Intracellular calcium ions facilitate dengue virus entry into endothelial cells and compromise endothelial barrier integrity
2021-12-20MengHooiShuPooiFongWongSingSinSamShihKengLoongBoonTeongTeohSazalyAbuBakar
Meng-Hooi Shu, Pooi-Fong Wong, Sing-Sin Sam,3, Shih-Keng Loong,3, Boon-Teong Teoh,3, Sazaly AbuBakar,3✉
1Tropical Infectious Diseases Research and Education Centre (TIDREC), Universiti Malaya, 50603 Kuala Lumpur, Malaysia
2Department of Pharmacology, Faculty of Medicine, Universiti Malaya, 50603 Kuala Lumpur, Malaysia
3World Health Organization Collaborating Centre for Arbovirus Reference and Research (Dengue and Severe Dengue) MAA-12, Universiti Malaya,50603 Kuala Lumpur, Malaysia
ABSTRACT
Objective: To investigate the involvement of Ca2+ in dengue virus(DENV)-infected human umbilical vein endothelial cells (HUVECs)and the disruption of endothelial integrity.
Methods: HUVECs were infected with DENV-2 in the presence of intracellular Ca2+ or endoplasmic reticulum Ca2+ chelators. Virus infectivity was measured by focus-forming assay and quantitative RT-PCR. Intracellular Ca2+ was measured using Fluo-4-AM dye.VE-cadherin and focal adhesion kinase (FAK) expressions were investigated by immunofluorescence and immunoblotting assays,respectively.
Results: DENV infection increased intracellular cytosolic Ca2+levels and caused disassembly of the adherens junction protein, VE-cadherin as evidenced by decreased VE-cadherin expression at the periphery of DENV-2 infected HUVECs. Depletion of intracellular Ca2+ stores, particularly those of the endoplasmic reticulum Ca2+,significantly decreased DENV yield in HUVECs. Decreased virus yield following the depletion of intracellular Ca2+ was caused by the inhibition of viral entry into HUVECs and not the inhibition of viral binding or attachment. DENV-2 infection also resulted in Ca2+-dependent activation of FAK.
Conclusions: Intracellular Ca2+ is required for the early phases of DENV infection in endothelial cells. Increased cytosolic Ca2+ levels in endothelial cells during DENV infection activated FAK, disrupted adherens junctions and compromised barrier integrity. Thus, Ca2+plays an important role in DENV infection in endothelial cells.
KEYWORDS: Endothelial cells; Calcium signalling; Dengue virus;Endothelium permeability; Intracellular calcium
Significance
Viruses modulate Caand calcium signaling for viral entry and replication. This study showed that increased intracellular Castores from the endoplasmic reticulum is vital for DENV entry and replication in endothelial cells. DENV infection compromised the integrity of endothelial barrier, with loss of VE-cadherin expression. FAK activation by DENV and loss of endothelial barrier integrity is Ca-dependent. Thus, DENV disrupts Cahomeostasis to increase permeability and cross endothelial barriers.
1. Introduction
Dengue is a mosquito-borne febrile illness endemic in many tropical and subtropical countries. The World Health Organization(WHO) estimated that dengue is now present in over 128 countries with over 3.9 billion people at risk of getting infected[1]. It is also estimated that over 390 million dengue cases are reported annually of which, 96 million clinically manifest the severe forms of dengue[2].Dengue virus (DENV) infection can cause clinical manifestations with increasing severity ranging from mild dengue fever to dengue hemorrhagic fever (DHF) and dengue shock syndrome. High fever,haemorrhagic phenomena, hepatomegaly and circulatory failure are four typical clinical features of DHF. Thrombocytopenia with concurrent haemoconcentration is a distinctive laboratory finding of DHF. In addition, evidence of vascular plasma leakage which include high haematocrit, hypoproteinemia, ascites and pleural effusion are normally present in patients with severe DHF[3].
Hypocalcemia was reported in patients with severe DHF[4].Changes in plasma calcium ions (Ca) reflect the importance of Caas a second messenger that regulates almost all aspects of cellular processes that are affected during DENV infection[5,6]. Alterations in host cell Cahomeostasis have been well documented in other viral infections[7-9]including flavivirus infections[10-12]. It is suggested that the complex interplay between viruses and Cain the infected host cell can occur via the following mechanisms: (1) viral proteins directly or indirectly disturb Cahomeostasis by altering membrane permeability and/or manipulating key components of the Ca-signaling apparatus; (2) viral proteins directly bind to Cafor structural integrity or functionality; and (3) alteration of cellular Ca-regulated proteins or pathways for critical virus-host interactions[9].
Several flaviviruses are known to stimulate and increase the influx of cytoplasmic Caduring infection either by virus-induced influx of extracellular Caor release of Cafrom internal stores. Elevation of cytoplasmic Caconcentration can promote virus binding or attachment, entry, uncoating and/or replication[10-13], virus maturation[14], cytopathic effect[15], interaction of viral particles with the cell membrane by fusion of membranes[16]and/or also capsid penetration[17,18]. The importance of Cainflux for viral infectivity was further exemplified in a study by Donate-Macian et al. which showed that the inhibition of calcium-permeable, non-selective transient receptor potential vanilloid 4 (TRPV4) cation channelmediated Cainflux leads to the inhibition of DEAD-box RNA-binding helicase, which is typically hijacked by RNA viruses for nuclear viral export and translation and the overall infectivity of dengue, hepatitis C and Zika viruses[19].
