Demethylbelamcandaquinone B from Marantodes pumilum var. alata (Blume) Kuntze inhibits osteoclast differentiation in RAW264.7 cells
2021-12-15HaryatiAhmadHairiJamiaAzdinaJamalNorAshilaAladdinKhairanaHusainNoorSuhailiMohdSofiNorazlinaMohamedIsaNainaMohamedAhmadNazrunShuid
Haryati Ahmad Hairi, Jamia Azdina Jamal, Nor Ashila Aladdin, Khairana Husain, Noor Suhaili Mohd Sofi,Norazlina Mohamed, Isa Naina Mohamed, Ahmad Nazrun Shuid
1Department of Biochemistry, Faculty of Medicine, Manipal University College Malaysia, Jalan Batu Hampar, Bukit Baru, 75150 Melaka, Malaysia
2Faculty of Pharmacy, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300 Kuala Lumpur, Malaysia
3Department of Pharmacology, Faculty of Medicine, Preclinical Building, Universiti Kebangsaan Malaysia, Jalan Yaacob Latiff, Bandar Tun Razak,Cheras, 56000 Kuala Lumpur, Malaysia
4Department of Pharmacology, Faculty of Medicine, Universiti Teknologi Mara, Jalan Hospital, 47000 Sungai Buloh, Selangor, Malaysia
ABSTRACT
Objective: To investigate the bone-resorbing effect of demethylbelamcandaquinone B (Dmcq B) extracted from Marantodes pumilum var. alata on osteoclast differentiation in RAW264.7 cells.
Methods: RAW264.7 macrophages were differentiated using RANKL into osteoclast-like cells. Then, they were treated with 10 μg/mL Marantodes pumilum var. alata crude aqueous extract, 5 μg/mL dichloromethane fraction, and 0.6 μg/mL Dmcq B and 0.06 μg/mL estradiol. Tartrate-resistant acid phosphatase 5b (TRACP 5b)as an osteoclast phenotypic marker was determined by TRACP staining and TRACP 5b colometric assay, and bone-resorbing pits were examined. The gene expression of pro-inflammatory cytokines(TNF-α and IL-6) was measured. Moreover, the protein expressions of pro-inflammatory cytokines (TNF-α and IL-6) and estrogen receptors were evaluated.
Results: Marantodes pumilum var. alata crude aqueous extract and Dmcq B inhibited RANKL-stimulated osteoclast differentiation as evidenced by size reduction of giant multinucleated osteoclast cells,decreased TRACP 5b activity as well as the subsiding of resorbed pit area compared with normal control. In addition, they reduced the gene and protein expressions of TNF-α and IL-6. Marantodes pumilum var. alata, Dmcq B, and estradiol treatments increased the protein expressions of estrogen receptors alpha and beta in osteoclasts.
Conclusions: Marantodes pumilum var. alata and its active compound, Dmcq B can inhibit osteoclast differentiation.
KEYWORDS: Marantodes pumilum var. alata; Phytoestrogen;R AW264.7 induced-osteoclast; Bone-resorbing effect
1. Introduction
Physiological bone remodeling is a continuous adaptation system that coordinates structural bone integrity and bone mineral homeostasis. Bone remodeling is stably maintained by an intricate balance between bone-forming osteoblast and bone-resorbing osteoclast activities[1]. However, excessive bone resorption by osteoclast leads to bone loss diseases such as osteoporosis,periodontitis, rheumatoid arthritis, and bone cancer[2]. Osteoclast bone-resorbing cells are multinucleated cells characterized by positive tartrate-resistant acid phosphatase (TRACP) staining, which are originally derived from hematopoietic precursor cells of the monocyte/macrophage lineage. They play a crucial role in balancing the bone remodeling process, which controls the amount of bone tissue by resorbing the mineralized bone matrix. The differentiating osteoclast cells are activated by receptor activator of nuclear factor kappa B ligand (RANKL), a member of the tumor necrosis factor(TNF) superfamily[3].
