Post COVID-19 mucormycosis: A case series
2021-12-20MalaVinodKaneriaKallappaBaligeriAbhijeetBudhe
Mala Vinod Kaneria, Kallappa Baligeri, Abhijeet Budhe
Department of Medicine, Topiwala National Medical College and B.Y.L. Nair Ch. Hospital, Mumbai, India
ABSTRACT
Objective: To evaluate the associated factors between COVID-19 and mucormycosis.
Methods: Twenty-two patients of COVID-19 associated mucormycosis (including 3 asymptomatic patients who were cured of COVID-19) from a single medical unit of our institute were included. A detailed history was noted, with special emphasis on the time of onset of mucormycosis symptoms,presence of comorbidities, including new onset diabetes, severity of COVID-19, oxygen requirement, details of receipt of steroids and immunomodulators such as tocilizumab, imaging findings,including the number of sinuses involved, bony erosions, orbital and cerebral involvement, microscopy, culture and histopathology reports and antifungals given. Surgical interventions including number of debridements, orbital exenteration, maxillectomy, and the vaccination status were noted.
Results: All 22 patients had rhino-orbital cerebral mucormycosis,27.27% in the first wave and 72.73% during the second wave.Diabetes was the commonest comorbidity, and 40.91% patients were newly detected diabetics. The time of presentation in relation to their COVID-19 symptoms was 8-15 days (average 12.5 days).Ten out of 22 (45.45%) had asymptomatic or mild COVID-19 and 40.91% did not require supplemental oxygen. Five out of 22(22.73%) did not receive steroids. Twelve out of 22 (54.55%) had orbital involvement, 3 (13.64%) had palatal ulcer and 4 (18.18%)had cerebral involvement and all these had progressed in spite of treatment with appropriate antifungals.
Conclusions: COVID-19 associated mucormycosis is a frequent,lethal, post COVID-19 complication, occurring even in mild and asymptomatic cases who have not received steroids or oxygen.
KEYWORDS: Mucormycosis; COVID-19; Steroids; Amphotericin B
Significance
COVID-19 may be followed by secondary fungal infections.Unlike Aspergillus and Candida, mucormycosis may occur even in mild and asymptomatic COVID-19 patients. Significantly, it may affect patients who have not received steroids, believed to be the most important risk factor. As the virus is getting adept at evading diagnostic tests, indirect evidence of COVID-19 may have to be sought, in order to establish the association, when RT-PCR for SARS-CoV-2 is negative.
1. Introduction
India was just emerging from a devastating second wave of COVID-19 in May 2021, but before the number could dwindle and the death toll counted, we were struck by another blow dealt by the receding wave. Mucormycosis, a fatal fungal infection, caused by ubiquitous environmental molds, was being reported from all over India as a COVID-19 associated infection. Mucormycosis mainly affects people who are immunocompromised, or patients already infected with other diseases. High risk groups include people with diabetes (especially diabetic ketoacidosis), solid organ transplantation, neutropenia, long-term systemic corticosteroid use,and iron overload (hemochromatosis). The risk is high in people living with HIV, and in those using immunomodulating drugs, and the anti-fungal voriconazole.
