Ethyl acetate extract of Smilax glabra Roxb roots and its major active compound astilbin promote osteoblastogenesis in vitro by upregulating bone cell differentiationassociated genes
2021-12-15HuyenNguyenMinhNguyenThuNguyenQuanPhamHaNguyenPhuongNguyen
Huyen T.T. Nguyen, Minh T.H. Nguyen, Thu X. Nguyen, Quan M. Pham, Ha X. Nguyen, Phuong T.M.Nguyen,4✉
1University of Science and Technology of Hanoi, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam
2Institute of Biotechnology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam
3Institute of Natural Products Chemistry, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam
4Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam
ABSTRACT
Objective: To investigate the osteoblastogenic activity of the ethyl acetate (EtOAc) extract of Smilax glabra Roxb roots and its major active compound astilbin.
Methods: Astilbin was isolated from EtOAc extract using silica gel chromatography combined with fraction crystallization. Chemical structure of astilbin was determined by analysis of the spectroscopic data in comparison with the literature. MTT method was used to detect the toxicity. Alkaline phosphatase (ALP) activity was determined by the spectrophotometric method at 405 nm using p-nitrophenyl phosphate as a substrate. Calcium deposition was stained with alizarin red-S, distained with cetylpyridium chloride,and quantified at 562 nm. In silico model for astilbin-ALP interaction was analyzed using AutoDock 4.2.6. The changes in expression of osteoblast differentiation related genes were determined using quantitative real-time PCR.
Results: Both the EtOAc extract and astilbin had no toxicity toward osteoblast MC3T3-E1 cells at 5.0, 10, 25, and 50 μg/mL. At 25 μg/mL, they enhanced ALP activity and mineralization of osteoblasts up to 30% and 55% for the EtOAc extract and 22% and 41% for astilbin, respectively. Molecular docking analysis of astilbin-ALP interaction revealed Arg167, Asp320, His324, and His437 were key residues participating in hydrophobic interaction; meanwhile, His434 and Thr436 residues were involved in hydrogen bond formation in the active site of human tissue-nonspecific ALP. Moreover, the expression level of genes opn, col1, osx, and runx2 were up-regulated in astilbin treated samples with the fold changes as 2.2; 3.7; 4.1; 2.3,respectively at 10 μg/mL (P<0.05).
Conclusions: The EtOAc extract and its major compound astilbin exhibit osteoblastogenic activity by up-regulating important markers for bone cell differentiation. It could be a new and promising osteogenic agent with dual actions for therapeutic applications.
KEYWORDS: Smilax glabra Roxb; Astilbin; Cytotoxicity;Osteogenesis; Osteoblast; Ethyl acetate extract
1. Introduction
Osteoporosis is a common global disease. Statistics show that over 200 million people worldwide suffer from the disease[1]. The number of people with osteoporosis is increasing in the world, especially older people. The use of current chemical medications to treat osteoporosis has limited effectiveness and side effects for long-term use[1,2]. Therefore, natural substances that can treat osteoporosis and maintain bone strength with fewer side effects are the potential alternatives.
Plants are rich in secondary metabolites, therefore they are promising sources for isolation of novel osteogenic agents that can induce osteoblast differentiation and result in new bone regeneration.The enhancement of alkaline phosphatase (ALP) and mineralization in the osteoblast cells are considered as the two important indicators for osteoblastogenic activity[3,4].
Smilax glabra (S. glabra) Roxb is traditionally used as a medicinal plant in Asia, including Vietnam, for the treatment of various chronic diseases, such as liver deficiency, coronary heart, and syphilis[5]. Our recent report indicated that the ethyl acetate (EtOAc) extract of this plant possessed antidiabetic activity in mice[6]. The major compound in S. glabra is astilbin, a flavonoid glucoside substance. It is known to have anti-inflammatory, antioxidant, and anti-hepatic fibrosis properties[7-9]. Recently, it has been reported to exhibit inhibitory activity against digestive enzymes alpha-glucosidase and alphaamylase[10,11]. For osteogenic activity, Jin et al.[12]recently found that astilbin exhibited osteoblastogenic activity in mice. However,osteoblastogenic activity of S. glabra extract and its constituents has not been investigated yet. This paper presents the results on isolation of astilbin from ethyl acetate extract of S. glabra grown in Vietnam and evaluation of osteoblastogenic activity of EtOAc extract, as well as astilbin in MC3T3-E1 osteoblast cells.
2. Materials and methods
2.1. Plant materials and reagents
The roots of S. glabra Roxb were collected in Thai Nguyen province, Vietnam in September 2016 and identified by Prof. Phan Ke Loc. The voucher specimen (SGR92016) was deposited at the Institute of Ecology and Biological Resource, Vietnam Academy of Science and Technology, Hanoi, Vietnam.