Several potential therapeutic signaling pathways in bone remodeling are targeted for the inhibition of bone resorption (antiresorption) and promotion of bone formation (osteoanabolic) with the ultimate goal of restoring the bone microstructure of patients at risk of bone fragility and fracture. Osteoclast cells are the identified targets for abolishing metabolic loss, particularly in osteoporosis.For instance, bisphosphonates (BPs) are the first line of antiresorptive agents used in treating bone disorders. In terms of cellular mechanism, BPs with a high affinity to hydroxyapatite strongly bind to mineralized tissues and induce apoptosis of osteoclasts. They are also able to inhibit cytokines that stimulate resorption indirectly affecting the development of osteoclast cells. The effectiveness of BPs can be increased by adding a nitrogen atom into the side chain,as in alendronate, risedronate, ibandronate, and zoledronate[4,5].Although BPs are most commonly used as anti-resorptive agents to treat osteoporosis, they can cause serious side effects such as osteonecrosis of the jaw and an increased incidence of atrial fibrillation[6,7]. Therefore, there is a high demand to search for new therapeutic options for the prevention and treatment of osteoporosis.Endogenous estrogen is a potent modulator of skeletal mass.Most estrogen-mediated effects are dependent on binding to estrogen receptors (ERs), which are the ligand of transcriptional regulators[8,9]. Estrogen is essential for regulating major proinflammatory cytokines such as tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) to trigger osteoclast formation and activity[10,11].In estrogen deficiency, TNF-α and IL-6 directly exaggerate osteoclast differentiation resulting in accelerated bone resorption, which attributes to an imbalance in bone remodeling[12]. This may lead to pathological bone loss such as osteoporosis. Thus, pro-inflammatory cytokines play crucial roles in the normal bone remodeling cycle and the pathogenesis associated with osteoporosis.
Among many studies on the health benefits of natural compounds,phytoestrogen has a promising therapeutic potential. Phytoestrogens are a class of nonsteroidal compounds derived from plants and exert various estrogenic and antiestrogenic effects, primarily through binding to ERs[13]. Interest in the action of phytoestrogen has focused on revealing osteoprotective mechanisms. Due to its estrogenic activities, phytoestrogen may mimic the endogenous estrogen actions, which physiologically inactivate osteoclast formation, differentiation, and survival[14]. Marantodes pumilum(M. pumilum) (Blume) Kuntze [synonym name: Labisia pumila(Blume) Fern-Vill], a medicinal herb from Malaysia, has been extensively studied regarding its phytoestrogenic properties. The plant could emulate similar endogenous estrogen effects such as raising the estradiol level and suppressing follicle-stimulating hormone and luteinizing hormone levels[15]. Besides, M. pumilum extract protected the bone from loss in the estrogen-deficient mouse model by reversing the biomechanical properties of the bone due to estrogen deficiency[16]. In addition, M. pumilum has been also proved to increase bone anti-oxidative enzymes and reduce oxidative stress in the estrogen-deficient rat model[17]. It was also reported that M. pumilum intervened in the osteoclastogenic activity by blunting RANKL-induced osteoclast differentiation[18]. Therefore,it is important to fill the gap in knowledge by substantiating the modulatory effects of M. pumilum var. alata crude aqueous extract and its active compound, demethylbelamcandaquinone B (Dmcq B),on pro-inflammatory cytokines related to osteoclastogenesis as well as the regulation of osteoclast differentiation and activity. Thus, the present study was aimed at determining the effects of M. pumilum var. alata crude aqueous extract and its active compounds on osteoclast activity and cytokine secretion in osteoclast precursor cells induced by RANKL, potentially through the ER signaling pathway.RAW264.7 cells, a widely used pre-osteoclast model derived from mouse macrophage cell line, was utilized in this study as it retains the ability to differentiate into osteoclast-like cells after reacted with RANKL[19,20]and highly expresses tartrate-resistant acid phosphatase 5b (TRACP 5b), an osteoclast phenotypic marker[3,21]. In a previous study, M. pumilum extract and its compound at a certain concentration were able to demonstrate the osteoanabolic activities[22]. Thus, this study was designed to exert significant effects on the inhibition of proliferation and differentiation of osteoclast cells.