This fungus, which is often associated with uncontrolled diabetes mellitus, has now found a new alliance. While Aspergillus(COVID-19 associated pulmonary Aspergillosis) and Candida(COVID-19 associated Candidiasis) played havoc in critical COVID-19, mucor slowly and surreptitiously gained ground and an escalating number of cases of mucormycosis were reported during the second wave of the pandemic, in patients who had recovered from COVID-19[1]. Unlike Aspergillus and Candida, which targeted critical patients in the ICU, mucormycosis was observed even in mild or asymptomatic COVID-19 cases, some who did not even require admission for COVID-19. The lymphopenia associated with SARS-CoV-2, the presence of uncontrolled diabetes mellitus, which is possibly triggered by the action of SARS-CoV-2 on the pancreas,injudicious use of steroids, possibly anti IL-6 directed therapies for COVID-19 (tocilizumab), and the use of broad spectrum antibiotics,lower the threshold for acquisition of mucor and have devastating consequences in this vulnerable population[2]. Steroids, which were projected as a miracle drug for COVID-19, after the first wave, are thought to play an important role in the causation of this fungal infection. Other factors thought to play a contributory role, are hypoxia, high iron levels (increased ferritins), the widespread use of zinc supplements (which are available over the counter) in order to boost the immunity, use of steam inhalation and the reuse of masks.The treatment of mucormycosis is disfiguring, expensive, prolonged and associated with adverse effects. In resource limited countries,the treatment of mucormycosis is particularly challenging. The number of cases of COVID-19 associated mucormycosis was unprecedented and hospitals had to set up special wards dedicated to the management of these patients. The Central Government of India was also prompted to declare a mucormycosis epidemic and make it a notifiable infection. We studied the profile of 22 patients of COVID-19 associated mucormycosis (CAM) in a single unit of our Dedicated COVID-19 Hospital in Mumbai.
2. Subjects and methods
This case series was approved by the ethics committee of Topiwala National Medical College. Informed consents were obtained from the patients for the publication of this research and any accompanying images. Information of the patients is anonymized in this report.
A total of 22 patients of mucormycosis who were diagnosed during or just after recovery from COVID-19, were included from a single unit of a Dedicated COVID-19 Hospital.
The data was prospectively collected from patients presenting in the first (April 2020 to December 2020) and the second COVID-19 waves (March 2021 to June 2021). Both patients who were diagnosed as mucormycosis during their COVID-19 treatment at our institute, and those who were transferred to our institute for the treatment of mucormycosis, after being treated for COVID-19 at other hospitals, were included. Besides the sociodemographic data, a detailed history was noted, with regards to (1) the day of mucormycosis symptom onset from the day of the first COVID-19 symptom (2) presence of comorbidities (3) detection of new onset diabetes (4) grade of severity of COVID-19 (5) requirement of supplemental oxygen and its duration (6) receipt of antiviral agents such as remdesivir, favipiravir, etc. (7) receipt of steroids,its form and duration (8) receipt of other immunomodulators such as tocilizumab, etc. (9) details of imaging (CT/MRI) of paranasal sinuses, orbit and brain, with emphasis on the number of sinuses involved, bony erosions, orbital, palatal and cerebral involvement(10) microscopy, culture and histopathology reports (11) antifungals given, their duration, route, cumulative dose, associated adverse effects if any (12) surgical interventions including debridement,orbital exenteration and maxillectomy, etc. (13) vaccination status.
3. Results
3.1. Sociodemographic details and comorbidities
A total of 22 consecutive patients of CAM were studied from one unit of the medicine department of a Dedicated COVID-19 Hospital. Six patients (27.27%) presented in the first wave and 16 (72.73%) during the second wave. All the patients had rhinoorbital cerebral mucormycosis. None of the patients had pulmonary mucormycosis or involvement of any other site. Seventeen were males (77.27%) and 5 were females (22.73%). The mean age was 55.4 years (ranging between 33 to 75 years). Only 6 (27.27%)patients were from Mumbai, while the rest had come to Mumbai for treatment of mucormycosis (72.73%). Thirteen (59.09%) patients were known diabetics for more than 5-10 years, with their diabetes being well controlled on oral hypoglycemic agents or insulin, prior to the COVID-19 diagnosis. Out of these 13 patients, 10 had only diabetes, 2 had hypertension in addition to diabetes and 1 patient had chronic kidney and liver disease in addition to hypertension and diabetes. Nine patients (40.91%) were newly detected to have diabetes, out of which 2 had hypertension, 2 had ischaemic heart disease and 5 had no known comorbidities prior to this (Table 1).None of the patients had haematological malignancy or a history of organ transplant.