The osteoblast cells MC3T3-E1 and human mesenchymal stem cells (MSCs) were obtained from American Type of Culture Collection (Manassas, VA, USA). Minimum essential medium α(MEM α) was obtained from Gibco BRL, Life Technologies (USA).3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT)reagent, trypsin-EDTA, penicillin/streptomycin, p-nitrophenyl phosphate (pNPP) substrate, ascorbic acid, dexamethasone,β-glycerol phosphate, and fetal bovine serum (FBS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals and reagents were of analytical grade.
2.2. Preparation of the EtOAc extract
The roots of S. glabra were dried at 50 ℃ and grounded to a fine powder then soaked with 96% ethanol (EtOH) at room temperature for 72 h (3 times) to obtain the EtOH extract after removing the solvent under reduced pressure. The extract was suspended in HO,partitioned with n-hexane (3 times), and then EtOAc (3 times). The EtOAc fraction, which was rich in astilbin was concentrated under reduced pressure to yield the EtOAc extract for further study.
2.3. Isolation of the main active compound astilbin
The isolation was carried out as previously described by Phuong et al[10]. Briefly, the EtOAc extract was subjected to column chromatography on silica gel eluting with dichlomethane (DCM)/MeOH, 8:2 (v/v) to obtain nine subfractions (F1-F9). Astilbin was obtained from fraction F6 by recrystallization from MeOH/HO (1/1,v/v). The purity of astilbin was determined by High Performance Liquid Chromatography (HPLC). Chemical structure was elucidated by analysis of nuclear magnetic resonance (NMR) and mass spectrometry (MS) data in comparison with the references.
2.4. Cell culture and cell viability
Cells were cultured in MEMα medium containing 10% FBS and antibiotics at 37 ℃ in a 5% COhumidified incubator. To induce osteogenic differentiation in MC3T3-E1 cells, culture medium was changed to osteoblast differentiated medium-ODM (supplemented with 50 μg/mL ascorbic acid, 10M dexamethasone, and 10 mM β-glycerolphosphate). Cells were seeded at 1×10cells/well in a 96-well plate. They were incubated after seeding and treating with different concentrations of samples for 48 h. After incubation time,the cells were harvested and incubated with a solution of 0.5 mg/mL MTT for 4 h at 37 ℃ and 5% CO. The formazan crystals were then solubilized in dimethyl sulfoxide, and the optical density was measured at 570 nm using a microplate reader (PowerWave XS model, BioTek Instruments, Inc., Winooski, VT, USA). The untreated cells were used as controls. For experiments with osteoblast human MSCs, the cells were seeded in MSC medium,which consisted of basal medium supplemented with FBS and MSC growth supplement (ScienCell, US), and then treated with astilbin at different concentrations. After incubation and treatment for 48 h,medium was removed and the cells were incubated with a solution of 0.5 mg/mL MTT for 4 h at 37 ℃ and 5% CO. Data of relative cell viability was calculated as percentage compared to the non-treated group (control).
2.5. ALP activity
To evaluate ALP activity, cells were seeded into 96-well plates in ODM supplemented with 10% FBS. The culture medium was then replaced by a new ODM supplemented with/without the agent to be tested and incubated for 7 d. Cells were then rinsed with phosphate-buffered saline and 100 μL pNPP was added. Finally,the ALP activity in the cells was measured at 405 nm using a spectrophotometer (Evolution 201 UV-Vis, Thermo Scientific). Data of ALP activity were calculated as percentage compared to the nontreated sample (control).

Where: A is the relative absorbance with sample, Ais the relative absorption without sample.
2.6. Mineralization
The degree of mineralization was determined using alizarin red-S staining method in the 6-well plates. MC3T3-E1 cells were cultured in MEMα medium containing vitamin C (50 μg/mL)and β-glycerolphosphate (10 mM) for 3 weeks with/without the appropriate concentration of the test sample. After that, the cells were washed twice with phosphate-buffered saline, fixed with 70%(v/v) ethanol for 1 h, and dried in the air, then stained with 40 mM alizarin red-S (pH 4.2) for 1 h and thoroughly washed with water.The cells were then distained for 15 min with 10% cetylpyridium chloride in 10 mM sodium phosphate buffer (pH 7.0). The stained cells indicated mineralization and optical densities were measured at 562 nm to determine the degree of cell staining in the control and samples. Data of mineralization activity were calculated based on the formula:
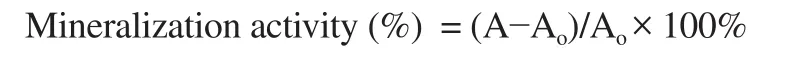
Where: A is the relative absorbance with sample, Ais the relative absorption without sample.