2. Materials and methods
2.1. Extraction of active compound
The active compound of M. pumilum var. alata crude aqueous extract was isolated as described in Ahmad Hairi et al[22]. Briefly,the aqueous extract of M. pumilum var. alata leaves (voucher specimen: UKMB 30006/SM 2622) was successively partitioned with dichloromethane (DCM) solvent under reflux with a ratio of 1:10 for 4 h. Following that, the DCM fraction was subjected to Si-gel column chromatography and eluted with a gradient system of chloroform (CHCl)-ethyl acetate (EtOAc) under an increasing percentage of EtOAc to obtain 15 sub-fractions. Sub-fraction of interest compound was further separated using Sephadex LH-20 and eluted with 1% methanol in chloroform (CHCl). Afterward, the interest compound was purified using Si-gel column chromatography and eluted continuously with CHCl-EtOAc (9:1). The interest compound was then identified as Dmcq B based on its NMR and HRESI-MS data[22].
2.2. Cell culture
RAW264.7 mouse monocyte-macrophage cell line (ATCC,Rockville, MD, USA) was grown in Minimum Essential Medium alpha (Gibco Life Technologies, Inc., Grand Island, NY, USA)containing 10% fetal bovine serum and 1% antibiotic-antimycotic(Gibco Life Technologies, Inc., Grand Island, NY, USA) at 37 ℃ in a humidified atmosphere of 5% COin the air. Culture media were changed for 7 d. The medium was changed on day 3 supplemented with 100 ng/mL of RANKL (Sigma, St. Louis, MO, USA) to induce osteoclast differentiation. For the protein expression of ER, 10%charcoal-dextran-treated fetal bovine serum (Hyclone Laboratories Inc., Logan, UT, USA) was used to replace heat-inactivated fetal bovine serum.
2.3. Treatment protocols
The concentration of each treatment [M. pumilum var. alata crude aqueous extract 10 μg/mL, DCM fraction 5 μg/mL, Dmcq B 0.6 μg/mL and estradiol (E) 0.06 μg/mL]was selected based on a previous cytotoxicity study on osteoblast[22]. Pre-osteoclast cells were then treated with different concentrations of treatments and culture media with treatments replaced every three days throughout the experimental period.
2.4. TRACP 5b
TRACP 5b is a specific biochemical marker with high sensitivity compared to other markers of bone resorption. It is determined by TRACP staining and TRACP 5b level using colometric assay. The differentiated osteoclast cells developed after 7 days of induction were stained by a standard TRACP (Cosmo Bio, Tokyo, Japan)staining according to the manufacturer’s instructions. Briefly, the cultured cells were washed with phosphate buffered saline and in 10%formalin neutral buffer for 5 min followed by rinsing with ultrapure water and incubated with tartrate staining solution for 1 h at 37 ℃in the dark. The stained cells were then rinsed with ultrapure water two times and allowed to air dry. TRACP-positive multinucleated cells appeared dark purple and contained either small (2-4 nucleus)or large (more than 5 nuclei) osteoclasts. Digital images of TRACP-positive cells were taken under brightfield microscopy using a confocal microscope (Nikon Eclipse Ti, Nikon Instrument Inc.,Melville, NY, USA). Quantitative measurement of TRACP 5b was performed using an acid phosphatase assay kit (Abcam Inc.MA, USA). Total TRACP 5b was measured using p-nitrophenyl phosphate as a substrate and quantified using a microplate reader at 405 nm.
2.5. Pit formation
The osteoclast bone resorption assay was performed by using a commercially available bone resorption assay kit (Cosmo Bio,Tokyo, Japan). Cells were seeded into calcium phosphate apatitecoated plates with various treatments of M. pumilum. After 7 days of induction, the cells were washed in 5% sodium hypochlorite to remove cells. The resorbed areas on the plate were washed with phosphate buffered saline and fixed with 10.0% formaldehyde for 10 min. The cells were rinsed with ultrapure water and incubated with 5% silver nitrate under ultraviolet light for 1 h. The cells were then rinsed with ultrapure water. Images of the resorbing pits were visualized under an inverted microscope. The area of the bone pits was analyzed by the image analyzer software Pro-Plus version 5(Media Cybernetics, Inc., Rockville, MD, USA).