Table 1. Sociodemographic and COVID-19 details.
Seventeen patients (77.27%) received treatment for COVID-19 elsewhere and were transferred to us for management of mucormycosis, 5 patients were treated for COVID-19 at our institute and developed symptoms suggestive of mucormycosis while in the wards. As far as symptoms related to COVID-19 were concerned, 3 were asymptomatic (cured COVID-19 patients), 7 had mild, 7 had moderate and 5 had severe COVID-19.
3.2. COVID-19 diagnosis
Eighteen patients (81.82%) were positive for SARS-CoV-2 by RTPCR, 1 patient was rapid antigen test (RAT) positive, 3 were RTPCR and RAT negative but had indirect evidence of COVID-19 in the form of compatible symptoms or raised inflammatory markers and raised antibodies to nucleocapsid protein of SARSCoV-2. One of these patients, in addition, had left superior cerebellar artery thrombosis and a large cerebellar infarct. Sixteen out of the 17 patients of COVID-19 associated mucormycosis who were transferred to our institute for the management of their mucormycosis, had turned RT-PCR negative by the time they came. The 5 patients from our institute were diagnosed to have mucormycosis, while they were still RT-PCR positive.
Four out of 22 patients had a CT severity score between 2 and 8(out of 25 points); 4 patients had no involvement on high-resolution computed tomography thorax; 14 patients had a score between 9 and 19.
3.3. Onset of mucormycosis
All of the patients who had symptomatic COVID-19, developed mucormycosis symptoms between 8-15 days (average 12.5 days)of their COVID-19 infection. Two out of 17 (11.76%) transferred patients were in a critical condition due to acute respiratory failure,though they had turned negative in RT-PCR test when diagnosed with mucormycosis. Five out of 22 patients (22.73%) had an ongoing high oxygen requirement (2 transferred patients, 3 from our institute) at the time of the diagnosis of mucormycosis. All the patients were managed in the wards. The intensive care units were full due to the intensity of the pandemic.
The patients presented with varied symptoms suggestive of mucormycosis, such as unilateral headache, facial pain, decreased vision, eye pain and swelling, unilateral ptosis, nasal stuffiness,epistaxis, loosening of teeth and blackish crusty discharge from the nose (Figure 1). Two patients had Bell’s palsy.

Figure 1. Left eye proptosis and blackish discolouration in a 52-year old female with COVID-19 associated mucormycosis
3.4. Investigations
All but 1 had raised C reactive protein, ranging from 1.5 to 228 mg/dL(median 55.65 mg/dL). The D-dimer was elevated in 17 patients(77.27%). Serum ferritin was also elevated in most, but was greater than 2 500 ng/mL in the 4 patients who had severe COVID-19.Galactomannan and beta-D-glucan assays were not performed in any of the patients.
Glycosylated haemoglobin was elevated in all (95.45%) but 1 patient (HbA1C 5.35%), with levels ranging from 6.3% to 14.7%.All the 22 patients had deranged blood glucose levels during their admission. However, none of the patients were in diabetic ketoacidosis.
3.5. Treatment details of COVID-19
Nine patients (40.91%) did not require any supplemental oxygen,whereas 13 patients (59.09%) required supplemental oxygen ranging from 1 to 15 L, for 5 to 15 days (or death). Two patients had a prolonged oxygen requirement of approximately 25 days.CT pulmonary angiography of 1 patient revealed acute pulmonary thromboembolism. The second patient had no new finding or evidence of progression on CT Thorax. All the patients were delivered oxygen by nasal prongs or bag and mask. Two patients with severe COVID-19 required BiPAP. None of the patients required mechanical ventilation.
Seventeen out of 22 patients (77.27%) received steroids for a duration ranging from 5-30 days (average 9.1 days). Most of the patients received methylprednisolone injection in a dose of 40 mg twice a day, which was switched over to oral prednisolone in tapering doses. One patient received injection dexamethasone. Five patients (22.73%) did not receive any steroids.
