (No. 2018YF D0900201), the Science and Technology Project of Guang- dong Province (No. 2018A050506080), the Shantou Uni- versity Scientific Research Foundation for Talents (No. NTF17006), and the Program for Innovation and Enhance- ment of School of Department of Education of Guangdong Province (No. 2017KCXTD014).
Abayed, F. A., Manor, R., Aflalo, E. D., and Sagi, A., 2019. Screen- ing forgenes from embryo to matureprawns., 282: 113205.
Balciuniene, J., Bardwell, V. J., and Zarkower, D., 2006. Mice mutant in the DM domain geneare viable and fertile but have polyovular follicles., 26 (23): 8891-8984.
Bellefroid, E. J., Leclere, L., Saulnier, A., Keruzore, M., Sirakov, M., Vervoort, M.,., 2013. Expanding roles for the evolutionarily conserved Dmrt sex transcriptional regulators during embryogenesis., 70: 3829- 3845.
Brunner, B., Hornung, U., Shan, Z., Nanda, I., Kondo, M., Zend- Ajusch, E.,., 2001. Genomic organization and expression of the doublesex-related gene cluster in vertebrates and detection of putative regulatory regions for., 77 (1-2): 8-17.
Chandler, J. C., Fitzgibbon, Q. P., Smith, G., Elizur, A., and Ven- tura, T., 2017. Y-linkedparalogue () in the East- ern spiny lobster,: The first invertebrate sex-linked., 430 (2): 337-345.
De Clercq, S., Keruzore, M., Desmaris, E., Pollart, C., Assimacopoulos, S., Preillon, J.,., 2016. DMRT5 together with DMRT3 directly controls hippocampus development and neo- cortical area map formation., 28 (2): 493-509.
Felsenstein, J., 1985. Confidence limits on phylogenies: An ap- proach using the bootstrap., 39: 783-791.
Fontana, J. R., and Crews, S. T., 2012. Transcriptome analysis ofCNS midline cells reveals diverse peptidergic pro- perties and a role for castor in neuronal differentiation., 372: 131-142.
Galindo-Torres, P., Garcia-Gasca, A., Llera-Herrera, R., Escobedo-Fregoso, C., Abreu-Goodger, C., and Ibarra, A. M., 2018.Sex determination and differentiation genes in a functional her-maphrodite scallop,.,37: 161-175.
Guan, G., Kobayashi, T., and Nagahama, Y., 2000. Sexually di- morphic expression of two types of DM (Doublesex/Mab-3)- domain genes in a teleost fish, the Tilapia ()., 272 (3): 662-666.
Guo, Y., Li, Q., Gao, S., Zhou, X., He, Y., Shang, X.,., 2004. Molecular cloning, characterization, and expression in brain and gonad ofof zebrafish., 324 (2): 569-575.
Henry, G. L., Davis, F. P., Picard, S., and Eddy, S. R., 2012. Cell type-specific genomics ofneurons., 40 (19): 9691-9704.
Hong, C. S., Park, B. Y., and Saint-Jeannet, J. P., 2007. The func- tion ofgenes in vertebrate development: It is not just about sex., 310 (1): 1-9.
Jiang, Q., Lu, B., Wang, G., and Ye, H., 2020. Transcriptional in- hibition of Sp-IAG by crustacean female sex hormone in the mud crab,., 21 (15): 5300.
Jones, D. T., Taylor, W. R., and Thornton, J. M., 1992. The rapid generation of mutation data matrices from protein sequences., 8: 275-282.
Kasahara, R., Aoki, F., and Suzuki, M. G., 2018. Deficiency inortholog causes behavioral abnormalities in the silkworm,., 53 (3): 381-393.
Kato, Y., Kobayashi, K., Oda, S., Colbourn, J. K., Tatarazako, N., Watanabe, H.,., 2008. Molecular cloning and sexually di- morphic expression of DM-domain genes in., 91 (1): 94-101.
Kato, Y., Kobayashi, K., Watanabe, H., and Iguchi, T., 2011. En- vironmental sex determination in the branchiopod crustacean: Deep conservation of a doublesex gene in the sex-determining pathway., 7: e1001345.
Kim, S., Kettlewell, J. R., Anderson, R. C., Bardwell, V. J., and Zarkower, D., 2003. Sexually dimorphic expression of multiple doublesex-related genes in the embryonic mouse gonad., 3 (1): 77-82.
Kondo, M., Froschauer, A., Kitano, A., Nanda, I., Hornung, U., Volff, J.,., 2002. Molecular cloning and characterization ofgenes from the medakaand the platy- fish., 295 (2): 213-222.