2.7. In silico studies
Crystal structure of human tissue-nonspecific alkaline phosphatase(h-TNAP) as previously modeled[13-15]was utilized for molecular docking studies. The structures of studied molecules were prepared using Marvin 6.1.2, ChemAxon (http://www.chemaxon.com), and PyMOL v2.4.0[16]. The energy minimization was carried out using Gabedit version 2.5.0[17].
The docking simulation procedure was performed by AutoDock 4.2.6 utilizing Lamarckian genetic algorithm and an empirical binding free energy function[18]. The Graphical User Interface program ‘AutoDock Tools’ was used to prepare the docking simulations. It was proved that the active site of h -TNAP contains two Zn ions and one Mg ion and is constituted by the residues Arg167, His434, His435, and His437. Therefore, the location and dimensions of the grid box for each protein were chosen such that they incorporate the active site. The detail of molecular docking simulation was given in Supplementary Method.
2.8. qRT-PCR
RNA was extracted from MC3T3-E1 cells using Trizol reagent(Invitrogen, Life Technology) as following the manufacturer’s protocols. RNA concentration and RNA purity were quantified by using NanoDrop (NanoDrop ND-1000, ThermoScientific). cDNA synthesized using superscript VILO Mastermix kit (Invitrogen,Thermo Fisher Scientific, Netherlands). The composition of cDNA synthesis contains 1 μg of RNA, 4 μL of master mix VILO, and RNase-free water to a final volume of 20 μL. The reaction followed the procedure by the manufacturer’s guidance. The primers for marker genes using in qRT-PCR were runx2, osx, opn, col1, and housekeeping gene gapdh as an internal control. The list of test genes and the primer sequences were shown in Table 1. Quantitative PCR(Thermo Fisher Scientific, US) was performed using SYBR green PCR master mix (NEB, US) and analyzed by relative quantification method.

Table 1. Primer sequences of study genes.
2.9. Statistical analysis
All data are presented as mean ± standard deviation (SD). Statistical analyses among the groups were determined by using a one-way ANOVA to assess statistical significance. The difference was significant as P<0.05.
3. Results
3.1. Isolation of astilbin from EtOAc extract
TheH andC NMR and MS data of the isolated compound are presented in Supplementary Table 1 and Supplementary Figure 1.Comparison with the reference[19]confirmed that it was astilbin with a molecular formula of CHO(MW = 450) (Figure 1).
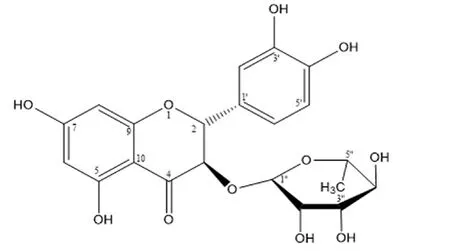
Figure 1. Structure of astilbin from Smilax glabra (C21H22O11; MW=450).
3.2. Cell toxicity
The results indicated that at concentrations of 5, 10, 25, and 50 μg/mL, either EtOAc (Figure 2A) or astilbin (Figure 2B) did not affect survival of MC3T3-E1 osteoblasts significantly. Astilbin at 50 μg/mL was possible to have a certain effect on cell survival but not statistically significant.