Seven out of 22 patients (31.82%) had received remdesivir for 5 days, with 1 of them having received both remdesivir and favipiravir; 1 patient received tocilizumab; and 1 patient received tocilizumab, bevacizumab and convalescent plasma therapy.
3.6. Diagnosis of mucormycosis
Most of the patients underwent imaging of the paranasal sinuses,orbit and brain (CT or MRI) more than once. Seven out of 22(31.82%) had pansinusitis. Most had involvement of more than two sinuses (Figure 2). In single sinus involvement, maxillary sinus was the most commonly involved one. All but 1 patient had unilateral involvement. Ten out of 22 patients (45.45%) had bony erosions(Figure 3A) and 1 patient (4.55%) had involvement of the skull base. Twelve out of 22 patients (54.55%) had orbital involvement on imaging studies (Figure 3B), 3 (13.64%) had palatal ulcer (Figure 4)and 4 patients (18.18%) had cerebral involvement (Figure 3C).
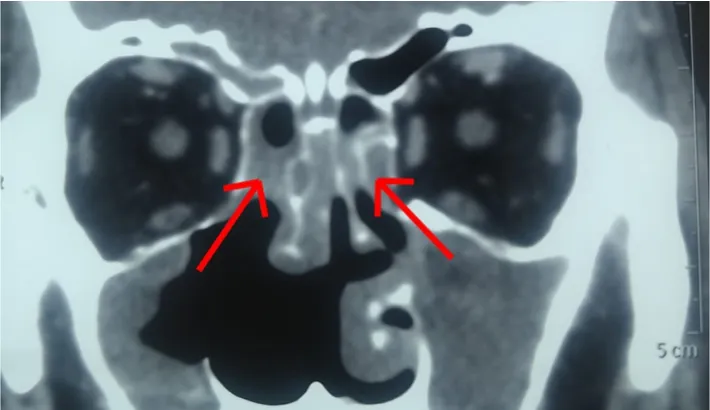
Figure 2. CT paranasal sinuses with bilateral sinusitis (arrows) in a 63-year old male with COVID 19 associated mucormycosis who had blackish nasal discharge on 12 days post COVID-19.

Figure 3. CT images of patients with COVID-19 associated mucormycosis. (A) CT paranasal sinuses showing right maxillary sinus involvement with bone erosion (arrow) in a female patient with COVID-19 associated mucormycosis. (B) CT showing orbital bone involvement (arrow) in a 62-year old male patient with COVID-19 associated mucormycosis on day 22 post COVID-19. (C) CT brain showing right temporal abscess (arrow) in a 56-year old male patient with COVID-19 associated mucormycosis, after 2 months of amphotericin B.

Figure 4. Palatal ulcer in a 54-year old male patient of COVID-19 associated mucormycosis, who also had left Bell’s palsy.
Potassium hydroxide mount showed broad aseptate hyphae suggestive of mucormycosis in 9 out of 21 patients (42.86%)in whom the reports were available. Both septate and aseptate hyphae were seen in 1 patient (4.76%). Eleven patients (52.38%)had no hyphae seen. Histopathology showed acute invasive fungal sinusitis with aseptate hyphae in 12 out of 22 patients (54.55%),with 1 patient having both septate and aseptate hyphae. Rhizopus was grown in 5 out of 20 patients (25.00%) in whom reports were available. Aspergillus did not grow in any specimen.
3.7. Treatment of mucormycosis
Twenty-one patients (95.45%) received conventional amphotericin B and 1 (4.55%) received liposomal amphotericin B. In addition,8 out of 22 (36.36%) received posaconazole delayed release tablets (PCZ DRT). Fourteen out of 22 (63.64%) received a single antifungal, 7 (31.82%) received 2 antifungals (AMB+PCZ)concurrently and 1 (4.54%) received 2 antifungals sequentially(AMB switched over to PCZ). On certain occasions, there was a switchover between liposomal and conventional AMB, depending on the availability of the antifungals, after adjustment of the dose.The cumulative dose of amphotericin B received was between 3.5 and 8 g. The total duration of amphotericin B ranged from 4 to 12 weeks. None of the patients received isavuconazole or intravenous posaconazole. Posaconazole serum levels were not performed in any of the patients due to financial constraints.