Kopp, A., 2012.genes in the development and evolution of sexual dimorphism., 28 (4): 175-184.
Kumar, S., Stecher, G., and Tamura, K., 2016. MEGA7: Mole- cular Evolutionary Genetics Analysis version 7.0 for bigger datasets., 33 (7): 1870-1874.
Le Vay, L., Ut, V. N., and Walton, M., 2007. Population ecology of the mud crab(Estampador) in an estuarine mangrove system; a mark-recapture study., 151 (3): 1127-1135.
Li, S., Li, F., Yu, K., and Xiang, J., 2018. Identification and characterization of a doublesex gene which regulates the expression of insulin-like androgenic gland hormone in., 649: 1-7.
Li, Y. Y., Ai, C. X., and Liu, L. J., 2018. Mud crab,China’s leading maricultured crab. In:. Gui, J. F.,., eds., Wiley Online Library, 226-233.
Lin, J., Yuan, Y., Shi, X., Fang, S., Zhang, Y., Guan, M.,., 2020. Molecular cloning, characterization and expression pro- files of a SoxB2 gene related to gonadal development in mud crab ()., 64 (2): 126-136.
Livak, K. J., and Schmittgen, T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCTmethod., 25 (4): 402-408.
Meng, A., Moore, B., Tang, H., Yuan, B., and Lin, S., 1999. Adoublesex-related gene, terra, is involved in somi- togenesis in vertebrates., 126 (6): 1259-1268.
Miller, S. W., Hayward, D. C., Bunch, T. A., Miller, D. J., Ball, E. E., Bardwell, V. J.,., 2003. A DM domain protein from a coral,, homologous to proteins important for sex determination., 5: 251- 258.
Muralidharan, B., Keruzore, M., Pradhan, S. J., Roy, B., Shetty, A. S., Kinare, V.,., 2017. Dmrt5, a novel neurogenic factor, reciprocally regulates Lhx2 to control the neuron-glia cell fate switch in the developing hippocampus., 37 (46): 11245-11254.
Murphy, M. W., Lee, J. K., Rojo, S., Gearhart, M. D., Kurahashi, K., Banerjee, S.,., 2015. An ancient protein-DNA interaction underlying metazoan sex determination., 22 (6): 442-451.
Ottolenghi, C., Fellous, M., Barbieri, M., and McElreavey, K., 2002. Novel paralogy relations among human chromosomes support a link between the phylogeny of doublesex-related ge- nes and the evolution of sex determination., 79 (3): 333-343.
Parlier, D., Moers, V., Van Campenhout, C., Preillon, J., Leclère, L., Saulnier, A.,., 2013. Thedoublesex-related geneis required for olfactory placode neurogenesis., 373 (1): 39-52.
Raymond, C. S., Murphy, M. W., Osullivan, M. G., Bardwell, V. J., and Zarkower, D., 2000., a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation., 14 (20): 2587-2595.
Raymond, C. S., Parker, E. D., Kettlewell, J. R., Brown, L. G., Page, D. C., Kusz, K.,., 1999. A region of human chromosome 9p required for testis development contains two genes related to known sexual regulators., 8 (6): 989-996.
Raymond, C. S., Shamu, C. E., Shen, M. M., Seifert, K. J., Hirsch, B., Hodgkin, J.,., 1998. Evidence for evolutionary conservation of sex-determining genes., 391: 691.
Rideout, E. J., Dornan, A. J., Neville, M. C., Eadie, S., and Good- win, S. F., 2010. Control of sexual differentiation and beha- vior by the doublesex gene in., 13 (4): 458-466.
Sato, T., Rocancourt, D., Marques, L., Thorsteinsdóttir, S., and Buckingham, M., 2010. A Pax3/Dmrt2/Myf5 regulatory cascade functions at the onset of myogenesis., 6 (4): e1000897.
Toyota, K., Kato, Y., Sato, M., Sugiura, N., Miyagawa, S., Miya- kawa, H.,., 2013. Molecular cloning of doublesex genes of four cladocera (water flea) species., 14: 239.
Urquhart, J. E., Beaman, G. M., Byers, H., Roberts, N. A., Cher- vinsky, E., Osullivan, J.,., 2016. DMRTA2 () is mutated in a novel cortical brain malformation., 89 (6): 724-727.
Veith, A. M., Schafer, M., Kluver, N., Schmidt, C., Schultheis, C., Schartl, M.,., 2006. Tissue-specific expression of dmrt genes in embryos and adults of the platyfish., 3 (3): 325-337.