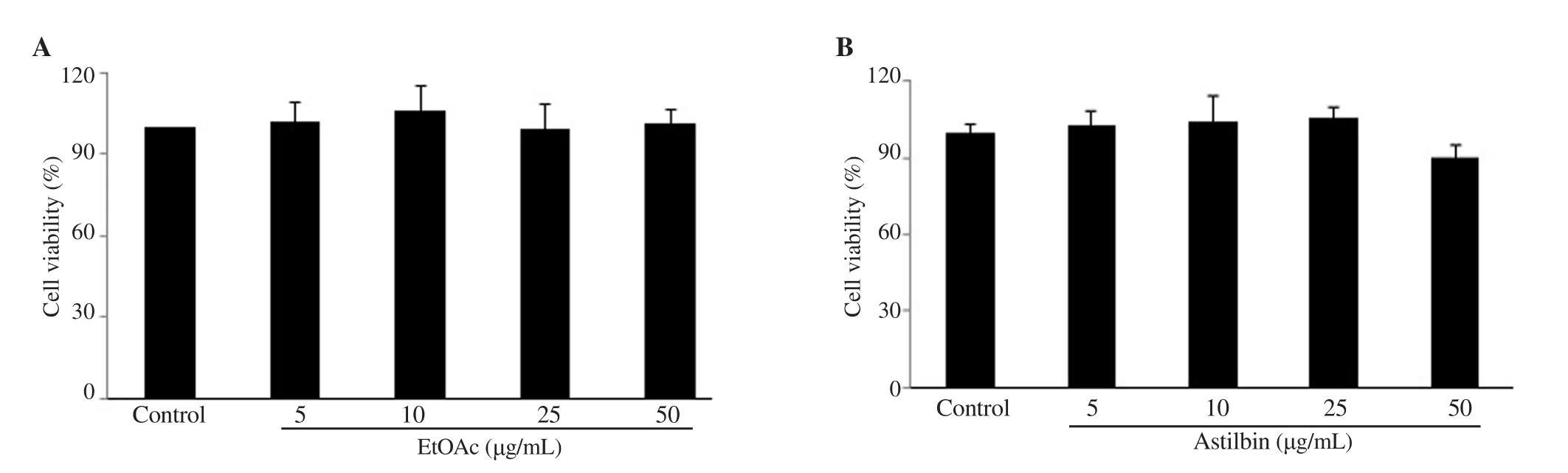
Figure 2. Toxicity of the ethyl acetate (EtOAc) extract (A) and astilbin (B) on osteoblast MC3T3-E1 cells. MC3T3-E1 cells were seeded in minimum essential medium α (MEM α) and then treated with EtOAc extract and astilbin at different concentrations. After incubation and treatment for 48 h, the medium was removed and the cells were incubated with a solution of 0.5 mg/mL MTT for 4 h at 37 ℃ and 5% CO2. Data of relative cell viability was calculated as percentage compared to the non-treated group (control). Results are presented as mean ± SD, n = 3.
3.3. Effect of EtOAc extract and astilbin on ALP activity
Data presented in Figure 3 showed that both the EtOAc extract and astilbin enhanced ALP activity of osteoblasts. Astilbin increased the activity up to 8%, 18%, 22% and 1% at 5, 10, 25, 50 μg/mL,respectively compared to the control (Figure 3B). The same pattern was also found for the EtOAc with the highest level at 25 μg/mL(Figure 3A). A similar result was found in MSC differentiation(Supplementary Figure 2). Thus, astilbin and the EtOAc extract clearly showed a stimulatory effect on ALP activity in osteoblasts.
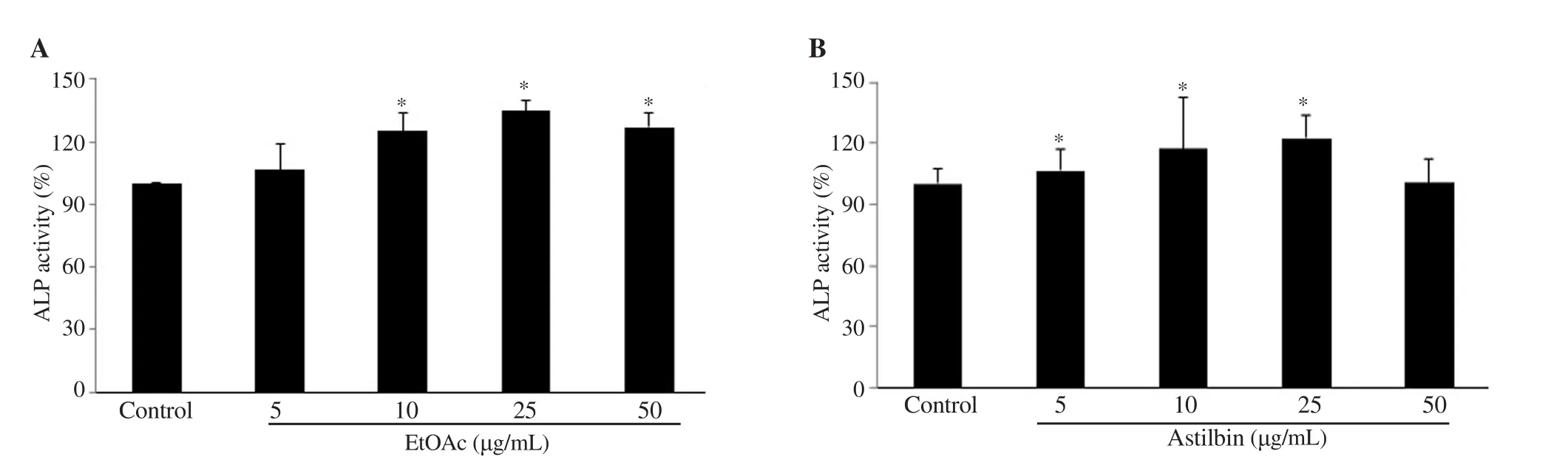
Figure 3. Effects of the EtOAc extract (A) and astilbin (B) on alkaline phosphatase (ALP) activity. MC3T3-E1 cells were seeded in ODM (supplemented with 50 μg/mL ascorbic acid, 10-8 M dexamethasone, and 10 mM β-glycerolphosphate) and treated with different concentrations of EtOAc extract and astilbin.Data of ALP activity were calculated as percentage compared to the non-treated sample (control). Each value is the average of triplicate cultures, and each bar indicates mean ± SD, n = 3, *P<0.05 compared with the control group.