Infusion reactions such as fever and chills were commonly observed, however, none of the patients required discontinuation of therapy. Hypokalemia and hypomagnesemia too were observed in many, but were correctible. Only 2 out of 22 (9.09%) had azotemia,where renal correction of amphotericin B was given and 1 (4.55%)had thrombocytopenia due to therapy. None required dialysis.
Most of the patients underwent diagnostic nasal endoscopy and/or functional endoscopic sinus surgery and surgical debridement more than once. Patients who were critical or RT-PCR positive could not undergo the procedure. One patient was advised orbital exenteration but refused. Dental opinion was taken in the patients with palatal ulcer and 2 of the patients were advised maxillectomy.
3.8. Outcome
Six out of 22 patients (27.27%) died, 2 in the first wave and 4 in the second wave. The first had indirect evidence of COVID-19,with RT-PCR and RAT being negative. Four patients had severe COVID-19 with acute respiratory distress syndrome at the time of mucormycosis diagnosis, which led to the fatality.
None of the patients were vaccinated except one male who had received the first dose of Covishield 3 weeks prior to his COVID-19 diagnosis (Table 1 and 2).

Table 2. Characteristics of COVID-19 associated mucormycosis patients.
4. Discussion
There are increasing case reports of rhino-orbital cerebral mucormycosis in patients with COVID-19, especially from India.COVID-19 infection, its treatment, resultant immunosuppression,and pre-existing comorbidities have made the patients vulnerable to secondary infections including mucormycosis[2]. Other theories put forward to explain the dubious association between COVID-19 and mucormycosis are mutation of the virus, a role of iron metabolism,use of industrial oxygen, overzealous use of steam inhalation leading to drying up of the nasal mucosa, reuse of masks, zinc supplement overuse and lack of vaccination.
In a systematic review of cases of CAM including 101 cases, 82 cases were reported from India and just 19 cases from the rest of the world[2]. A majority (77.27%) of the patients in our study were expectedly males, as males have been preferentially targeted by SARS-CoV-2[2-5]. Majority of our patients (72.73%) had come to Mumbai from other parts of Maharashtra, for the management of post COVID-19 mucormycosis. This observation has been made by other physicians too, the possible reasons being the inappropriate and prolonged use of steroids, failure to monitor the associated hyperglycemia, high fungal spore burden in the air in peripheral towns and districts and the presence of the Delta plus variant.
Three of the patients (13.63%) were regarded as asymptomatic(who were possibly cured of COVID-19) and had indirect evidence of COVID-19 in the form of elevation of antibodies to nucleocapsid protein, raised inflammatory markers, new onset diabetes or thrombosis in the absence of traditional risk factors. Since CAM has been observed in asymptomatic and mild cases too and RT-PCR may be false negative due to various reasons, one must have a high index of suspicion and try to establish the diagnosis of COVID-19 by indirect means, if necessary.
All the symptomatic COVID-19 patients developed mucormycosis symptoms, between 8 to 15 days (average 12.5 days) of COVID-19 symptoms. A similar finding was observed in a multicentric epidemiological study by Patel et al., where 84.2% of the patients were identified as late CAM, i.e. presenting >8 days after the onset of COVID-19 symptoms[6-8]. Most patients had recovered from their COVID-19 symptoms and had turned RT-PCR negative. Only 5 out of 22 (22.73%) had an ongoing oxygen requirement at the time of the diagnosis of mucormycosis. In the study by Patel et al.,1.6% of the CAM patients were in the ICU[6]. Mekonnen et al. have reported a patient of CAM with ARDS, where the mucormycosis developed 9 days after the COVID-19 symptoms[8].