Cyclophilin-binding ligand regulates intracellular Calevels[20]and is involved in Ca-mediated signalling pathways[21]. Thus far, it is known that DENV manipulates intracellular Calevels and cyclophilin-binding ligand in human cells to protect cells from undergoing apoptosis which in turn favours efficient virus production[20]. Calmodulin is a multifunctional intermediate messenger protein that binds calcium ions and transduces Casignals by causing its confirmational change to interact with various target proteins[22]. Ca-calmodulin complex was found to be important for DENV replication as inhibition of calmodulin with its antagonist W-7 inhibited NS1 secretion, viral RNA, protein synthesis and overall viral yield[23]. Ubiquitin ligase E3 component N-recognin 4 is a member of a family of predicted E3 ligases which is dependent on the function of Ca-calmodulin. It was shown that ubiquitin ligase E3 component N-recognin 4-mediated STAT3 degradation by DENV NS5 inhibits anti-viral type 1 interferon response[24]. In addition, the Ca-calmodulin dependent proteins kinaseⅡ(CaMK-Ⅱ) is also important for phosphorylation and reorganization of vimentin needed for DENV replication[25]. In this regard, inhibitors of CaMK-Ⅱ such as benzenesulfonamide derivatives have been shown to significantly inhibit CaMK-Ⅱactivity, leading to the inhibition of DENV and ZIKV infections of human neuronal BE(2)-C cells. Besides that, the viremia levels were markedly reduced and experimented animals survived longer in mouse-challenge models[26]. Reduction of Cain the trans-Golgi network also affects protease activity required to process viral glycoprotein of flaviviruses[27]. These suggest that viruses alter Calevels and Casignalling to aid their entry and/or replication.
Alteration in the membrane permeability and factors leading to endothelium dysfunction are possible mechanisms contributing to the vascular plasma leakage in severe dengue[28,29]. The role of Caon DENV-induced vascular permeability remains to be clarified.The present study sought to investigate how DENV modulates Cahomeostasis to facilitate its infection of human endothelial cells and the disruption of endothelial barrier integrity. Findings from this study revealed that DENV-2 increases Cainflux into endothelial cells, the importance of intracellular Castores for the production of infectious viral particles and finally, Ca-mediated kinases activation and adherens junction disruption compromising barrier integrity during DENV infection.
2. Materials and methods
2.1. Cell line
Human umbilical vein endothelial cells (HUVECs) were passaged in endothelial cell medium (ECM) containing 5% heat-inactivated fetal bovine serum (FBS), endothelial cell growth supplement and penicillin/streptomycin (ScienCell Research Laboratories,California, USA) at 37 ℃ in a humidified incubator with 5% CO.Cells were cultured for not more than five passages when used in the experiments to ensure similar cell passage number and to avoid inclusion of senescent cells[30]. African green monkey kidney epithelial (Vero) cells were cultured in Eagle’s minimum essential medium (EMEM) containing 10% FBS at 37 ℃ in a humidified condition with 5% CO. Aedes albopictus mosquito (C6/36) cells maintained in the Department of Medical Microbiology, Faculty of Medicine, Universiti Malaya were used. C6/36 cells were maintained in similar medium as Vero cells but were incubated at 28 ℃ with 3% CO.
2.2. Preparation of virus stock and calcium-free virus stock
C6/36 cells were infected with the clinical isolate of DENV type-2 (DENV-2) strain MY91-99133 (Department of Medical Microbiology, University Malaya Medical Centre Virology Repository) for 1 h at room temperature with constant gentle agitation to allow virus adsorption. Following that, virus inoculum was discarded, replenished with fresh EMEM containing 2% FBS and incubated for 7 days. For virus stock preparation, DENV-2 infected culture supernatant was centrifuged at 2 000 × g to remove cell debris, filtered and stored at -80 ℃ for subsequent use. For Ca-free virus stock preparation, DENV-2 infected culture supernatant was firstly subjected to centrifugation at 2 000 × g, followed by ultracentrifugation at 105 000 × g at 4 ℃. The virus pellet was washed, resuspended with Ca-free ECM, filtered and stored at-80 ℃. Both virus stocks were titrated by focus-forming assay.
2.3. Determination of drugs cytotoxicity by 3-(4,5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide(MTT) assay
HUVECs were seeded into 96-well cell culture plates at a density of 5 × 10cells/well. On the following day, medium containing either the intracellular Cachelator, 1,2-bis-(2-aminophenoxy)ethane-N,N,N',N'-tetra acetic acid tetra (acetoxy methyl) ester(BAPTA-AM) (Sigma-Aldrich, Missouri, USA), at concentrations ranging from 0-200 μg/mL or endoplasmic reticulum Cachelator,2-aminoethoxy diphenylborinate (2-APB) (Sigma-Aldrich, Missouri,USA), at concentrations ranging from 0-50 μg/mL were added into the cells and incubated for 24 h. Media containing only the chelator or blocker diluents were used as vehicle control. Following treatment, the quantification of HUVECs proliferation rate was determined using the CellTiter 96 Non-Radioactive Cell Proliferation Kit (Promega, Wisconsin, USA) following the manufacturer’s recommended protocol.