3.4. Effect of EtOAc extract and astilbin on mineralization of osteoblasts
The matrix mineralization is the final stage of the osteoblast differentiation characterized by calcium deposition. Data presented in Figure 4 indicated that the treatment of all samples enhanced mineralization activity in a dose-dependent manner. For astilbin, the activity which was calculated as the percentage of absorbance at 562 nm of the treated and untreated cells, was enhanced up to 14%, 20%,41%, and 44% at 5, 10, 25, 50 μg/mL, respectively compared to the control. The stronger red color of cell plates was observed in the astilbin treated samples (Figure 5), indicating an elevated calcium deposition in the treated cells.

Figure 4. Mineralization activity of EtOAc (A) and astilbin (B) on MC3T3-E1 cells. MC3T3-E1 cells were cultured in Dulbecco's modified eagle medium containing vitamin C (50 μg/mL) and β-glycerolphosphate (10 mM) for 3 weeks with/without different concentrations of test sample and then stained with 40 mM alizarin red-S (pH 4.2) for 1 h. The cells were distained for 15 min with 10% cetylpyridium chloride in 10 mM sodium phosphate buffer (pH 7.0), then optical density was observed and determined spectrophotometrically at 562 nm. Data of mineralization activity were calculated as percentage compared to the non-treated sample (control). Each value is the average of triplicate cultures, and each bar indicates mean ± SD, *P<0.05, **P<0.01.
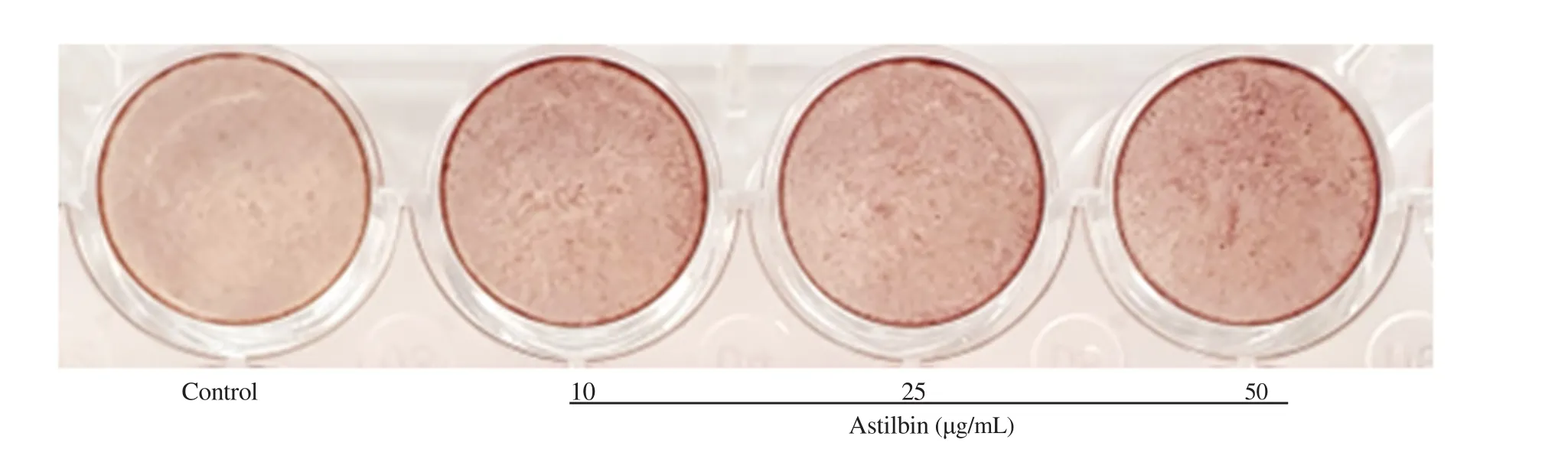
Figure 5. Calcium formation observed in MC3T3-E1 cell culture under treatment condition with different concentrations of astilbin. Alzarin red-S staining was used for determination of the mineralization level in the cells.
3.5. In silico study
The docking score (binding free energy) of astilbin was 11.08 kcal/mol. According to the ranking criteria of Autodock 4.2.6, the more negative value of docking energy, the better binding affinity of the compound towards targeted receptor[20,21]. Astilbin thus, proved to yield good docking energy and could be considered as a potential target for further drug development.