Diabetes mellitus (DM) was the commonest comorbidity observed in our study, similar to other studies[2,6,9]. Thirteen (59.09%) had pre-existing long standing DM and 9 (40.90%) had new onset diabetes. In the multicentric study by Patel et al., new onset DM was observed in 20.9% of the CAM subjects[6]. A bidirectional relationship exists between COVID-19 and diabetes, which is fuelled by the exuberant use of steroids, the only treatment known to confer mortality benefits[10,11]. Low pH due to acidosis is a fertile media for mucor spores to germinate and diabetic ketoacidosis is a risk factor for mucormycosis, however, none of our patients had diabetic ketoacidosis[2]. The study by Patel et al. observed that the incidence of diabetic ketoacidosis is much lesser in CAM patients than in non CAM patients[6].
COVID-19 was the only underlying disease in 5 out of 22 patients(22.72%), while a figure of 32.6% has been reported by Patel et al[6].
Steroids have been the common culprit in practically all the case of CAM which have been reported. Mishra et al. have reported steroid usage in 60% of CAM patients, Singh et al. have reported 76.3% usage, whereas it was 77.27% in our study[6,9,12].
The Recovery trial has shown mortality benefits attributed to steroids, provided it is used in hypoxic patients, in the appropriate dose (6 mg dexamethasone or equivalent) and for the appropriate duration (10 days or lesser)[11]. However, the rampant misuse of steroids in COVID-19 has aggravated the already existing hyperglycemia and fuelled the fungal infection[2]. Both long term and short term use of corticosteroids have been associated with several opportunistic fungal infections, especially in people with DM[13,14]. Patel et al., in their study, have concluded that inappropriate steroid use along with the presence of hypoxia was associated with the development of late CAM[6].
The concurrent use of immunomodulatory drugs like tocilizumab(2 patients), bevacizumab (1 patient) possibly added to the immunosuppression caused by steroids.
There are reports implicating the use of industrial oxygen in CAM in the study by Patel et al., 55.6% of the patients were hypoxic in their study and 59.09% in our study[6]. It is possible that steroids also have a causative role here, as they are indicated in hypoxic patients.
Five (22.72%) patients of CAM in our study, did not receive any steroids and 9 (40.90%) patients did not require supplemental oxygen. Additionally, 10 patients were asymptomatic or had mild disease, thus suggesting that mucormycosis may be seen in mild COVID-19 patients without underlying comorbidities, who have not received steroids or oxygen. In fact, the first patient of CAM in the first wave, presented with mucormycosis and was incidentally detected to be RT-PCR positive. Revannavar SM et al. have reported a similar case of CAM with incidental RT-PCR positivity[15]. In a systematic review by Garg et al., 2 patients presented in a similar manner[9].
This is concerning and highlights, firstly, the major contribution of hyperglycemia due to SARS-CoV-2 and the stress of infection[10].Secondly, hyperglycemia induced availability of free iron, which is an ideal resource for mucor[2]. Furthermore, interleukin 6 also increases free iron by increasing ferritin levels[2]. High ferritin level in COVID-19, which is viewed as an inflammatory marker, may have a more sinister role to play. The role of serum ferritin and iron metabolism in CAM needs to be studied in larger randomized controlled trials.
All but one patient in our study had raised C reactive protein (mean 60.33 mg/dL), an inflammatory marker which is elevated in both inflammation and infection. In a study on molecular epidemiology in zygomycosis by Parisa et al., 73.6% of patients of mucormycosis had CRP >6 mg/L, with the range being 2-150 mg/L[16]. Since CRP elevation is a part of the inflammatory response in COVID-19, it was difficult to assess the contribution of the fungal infection to the rise in CRP. However, the CRP was higher in the severe COVID-19 patients.