2.4. Treatment of virus-infected cells with drugs
HUVECs were seeded into 24-well cell culture plate and incubated overnight. On the following day, the confluent cell monolayer was incubated with Ca-free ECM in the absence or presence of either the chelator or blocker. Cells were then infected with Ca-free DENV-2 at a multiplicity of infection (MOI) of 3 in the absence or presence of the same concentration of the chelator or blocker.Following infection, cells were washed thrice to remove unadsorbed virus and then incubated with either fresh Ca-free ECM containing 2% FBS (Ca-free medium) or fresh non-Ca-free ECM containing 2% FBS (complete medium) for 48 h at 37 ℃. The culture supernatant was then harvested to measure virus infectivity by focusforming assay and quantitative reverse transcription-polymerase chain reaction (RT-PCR).
2.5. Focus-forming assay
Focus-forming assay was performed to titrate DENV-2 using the Vero cells as previously described[31,32].
2.6. Quantitative RT-PCR
Quantitative RT-PCR amplification was performed to quantitate DENV-2 RNA copy number using a customized one-step TaqMan real-time RT-PCR (Applied Biosystems, California, USA) following the manufacturer's recommended protocol.
2.7. Cell viability assay using trypan blue exclusion assay
HUVECs were seeded into 96-well cell culture plate and incubated overnight. On the following day, the confluent cell monolayer was treated and infected as described above. At the end of the incubation,cells were washed with phosphate buffered saline (PBS) and stained with 0.4% trypan blue (Sigma-Aldrich, Missouri, USA). The number of viable cells that were not stained (colourless) and the number of non-viable cells that were stained blue were counted using an inverted microscope (Nikon, New York, USA).
2.8. Transmission electron microscopy
Confluent HUVECs monolayer was incubated with Ca-free ECM in the presence of 50 μg/mL BAPTA-AM. Cells were then infected with Ca-free DENV-2 at MOI of 3 in the presence of the same concentration of the BAPTA-AM. For binding or attachment assay,cells were harvested by trypsinisation and pelleted by centrifugation.For entry assay, cells were incubated with fresh Ca-free ECM containing 2% FBS. The cells were then harvested as previously described. Non-infected and non-treated cells were included as negative controls.
The pelleted cells were fixed with 4% glutaraldehyde (Agar Scientific, Essex, United Kingdom) in 0.1 M sodium cacodylate buffer (Agar Scientific, Essex, United Kingdom) overnight at 4 ℃. Following that, the cell pellets were washed with distilled water and dehydrated with increasing concentrations of ethanol(Merck Millipore, Massachusetts, USA). The cell pellets were then embedded in epoxy resin for 5 h at 37 ℃ and polymerized overnight at 60 ℃. Subsequently, the embedded block was cut into ultrathin sections using a diamond knife (Diatome, Pennsylvania,USA) on an EM UC7 Ultramicrotome (Leica, Hesse, Germany).Sections were then mounted on a 200 mesh copper grid (Ted Pella,California, USA) and stained with 8% uranyl acetate (Polyscience,Pennsylvania, USA), followed by imaging using a HT7700 Transmission Electron Microscope (Hitachi, Tokyo, Japan)[33].
2.9. Ca2+ measurements
HUVECs were seeded into 96-well cell culture plate. On the following day, the cell culture medium was removed and the cells were incubated with 2 μg/mL Hoechst dye (Invitrogen, New Hampshire, USA) for 30 min. Following incubation, the Hoechst dye was discarded and the cells washed with Hanks’ balanced salt solution (Sigma-Aldrich, Missouri, USA), loaded with 2 μM Fluo-4-AM (Invitrogen, California, USA), followed by infection with DENV-2 or Ca-free DENV-2 at MOI of 0, 1, 3 and 5. The intensity of Fluo-4-AM dye in the cells were captured and recorded using the Operetta High-Content Imaging System (PerkinElmer,Massachusetts, USA) at 15, 45 and 105 min.
2.10. Immunoblotting
HUVECs were seeded into 75 cmcell culture flask at a density of 1 × 10cells/flask and incubated overnight. Following that, for FAK phosphorylation assay, the confluent cell monolayer was incubated with Ca-free ECM containing calcium chloride (CaCl) or without CaClin the absence or presence of the Ca-free DENV-2 at MOI of 3 or ECM in the absence or presence of the DENV-2 at MOI of 3 for 15, 30 and 120 min.
For VE-cadherin assay, the confluent cell monolayer was incubated with Ca-free ECM with or without BAPTA-AM for 2 h. Cells were then infected with Ca-free DENV-2 at MOI of 3 for 2 h.Following infection, cells were washed and then incubated with fresh Ca-free ECM containing 2% FBS for 24 and 48 h. At the end of the incubation, cells were washed in cold PBS and pelleted by centrifugation. Total cellular proteins were then extracted by incubating the cells with RIPA Lysis buffer (Santa Cruz Biotechnology, Texas, USA) for 45 min on ice. Protein concentration was quantified using the Micro BCA Protein Assay Kit (Thermo Fisher Scientific, Massachusetts, USA) following the manufacturer’s instructions.