Binding orientation analysis of astilbin (Figure 6) revealed that an array of hydrophobic interaction was observed as contributed by Arg167, Asp320, His324, and His437, meanwhile, His434 and Thr436 were the key residues involved in hydrogen bond formation.It should be noted that Arg167, His434, and His437 are key residues that constitute the active site of h-TNAP, therefore, interaction forming with these amino acids could affect the function of this protein. The obtained results indicate that astilbin may potentially interact with h-TNAP resulting in changes in its activity.
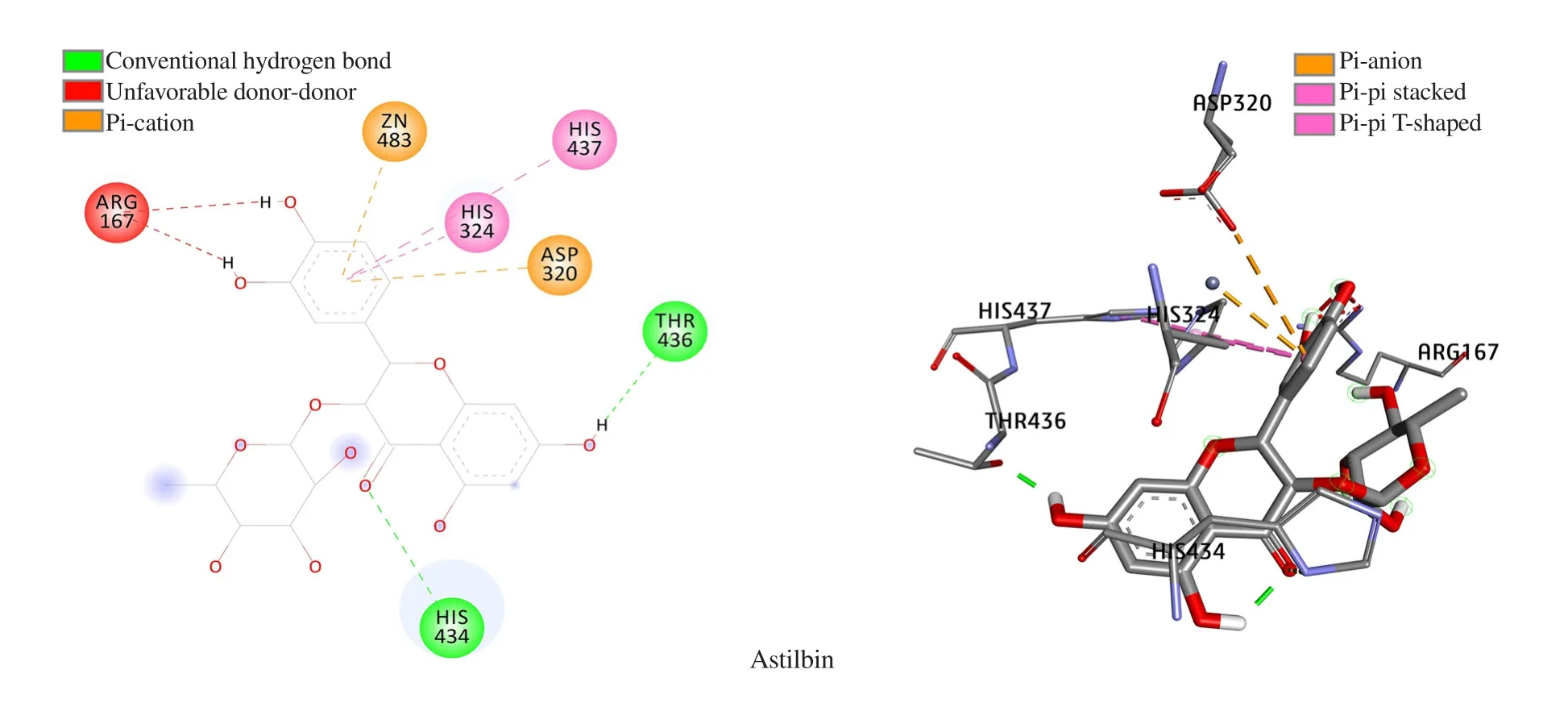
Figure 6. Docking pose of astilbin with h-TNAP protein.
3.6. Effects of astilbin on gene expression
Data shown in Figure 7 and Supplementary Figure 3 pointed out that all tested genes relating to osteoblast differentiation were successfully amplified in the qRT-PCR reaction and had elevated expression levels under condition of astilbin treatment.