Both microscopy and histopathology showed aseptate and septate hyphae in 1 patient, though culture grew only Rhizopus in 5 out of 20 (25.00%) patients in whom the culture reports were available. Mucorales are fragile and any mishandling or grinding of the specimen, may destroy the hyphae and cultures may also be negative due to unviable organism in necrotic tissues[17]. Aspergillus was not grown in any specimen. We have a series of patients who have grown isolated Aspergillus in post COVID-19 rhino-orbital involvement and also patients who have grown both Mucor and Aspergillus and one must be cautious in starting voriconazole,as it has no effect on mucor and in fact can cause breakthrough mucormycosis[18].
The commonest sinus involved was the maxillary sinus, 54.54% had orbital involvement and 18.18% had cerebral involvement.Sharma et al. in their case series of 23 CAM patients, found the ethmoids (100%) to be the commonest sinus affected, intra-orbital extension in 43.47% of cases, while intracranial extension was only seen in 8.69%[19]. Disease extension was observed in most patients despite antifungals. Combination therapy with amphotericin B and PCZ was given to 7 (31.82%) patients in view of disease progression. The role of combination therapy for mucormycosis is not conclusively proved except as salvage therapy and lipid polyeneechinocandin therapy is the most promising regimen, which has been studied in trials[20]. Patel et al., have also reported a higher use of combination therapy (50%) in their CAM subjects, contrary to the prevailing practice and attribute it to the apprehension associated with COVID-19[6]. Six patients were given retrobulbar injections of amphotericin B deoxycholate, in addition to systemic antifungals.
Six out of 22 (27.27%) patients died in our series. Four out of the 6 deaths could be attributed to the acute respiratory distress syndrome due to COVID-19. Sarkar et al. have reported a mortality of 40% in their CAM case series[21]. In our study, neither extensive involvement of organs due to mucormycosis nor severity of COVID-19 were statistically significant predictors of outcome.
The pandemic was a challenging time and there was a steep rise in cases of CAM. This led to an acute shortage of antifungals,including conventional and liposomal amphotericin B and isavuconazole. The presence of active COVID-19 and its associated infectivity precluded timely surgical intervention in some patients.Though mild and moderate cases are deemed to be non-infectious after day 10 and the RT-PCR positivity thereafter may reflect shedding of non-viable virus, this proved to be an obstacle to surgical debridement in some situations.
Majority of the patients were not vaccinated, as the general public became eligible for vaccination only in March end. There is an unprecedented rise in COVID-19 associated mucormycosis and this holds an enormous public health significance due to the associated fatality[22]. India is gearing up for an anticipated third wave and has to prepare itself for the shortfall of various drugs and infrastructure and CAM has dealt a severe blow by burdening us with the additional task of procuring expensive antifungals which have to be delivered for prolonged periods. Further challenges are posed by the highly infectious and transmissible nature of the SARS-CoV-2, which prevents timely surgical intervention, due to the fear of transmission of the virus to healthcare workers. The dual consideration of the optimal time of surgery to debulk such patients,in the presence of COVID-19 and to reduce the risk of transmission to the operating team is a debatable issue. The ideal duration of therapy, the ideal route of antifungals, the role of combination therapy, the number of surgical debridements (which may be disfiguring) needed, are all questions which need urgent answers, if we are to win this war against CAM.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Authors’ contributions
M.V. K. collected the data, analysed the data and drafted and typed the final manuscript; K. B. helped in collecting the data and the images and in drafting the article; A. B. helped in collecting the data and the images and in drafting the article.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Recent emergence and outbreak of rotavirus gastroenteritis in Samoa: A scoping review of risk factors, containment measures and public health preparedness
- Tick-borne pathogens in Iran: A meta-analysis
- Intracellular calcium ions facilitate dengue virus entry into endothelial cells and compromise endothelial barrier integrity
- Vaccine induced thrombotic thrombocytopenia: Coagulation after administration of COVID-19 vaccine