Total cellular proteins (30 μg/mL) were separated on 8% (for protein size between 40-250 kDa), 10% (for protein size between 30-200 kDa), 12.5% (for protein size between 20-150 kDa) and 14%(for protein size between 10-80 kDa) SDS-PAGE gel under reducing conditions and electro-transferred onto nitrocellulose membrane(Merck Millipore, Massachusetts, USA). Membrane was then blocked with 3% bovine serum albumin (Sigma-Aldrich, Missouri,USA), overnight at 4 ℃. After blocking, membrane was washed with tris-buffered saline (TBS) containing 0.05% Tween 20 Detergent (T)(Merck Millipore, Massachusetts, USA). The membrane was then incubated with 1:1 000 dilution of rabbit monoclonal FAK antibody(Cell Signaling Technology, Massachusetts, USA) or 1:1 000 dilution of rabbit monoclonal phosphorylated FAK (pFAK) [tyrosine 397(Tyr397)]antibody (Cell Signaling Technology, Massachusetts,USA) or 1:5 000 dilution of mouse monoclonal β-actin antibody(Sigma-Aldrich, Missouri, USA) or 1:1 000 dilution of mouse monoclonal VE-cadherin antibody (Merck Millipore, Massachusetts,USA). The membrane was subsequently washed thrice prior to incubating with 1:1 000 dilution of horseradish peroxidase conjugated goat anti-rabbit IgG antibody (Calbiochem, Merck Millipore, Massachusetts, USA) or 1:1 000 dilution of horseradish peroxidase conjugated goat anti-mouse IgG antibody (Calbiochem,Merck Millipore, Massachusetts, USA). Thereafter, the membrane was washed thrice with TBS-T, twice with TBS and developed using the Metal-Enhanced DAB Substrate Kit (Thermo Fisher Scientific,Massachusetts, USA) strictly following the manufacturer’s recommended protocol. The protein of interest band intensity was quantitated using ImageJ (National Institute of Health, Maryland,USA). The level of FAK phosphorylation was determined as the ratio of phosphorylated FAK to the total FAK, whereas the level of VE-cadherin expression was determined as the ratio of VE-cadherin to β-actin.
2.11. Immunofluorescence staining of VE-cadherin
Confluent HUVECs monolayer were infected with DENV-2 at MOI of 0 and 5 for 2 h. Following infection, cells were washed and then incubated with fresh ECM containing 2% FBS for 48 h. Cells were then washed with PBS and fixed with 4% paraformaldehyde (Sigma-Aldrich, Missouri, USA). After fixing, cells were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, Missouri, USA), washed and blocked with 3% bovine serum albumin (Calbiochem, Merck Millipore, Massachusetts, USA). The cells were subsequently washed and immunolabelled with 2 μg/mL mouse monoclonal antihuman VE-cadherin antibody (Merck Millipore, Massachusetts,USA). After three washes, 6 μg/mL Alexa Fluor 555 goat anti-mouse IgG (heavy and light chains) (Molecular Probes, Oregon, USA) were added to the cells. Cells were then washed thrice and stained with 2 μg/mL Hoechst dye for 5 min. The cells were viewed and images were captured using the Operetta High-Content Imaging System(PerkinElmer, Massachusetts, USA).
2.12. Statistical analysis
The data from the cytotoxicity assay, cell viability assay, focusforming assay, quantitative RT-PCR, Cameasurements and immunoblotting (ratio of phosphorylated FAK to total FAK) were expressed as mean ± standard deviation (SD) from repeated assays.The data were subjected to two-way analysis of variance (ANOVA)and Bonferroni’s post-test to assess statistical significance. All the statistical analyses were performed using GraphPad Prism 4.0 software (California, USA).
3. Results
3.1. DENV-2 infection causes Ca2+ influx and disassembly of VE-cadherin on HUVECs
A calcium fluorescence-based flux assay was used to assess Camobilization following DENV infection. Fluo-4-AM diffuses rapidly into cells and binds free cytosolic Caand fluoresces. In the absence of Cain media, there was no significant increase in the intensity of Fluo-4-AM for both mock- and DENV2-infected HUVECs.However, in the presence of extracellular Ca, DENV-2 infection significantly increased the mean intensity of Fluo-4-AM levels at 15 and 45 min compared to the mock-infected cells (Figure 1A).These results show that DENV infection increased the influx of extracellular Cainto cells.

Figure 1. DENV-2 infection causes Ca2+ influx and VE-cadherin disassembly. (A) Fluo-4-AM fluorescence intensity and Ca2+ influx in infected HUVECs.(B) VE-cadherin fluorescence distribution (arrows). (C) Virus titer in infected HUVECs. Values are expressed as mean ± SD (n=3). *P<0.05. DENV: dengue virus; MOI: multiplicity of infection; HUVECs: human umbilical vein endothelial cells. a.u.: arbitrary unit.