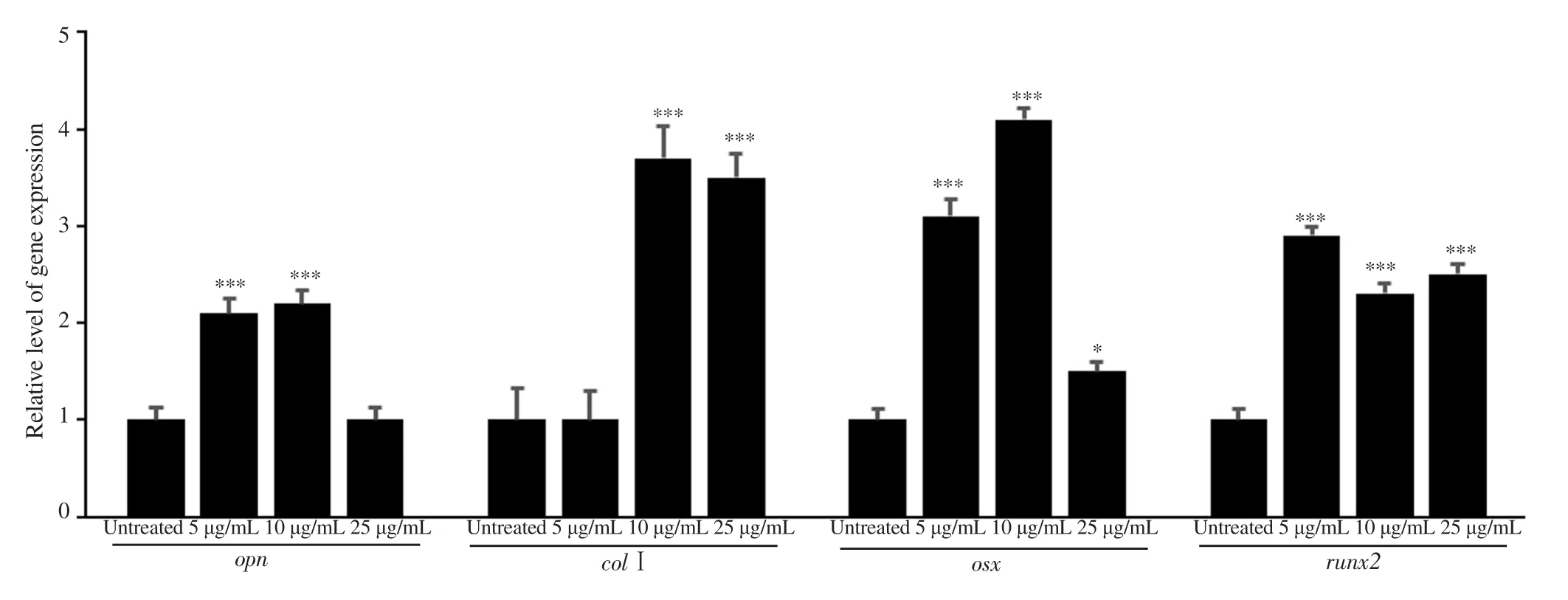
Figure 7. Astilbin enhanced the expression of several marker genes required for osteoblast differentiation. Osteoblast cells were cultured in 6-well plates at a density of 5×105 cells/mL in each well. Cells were treated with different concentrations of astilbin for 1 d before RNA extraction. cDNA synthesized using superscript VILO Mastermix kit. Gapdh served as a house-keeping gene for normalization of the test genes. Each bar indicates mean ± SD, n=3, *P<0.05,***P<0.001.
4. Discussion
Since the use of current synthetic drugs to treat osteoporosis has some limitations and causes undesirable side effects for long-term use, natural substances, especially those naturally from traditional medicinal plants that have long been used to treat osteoporosis and/or osteoarthritis, have been getting more attraction of researchers.In this study, the EtOAc extract and its major active compound astilbin have been investigated for the bone regeneration activity in MC3T3-E1 cells.
ALP is an important factor in hard tissue formation, highly expressed in mineralized tissue. The mechanism of its function is not completely understood, but it appears to increase the local concentration of inorganic phosphate, a mineralization promoter,and to decrease the concentration of extracellular pyrophosphate,an inhibitor of mineral formation. ALP has also been reported to be implicated in cardiovascular calcification which appears to proceed by an osteogenic mechanism[22]. Bone cell differentiation can be defined through three stages: i) cell growth; ii) maturation of the substrate; iii) mineralization of the substrate. Stage ii was determined by the expression of maximum ALP activity. Our results show that both the EtOAc extract and astilbin enhanced ALP activity of osteoblasts, indicating that they possess the osteoblastogenic activity.Moreover, the activity was also found with the same pattern in MSCs in presence of astilbin, suggesting its osteoblastogenic effect on different osteoblast cell lines.