Volff, J., Zarkower, D., Bardwell, V. J., and Schartl, M., 2003. Evolutionary dynamics of the DM domain gene family in me- tazoans., 57 (1): S241-S249.
Wang, M., Xie, X., Xu, D., Wang, Z., Yu, G., Jin, Z.,., 2020. Molecular characterization of the sex-lethal gene in mud craband its potential role in sexual develop- ment., 250: 110486.
Wang, Y., Jin, S., Fu, H., Qiao, H., Sun, S., Zhang, W.,., 2019. Identification and characterization of thegene in the oriental river prawn., 20 (7): 1734.
Wexler, J. R., Plachetzki, D. C., and Kopp, A., 2014. Pan-me- tazoan phylogeny of the DMRT gene family: A framework for functional studies., 224 (3): 175-181.
Williams, M. J., and Primavera, J. H., 2001. Choosing tropical portunid species for culture, domestication and stock enhance- ment in the Indo-Pacific., 14 (2): 121- 142.
Winkler, C., Hornung, U., Kondo, M., Neuner, C., Duschl, J., Shi- ma, A.,., 2004. Developmentally regulated and non-sex- specific expression of autosomal dmrt genes in embryos of the Medaka fish ()., 121(7): 997-1005.
Wu, Q., Waiho, K., Huang, Z., Li, S., Zheng, H., Zhang, Y.,., 2020. Growth traits and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab,., 515: 734560.
Yang, X., Ikhwanuddin, M., Li, X., Lin, F., Wu, Q., Zhang, Y.,., 2018. Comparative transcriptome analysis provides insights into differentially expressed genes and long non-coding RNAs between ovary and testis of the mud crab ()., 20 (1): 20-34.
Yoshizawa, A., Nakahara, Y., Izawa, T., Ishitani, T., Tsutsumi, M., Kuroiwa, A.,., 2011. Zebrafish Dmrta2 regulates neurogenesis in the telencephalon., 16 (11): 1097-1109.
Yu, Y., Ma, W., Zeng, Q., Qian, Y., Yang, J., and Yang, W., 2014. Molecular cloning and sexually dimorphic expression of two Dmrt genes in the giant freshwater prawn,., 3 (2): 181-191.
Yu, Y., Zhang, X., Yuan, J., Li, F., Chen, X., Zhao, Y.,., 2015.Genome survey and high-density genetic map construction pro- vide genomic and genetic resources for the Pacific White Shrimp., 5: 15612.
Yu, Y., Zhang, X., Yuan, J., Wang, Q., Li, S., Huang, H.,., 2017. Identification of sex-determining loci in Pacific White Shrimpusing linkage and association analysis., 19 (3): 277-286.
Zhang, E., and Qiu, G., 2010. A novel Dmrt gene is specifically expressed in the testis of Chinese mitten crab,., 220 (5): 151-159.
Zhang, T., and Zarkower, D., 2017. DMRT proteins and coordi- nation of mammalian spermatogenesis., 24: 195-202.
Zhong, P., Zhou, T., Zhang, Y., Chen, Y., Yi, J., Lin, W.,., 2019. Potential involvement of a DMRT family member (Mr- Dsx) in the regulation of sexual differentiation and moulting in the giant river prawn., 50 (10): 3037-3049.
Zhu, L., Wilken, J., Phillips, N. B., Narendra, U., Chan, G., Strat- ton, S. M.,., 2000. Sexual dimorphism in diverse metazoans is regulated by a novel class of intertwined zinc fingers., 14 (14): 1750-1764.
Zwarts, L., Broeck, L. V., Cappuyns, E., Ayroles, J. F., Magwire, M. M., Vulsteke, V.,., 2015. The genetic basis of natural variation in mushroom body size in., 6 (1): 10115-10115.
September 16, 2020;
December 21, 2020;
February 22, 2021
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2021
. Tel: 0086-754-86503471
E-mail: mahy@stu.edu.cn
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Meshless Method with Domain Decomposition for Submerged Porous Breakwaters in Waves
- Facial Features of an Air Gun Array Wavelet in the Time-Frequency Domain Based on Marine Vertical Cables
- Magma Evolution Processes in the Southern Okinawa Trough:Insights from Melt Inclusions
- Summery Intra-Tidal Variations of Suspended Sediment Transportation–Topographical Response and Dynamical Mechanism in the Aoshan Bay and Surrounding Area, Shandong Peninsula
- High-Resolution Geochemical Records in the Inner Shelf Mud Wedge of the East China Sea and Their Indication to the Holocene Monsoon Climatic Changes and Events
- Geological Guided Tomography Inversion Based on Fault Constraint and Its Application