Immunofluorescence staining of the DENV-2 infected HUVECs revealed multiple large intercellular gaps between adjacent cells and highly irregular shaped cells indicating morphologic alterations of the infected HUVECs monolayer as compared to mock-infected HUVECs which retained normal morphology, cobblestone-like cell shape (Figure 1B). VE-cadherin expression decreased in HUVECs infected with DENV-2 at MOI of 5, resulting in speckles disposition of VE-cadherin at the periphery of the cells compared with the condensed contiguous staining pattern in the mock infected cells progressively over the 72 h infection period. Correspondingly, virus titers had also increased over time with the highest titer observed at 48 h after infection at MOI of 5 (Figure 1C). These results suggest that DENV-2 infection increased Cainflux and disrupted membrane integrity of HUVECs.
3.2. Intracellular Ca2+ chelator affects DENV yield in HUVECs
To determine if intracellular level of Caaffects DENV replication,HUVECs were pretreated with BAPTA-AM prior to and during infection. Extracellular virus at 48 h post infection was titrated using focus-formation and quantitative RT-PCR assays. ICvalue of BAPTA-AM, an intracellular Cachelator was 54.74 μg/mL in HUVECs (Figure 2A). Almost 100% of the HUVECs were still viable following treatments with 20 μg/mL and 50 μg/mL BAPTAAM (Figure 2B) and hence, all subsequent experiments used these two concentrations for treatment. Treatment of HUVECs with 20 μg/ mL and 50 μg/mL BAPTA-AM prior to and during DENV infection, followed by incubation in Ca-free medium significantly reduced the viral titer by ~7-fold [(116.67 ± 7.64) ffu/mL]and~6-fold [(143.33 ± 20.21) ffu/mL], respectively, compared to the viral titer of non-BAPTA-AM-treated infected HUVECs [(850.00± 70.71) ffu/mL](Figure 2C). There were slight dose-dependent reductions in the viral RNA copies from (1 945.35 ± 20.79) copies of non-BAPTA-AM-treated HUVECs to (1 510.65 ± 52.63) copies of 50 μg/mL BAPTA-AM-treated HUVECs (Figure 2D).

Figure 2. Chelation of intracellular Ca2+ decreases DENV yield. (A) Cytotoxicity of BAPTA-AM. (B) Cell viability after treatment. Virus infectivity/replication as determined by (C) focus-forming assay and (D) quantitative RT-PCR. Values are expressed as mean ± SD. (n=3).*P<0.001. DENV: dengue virus; BAPTA-AM: 1,2-bis-(2-aminophenoxy)ethane-N,N,N',N'-tetra acetic acid tetra (acetoxy methyl) ester.
In another set of treated HUVECs that were incubated in complete medium (non-Ca-free) after DENV-2 infection, the viral titer have also significantly reduced by ~2-fold [(495.00 ±99.62) ffu/mL]in the 20 μg/mL BAPTA-AM treatment and by~3-fold [(336.67 ± 40.10) ffu/mL]in the 50 μg/mL BAPTA-AM treatment compared to the viral titer of non-BAPTA-AM treatment[(1 066.67 ± 251.66) ffu/ mL](Figure 2C). In addition, the amount of extracellular DENV-2 specific RNA has significantly reduced by ~2-fold [(2 923.46 ± 74.24) copies of RNA]for the 50 μg/mL BAPTAAM-treated HUVECs compared to the non-BAPTA-AM-treated HUVECs [(6 418.17 ± 813.41) copies of RNA](Figure 2D).
Treatment of HUVECs with 20 μg/mL and 50 μg/mL BAPTAAM prior to DENV infection but not during infection, followed by incubation in Ca-free medium also resulted in slight decreases,with viral titers of [(540.00 ± 88.88) ffu/mL]and [(427.50 ±38.89) ffu/mL], respectively, compared to the viral titer of [(823.33± 75.06) ffu/mL]of untreated infected HUVECs (Figure 2C).The viral RNA copies have also slightly reduced from (2 112.67± 111.35) copies for the non-BAPTA-AM-pretreated HUVECs to(1 671.76 ± 141.17) copies for the 50 μg/mL BAPTA-AM-pretreated HUVECs (Figure 2D). In the same treatment, but with Ca-free medium substituted with complete medium (non-Ca-free) after DENV infection, there were significant reductions in the viral titers and amount of extracellular viral specific RNA by ~4-fold [(273.33± 65.26) ffu/mL]and ~2-fold [(2 659.11 ± 153.46) copies of RNA],respectively, in the 50 μg/mL BAPTA-AM-pretreated HUVECs compared to the viral titer and RNA copies of non-BAPTA-AM-pretreated HUVECs [(1 166.67 ± 202.07) ffu/mL]and (5 307.08 ±188.02) copies, respectively (Figure 2C and 2D). These findings suggest that depletion of intracellular Calevel significantly hampered DENV replication and infectious virus production which can be reversed with Casupplementation.