In silico analysis by combining both docking score and binding mode analysis criteria suggested that astilbin could be a promising inducer for h-TNAP activity by binding with key amino acids holding metal cofactors Caand Mgions in the active site. This may explain for the enhancement of enzyme activity when the cells are treated with this compound.
The other important indicator for bone regeneration is the mineralization with the deposition of calcium by bone cells. In our study, the mineralization of osteoblasts clearly observed in treated samples reconfirmed osteoblastogenic activity of the EtOAc extract and astilbin. It is known that Cais a cofactor of ALP enzyme.Thus, it seems to have an interaction between the induction of the mineralization in osteoblasts by astilbin and the enhancement of cell ALP activity. A further investigation needs to be carried out to answer this question.
Similar investigations for osteogenic activity of natural compounds have also been previously reported[3,23,24]. Berberin, an alkaloid from medicinal plant Coscinium usitatum, has been intensively studied on bone-inducing activity in different osteoblast cell lines[25,26].Tai et al. isolated chrysoeriol compound from the leaves of Eurya ciliata Merr and reported that it strongly stimulated MC3T3-E1 cell growth at 0.2 -5.0 μg/mL. The compound at 5 μg/mL increased both ALP activity (up to 122%) and mineralization compared to the control[27]. In this study, the in vitro molecular mechanism of action of major active compound astilbin on induction of bone formation was studied by evaluating its effects on the marker gene expressions relating to bone cell differentiation using qRT-PCR technique. The selected marker genes were opn (a marker gene for early stage of matrix mineralization), osx (a marker gene for matrix maturation),colI (a marker gene for osteoblast differentiation and abundant expression in bone matrix), runx2 (a marker gene for expression of a major transcription factor)[28]. Our data on induction of osteogenic genes by astilbin is in line with data reported for berberine[25]. Han et al.[29]also showed that a derivative of berberine (Q8) induced osteoblast differentiation in vitro by increased ALP activity via induction of bone morphogenetic protein 4 (BMP4), and expression of genes encoding for ALP, bone sialoprotein (BSP), and osteocalcin.During the differentiation process, Q8 stabilizes the Runx2 and Osterix proteins by blocking the ubiquitin-proteasome pathway,thereby inducing the transcriptional activity of runx2 and osx genes. Our finding suggests that astilbin might block the ubiquitinproteasome pathway, resulting in up-regulation of runx2 and osx genes. Clearly, the effect of astilbin through other signal pathways,such as ubiquitin-proteasome or other MAPK and Smad pathways should be further examined to fully understand its mechanism of action as an osteoblastogenesis compound. Jin et al.[12]reported the osteoblastogenic activity of astilbin through the repression of NF-κB and MAPK pathways. Thus, this compound may have dual action:osteoclastogenesis and osteoblastogenesis, suggesting it could be a potential candidate for treating osteoporosis. Further works on mechanisms of action, toxicity, and the combined effects of astilbin with other bioactive compounds are needed to fully understand the osteoblastogenic activity and potential application of this compound,as well as of the EtOAc extract.
In conclusion, this study reports new bone-regeneration induction activity of astilbin and the EtOAc extract of S. glabra in MC3T3-E1 osteoblast cells. The compound enhances ALP activity and mineralization and up-regulates marker genes involved in differentiation of bone-forming cells. This finding is a basis for further research on underlying mechanisms of action and therapeutic applications of astilbin and the extract.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgments
The authors are highly appreciative of the Vietnam Academy of Science and Technology under grant NCCC 08.10/20-20 and the Institute of Biotechnology under grant CS20-01 for financial support. The authors would like to thank Prof. Jamshed Iqbal et al.(Institute of Information Technology, Pakistan) for their contribution of homology modeling studies.
Funding
This study is supported by the the Vietnam Academy of Science and Technology under grant NCCC 08.10/20-20 and the Institute of Biotechnology under grant CS20-01.
Authors’ contributions
PTMN designed the project, supervised, analyzed data, and wrote the manuscript. HTTN, MTHN, and TXN performed the experiments and analyzed data. QMP and HXN performed molecular docking analysis. All authors have read and approved the final manuscript.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Chemical constituents and biological activities of essential oils of Amomum genus(Zingiberaceae)
- Salsola imbricata Forssk. ameliorates acetic acid-induced inflammatory bowel disease by modulating dysregulated antioxidant enzyme system and cytokine signaling pathways in mice
- Demethylbelamcandaquinone B from Marantodes pumilum var. alata (Blume) Kuntze inhibits osteoclast differentiation in RAW264.7 cells
- An in vitro and in silico study of anti-dermatophytic activity of gossypol from fruits of Thespesia populnea (L.) Sol. ex Correa