3.3. Endoplasmic reticulum Ca2+ chelator affects DENV yield in HUVECs
Activation of the phospholipase C inositol 1,4,5-trisphosphate (IP)signaling pathway mobilizes sequestered endoplasmic reticulum Capool. Hence, the IPreceptor antagonist, 2-APB was used to inhibit endoplasmic reticulum Capool mobilization. The ICvalue of 2-APB was 34.54 μg/mL in HUVECs (Figure 3A). More than 85% of the HUVECs were still viable following treatments with 20 μg/mL and 40 μg/mL 2-APB treatments (Figure 3B) and hence,these two concentrations were chosen for all subsequent treatments.The DENV-2 titers produced by HUVECs treated with 20 μg/ mL and 40 μg/mL 2-APB prior to and during infection, followed by maintenance in Ca-free medium were significantly decreased dose-dependently from [(980.00 ± 242.49) ffu/mL]in non-2-APB-treated infected HUVECs to [(45.00 ± 47.70) ffu/mL]and [(0.00 ±0.00) ffu/ mL], respectively (Figure 3C). The amount of extracellular DENV RNA were slightly reduced dose-dependently from (2 646.26± 263.37) copies of RNA for the non-2-APB treated HUVECs to(1 592.02 ± 122.98) copies of RNA for the 40 μg/mL 2-APB treated HUVECs (Figure 3D). In the same treatment in complete medium(non-Ca-free), the DENV-2 titer was significantly decreased by ~13-fold (161.67 ± 36.17) in the 20 μg/ mL 2-APB-pretreated HUVECs and ~1 267-fold [(1.67 ± 2.89) ffu/mL]in the 40 μg/mL 2-APB-pretreated HUVECs compared to the viral titer of non-2-APB-pretreated HUVECs [(2 116.67 ± 275.38) ffu/mL](Figure 3C).The amount of extracellular DENV RNA has significantly reduced by ~3-fold (2 347.11 ± 80.59) copies of RNA for the 20 μg/mL 2-APB-treated HUVECs and (2 407.19 ± 289.32) copies of RNA for the 40 μg/mL 2-APB-treated HUVECs compared to the non-2-APB-treated HUVECs (6 457.37 ± 608.72) copies of RNA (Figure 3D).These findings suggest that inhibition of endoplasmic reticulum Carelease significantly inhibited DENV infectivity and replication resulting in reduced extracellular virus yield. Supplementation of Cacan compensate this inhibition effect but at a lesser extent when a high 2-APB dose is used to deplete endoplasmic reticulum Castores.

Figure 3. Inhibition of Ca2+ mobilization from the endoplasmic reticulum (ER) reduces DENV yield. (A) Cytotoxicity of 2-APB. (B) Cell viability after treatment. Virus infectivity/replication as measured by (C) focus-forming assay and (D) quantitative RT-PCR. Values are expressed as mean ± SD. (n=2).*P<0.001. DENV: dengue virus; 2-APB: 2-aminoethoxy diphenylborinate.
The DENV titers produced by HUVECs pretreated with 40 μg/mL 2-APB and then infected with DENV in the absence of the 2-APB,followed by maintenance in Ca-free medium or complete medium(non-Ca-free) were significantly reduced by ~4-fold [(295.00 ±39.69) ffu/mL]or ~2-fold [(586.67 ± 61.71) ffu/mL], respectively,compared to the virus titer of [(1 066.67 ± 301.39) ffu/ mL]maintained in Ca-free medium or [(1 366.67 ± 104.08) ffu/mL]maintained in complete medium for the non-2-APB-treated infected HUVECs (Figure 3C). The amount of extracellular DENV RNA were slightly reduced dose-dependently from (2 863.23 ± 597.54)copies of RNA in infected HUVECs maintained in Ca-free medium or (3 925.83 ± 460.52) copies of RNA in infected HUVECs maintained in complete medium for non-2-APB treatment to(1 717.40 ± 208.10) copies of RNA in HUVECs maintained in Ca-free medium or (3 298.80 ± 239.18) copies of RNA in HUVECs maintained in complete medium for 40 μg/mL 2-APB treatment(Figure 3D). These findings suggest that endoplasmic reticulum Capool is essential for the viral replication and production of infectious DENV particles.
3.4. Intracellular Ca2+ chelator affects DENV entry into HUVECs
To determine if depletion of intracellular Calevel hampers DENV attachment and entry, the infected cells were visualised using transmission electron microscopy. Clusters of virus-like particles with the typical size of 40-60 nm in diameter were found attached on the HUVECs cell membrane that were pretreated with or without BAPTA-AM and infected in the absence or presence of BAPTA-AM (short black arrows, Figure 4A and 4B). In these cells, the formation of endocytic invagination was detected (data not shown). However, in untreated HUVECs, numerous virus particles containing endocytic vesicles (long black arrow) were present in the cell cytoplasm (Figure 4C), but these vesicles were absent in the presence of BAPTA-AM (Figure 4D). These findings show that intracellular Cais required for viral entry but not attachment.
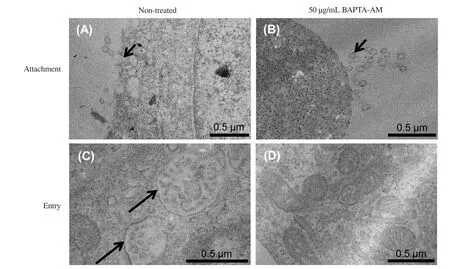
Figure 4. Effects of the inhibition of intracellular Ca2+ on DENV attachment and entry. Confluent monolayer HUVECs pretreated with BAPTA-AM, followed by infection with DENV-2 in the presence of BAPTA-AM. (A-B) Viral binding or attachment and (C-D) viral entry. Black arrows show viral particles. DENV:dengue virus; HUVECs: human umbilical vein endothelial cells; BAPTA-AM: 1,2-bis-(2-aminophenoxy)ethane-N,N,N',N'-tetra acetic acid tetra(acetoxy methyl) ester.
3.5. DENV infection triggers FAK phosphorylation
FAK activation can be mediated by increases in cytosolic Calevels[34]and triggers adherens junction disassembly[35]. In the present study, the expression of phosphorylated FAK, 15 min after DENV-2 infection in medium that contained or supplemented with Cawas significantly increased by ~1-fold [phosphorylated/total protein abundance ratio of (0.58 ± 0.04)]in complete medium,~2-fold [ratio of (0.63 ± 0.09]in Ca-free medium supplemented with 2 mM CaCland ~2-fold [ratio of (0.72 ± 0.01]in Ca-free medium supplemented with 5 mM CaClas compared to in Ca-free medium [ratio of (0.40 ± 0.11)](Figure 5A and 5D). The addition of CaCl(2 mM or 5 mM) into Ca-free medium only contributed a subtle increase in the phosphorylation of FAK at 15 min in mock-infected cells. Nevertheless, the expression of p-FAK was higher at 30 min compared to 15 min in mock-infected cells with CaClsupplementation (Figure 5D). Phosphorylation of FAK was also significantly increased by ~2-fold, 30 min after DENV-2 infection but only in complete medium (phosphorylated/total protein abundance [ratio of (1.04 ± 0.01)]and Ca-free medium supplemented with 5 mM CaCl[ratio of (1.04 ± 0.04)]as compared to in Ca-free medium [ratio of (0.64 ± 0.08)](Figure 5B and 5D).Similarly, 120 min after DENV-2 infection, significant increases in the phosphorylated/total protein abundance ratio of phosphorylated FAK were observed in complete medium (0.58 ± 0.06) and Ca-free medium supplemented with 5 mM CaCl(0.64 ± 0.01) as compared to in Ca-free medium (0.32 ± 0.00) (Figure 5C and 5D). These findings suggest that DENV infection activated FAK signalling in HUVECs and its activation is Ca-dependent.

Figure 5. DENV infection activates focal adhesion kinase (FAK). HUVECs incubated with different medium, CaCl2 supplementation and DENV-2 for (A) 15,(B) 30 and (C) 120 min. In (A-C), (M): molecular weight marker, (1): Ca2+-free medium, (2): Ca2+-free medium + DENV, (3): complete medium, (4): complete medium + DENV, (5): Ca2+-free medium + 2 mM CaCl2, (6): Ca2+-free medium + 2 mM CaCl2 + DENV, (7): Ca2+-free medium + 5 mM CaCl2, and (8): Ca2+-free medium + 5 mM CaCl2 + DENV. (D) Ratio of phosphorylated FAK to total FAK proteins. Values are expressed as mean ± SD. (n=3). *P<0.01. DENV:dengue virus; HUVECs: human umbilical vein endothelial cells; pFAK: phosphorylated FAK; Tyr397: tyrosine 397.
4. Discussion
Cais a highly versatile intracellular messenger in signalling cascades and plays a key role in regulating almost every aspect of cellular processes in the host cell. Although Cais required for the maintenance of endothelial cell integrity, increased cytosolic Cain endothelial cells promotes disruption of endothelial cell barrier[36].Increased Calevel can be mediated through the generation of inositol 1,4,5-triphosphate (IP, activation of IP-receptors, release of stored intracellular Caand entry through plasma membrane channels[37].
DENV infection is known to increase endothelial permeability[38,39],hence, altering the homeostasis of Cain host cells could be its mechanism to facilitate infectivity and crossing of endothelial cell barrier. Indeed, it has been reported that flaviviruses stimulate and increase the influx of cytoplasmic Caduring infection[10-12]by manipulating Casignalling mediators[12,23,25].
In the present study, results showed that intracellular cytosolic Calevels significantly increase progressively in HUVECs infected with DENV at high MOI in medium, particularly in the presence of extracellular Ca. This finding supports the notion of DENV infection increases Calevel and is in agreement with the observation in liver cells infected with DENV[11]and kidney cells infected with West Nile virus[12]. Depletion of intracellular Caby BAPTA-AM resulting in the significant reduction of DENV-2 replication in HUVECs is also in agreement to that observed in liver cells infected by DENV-4[11]. Although Casupplementation after infection also showed significant reduction of DENV replication in HUVECs, the reduction was not as great as the total depletion of intracellular Ca. This finding suggests that Cais essential for DENV infection in endothelial cells and supplementation with extracellular Cacan facilitate production and replication of infectious virions. Efficient DENV replication is dependent on the ability of viral binding or attachment onto the host cell.Hence, we then investigated the effects of intracellular Caon viral attachment and entry using transmission electron microscopy.Depletion of intracellular Causing BAPTA-AM resulted in the significant reduction of DENV replication in HUVECs that was due to the inhibition of viral entry but not viral attachment. These results suggest that intracellular Cais important for viral entry into the endothelial cells and facilitates viral replication in endothelial cells. Collectively, these results support the hypothesis that influx of extracellular Caand increased intracellular Caare important to support the early phase DENV infection in endothelial cells.Cais not only important for efficient DENV infection but is also mobilized by DENV during infection in HUVECs.
Endoplasmic reticulum is the main intracellular storage site for intracellular cytosolic Ca. Using the specific endoplasmic reticulum Cachelator, 2-APB, which is an IPreceptor antagonist that prevents IP-mediated release of endoplasmic reticulum Ca[40,41], we showed that inhibition of endoplasmic reticulum Castores resulted in significant decrease of DENV replication in HUVECs, which is in agreement to that observed in liver cells infected by DENV-4[11]. The production of the infectious virus particles, particularly, was greatly reduced following the depletion of endoplasmic reticulum Ca. This could be due to the interference of calcium-dependent furin activities for prM cleavage, which is known to be important for the virus maturation and infectivity[42,43].Similarly, inhibition of endoplasmic reticulum Castores prior to DENV infection but not during infection or Casupplementation after infection also significantly reduced DENV replication in HUVECs, however, the reduction was more apparent than the total inhibition of endoplasmic reticulum Castores. These results indicate that intracellular Castores from endoplasmic reticulum is important for the early phase of DENV infection in endothelial cells.Endothelial cell integrity is maintained mainly by the adherens and tight junction proteins, in which the tight junction serves as a linker between the adherens junction and the actin cytoskeleton[44-46].VE-cadherin is an adherens junction protein that predominates in adherens junction and plays a critical role in maintaining the endothelial cell-cell contact and monolayer integrity[47,48]. It mediates homotypic calcium-dependent cell-cell interactions[49]. We observed the presence of multiple large intracellular gaps between cells in the monolayer of HUVECs following infection with DENV in addition to a progressive loss of VE-cadherin expression at the periphery of the infected HUVECs with time, as similarly observed in other human endothelial cells[39,46]. These observations suggest that DENV infection triggers adherens junction disassembly and compromised barrier integrity of endothelial cells.
FAK is a non-receptor tyrosine kinase that is localized with integrin receptors at focal contact sites[50]. FAK phosphorylation at Try397 is linked to signal transduction events, hence serves as a binding site for the SH2- and SH3-domain-mediated binding of protein such as sarcoma-family kinases (Src)[34,51-53]. Recruitment and activation Src family kinases, phosphorylates FAK further to enhance FAK activity. FAK and Src phosphorylations are associated with the disruption of tight junction, cytoskeleton organization and attachment of host cell monolayers to maintain cell integrity[54-56].Indeed, research showed that infections with flaviviruses such as West Nile virus, resulted in an early and sustained activation of FAK in mammalian cells. It was suggested that FAK activation contributes to the survival of flavivirus-infected cells which is inversely correlated with the level of caspase-3 cleavage, a marker of apoptosis[12]. A more recent study showed that direct DENV infection caused Src, RhoA and VE-cadherin phosphorylations and increased trans-endothelial cell permeability. Inhibition of Src and RhoA phosphorylations were further shown to abrogate cell permeability and restore the expression of VE-cadherin[39].
Several previous studies reported that FAK phosphorylation increases when intracellular Calevel is increased[57,58]. In the present study, FAK phosphorylation levels were shown to be increased in DENV-infected HUVECs and the increase was greater in DENV-infected HUVECs supplemented with increasing Caconcentration. These observations suggest that FAK activation by DENV infection is Ca-dependent. FAK has been reported to bind to VE-cadherin, triggering adherens junction disassembly[35]and its activation leads to a loss of VE-cadherin-mediated intercellular contacts[59]. Increased FAK activation during DENV infection, thus explains the loss of VE-cadherin expression along the periphery of infected HUVECs.
In summary, findings from the present study illustrated that DENV takes full advantage of intracellular Castores in endothelial cells for efficient DENV entry and replication. Our results also revealed the disruption of endothelial barrier integrity as a result of FAK activation and the loss of VE-cadherin expression is dependent on Ca. Thus, Caplays an important role in DENV infection in endothelial cells, whereby, DENV disrupts Cahomeostasis to increase permeability and cross endothelial barriers.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This study received funding from the Ministry of Higher Education Malaysia via the Higher Institution Centre of Excellence (HICoE)program (MO002-2019) and Development of Research Institute for Excellent Enterprises (ATC+) Project, Republic of Korea (IF001-2021).
Author’s contributions
PFW and SAB conceived the study and designed the experimental approach. MHS conducted all experiments and data analyses. PFW,SSS, SKL and BTT verified data analyses and performed data interpretation. PFW and MHS drafted the manuscript. SSS, SKL,BTT and SAB performed critical revisions to the draft. PFW and SKL prepared the final version for submission.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Recent emergence and outbreak of rotavirus gastroenteritis in Samoa: A scoping review of risk factors, containment measures and public health preparedness
- Tick-borne pathogens in Iran: A meta-analysis
- Post COVID-19 mucormycosis: A case series
- Vaccine induced thrombotic thrombocytopenia: Coagulation after administration of COVID-19 vaccine