2.6. Quantitative analysis of gene expression by real-time PCR
Total RNA was extracted using TRI reagent according to the manufacturer’s instructions (Molecular Research Centre, OH, USA),which was subsequently purified by ethanol precipitation. RNA samples were analyzed using Script Two-Step RT-PCR kit with SYBR Green (Biorad, Hercules, CA, USA). Primers were designed with Primer 3 software and blasted with National Centre for Biotechnology Information database sequences (Table 1). For each sample, 100 ng from the total RNA was used to synthesize cDNA.RNA was initially synthesized with iScriptReverse Transcription Supermix (Bio-Rad, Hercules, CA, USA), incubated for 5 min at 25 ℃, 30 min at 42 ℃, and 5 min at 85 ℃ to obtain the cDNA template.PCR amplification for each gene was then performed using SYBR-green detection by iQ5 multicolor real-time PCR system (Bio-Rad,Hercules, CA, USA) with the following program: initial denaturation of DNA (95 ℃, 3 min), 60 cycles amplification for the denaturation(95 ℃, 10 s), as well as annealing and extension (55 ℃, 30 s). The data collection and real-time analysis were performed at 95 ℃ for 1 min and 55 ℃ for 1 min, respectively. The expression level of each target gene was normalized againstβ
-actin (a housekeeping gene). The PCR specificity was examined using a 2% agarose gel.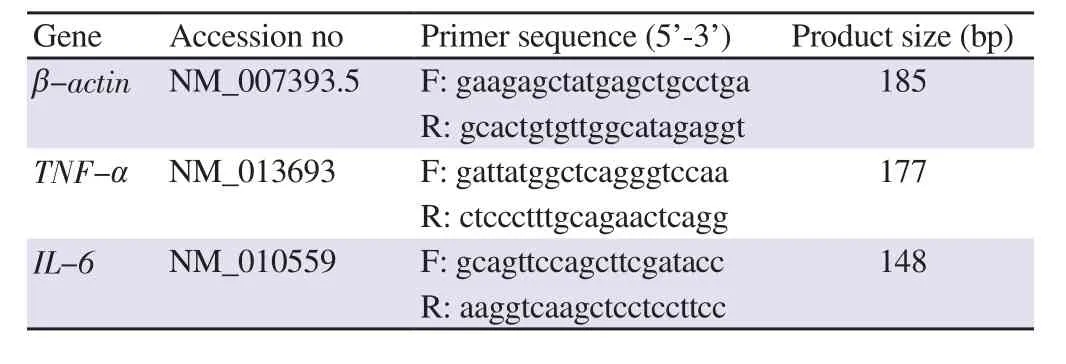
Table 1. Primer sequences for quantitative gene expression analysis.
2.7. Enzyme-linked immunosorbent assay (ELISA)
ELISA kits were used to detect TNF-α, IL-6, ERα, and ERβ according to the manufacturer’s instruction (Elabscience Biotechnology, Wuhan, China). Initially, 5×10cells were treated with various concentrations of treatments for 24 h (TNF-α and IL-6)and 72 h (ERα and ERβ). After treatments, the culture cell pellet was then lysed using a freeze-thaw method three times. The standard and samples were put to a 96-well microtitre plate coated with the antibodies specific to the protein marker and incubated for 90 min at room temperature. Horseradish peroxidase-conjugated streptavidin was added to each well successively after washing three times with washing buffer. Horseradish peroxidase catalyzed the conversion of a chromogenic substrate to a colored solution. The optical density(OD) of each well was recorded using a microplate reader at 405 nm. The content of protein was calculated according to the standard curve.
2.8. Statistical analysis
All data were expressed as mean ± SEM of at least six replicates.Statistical analysis was performed using the program Statistical Package for Social Sciences (SPSS) program version 20 (IBM, New York, NY, USA) with one-way ANOVA followed by Tukey’s HSD test as a post-doc test. A significant difference was considered as P< 0.05.
3. Results
3.1. Reduction of the formation of TRACP-positive multinucleated cell and TRACP 5b activity by M. pumilum var. alata treatments
The microscopic photographs were shown at the same magnification for all groups. Control cultures with RANKL induced the formation of TRACP-positive giant multinucleated cells, most of which had more than 10 nuclei (Figure 1); the sizes of giant cells were extremely large. M. pumilum var. alata treatments, as well as Etreatment, reduced the size of RANKL-induced TRACP-positive giant multinucleated cells (2-4 nucleus). In addition, all the treatments caused a significant decrease in TRACP 5b activity(Figure 2).
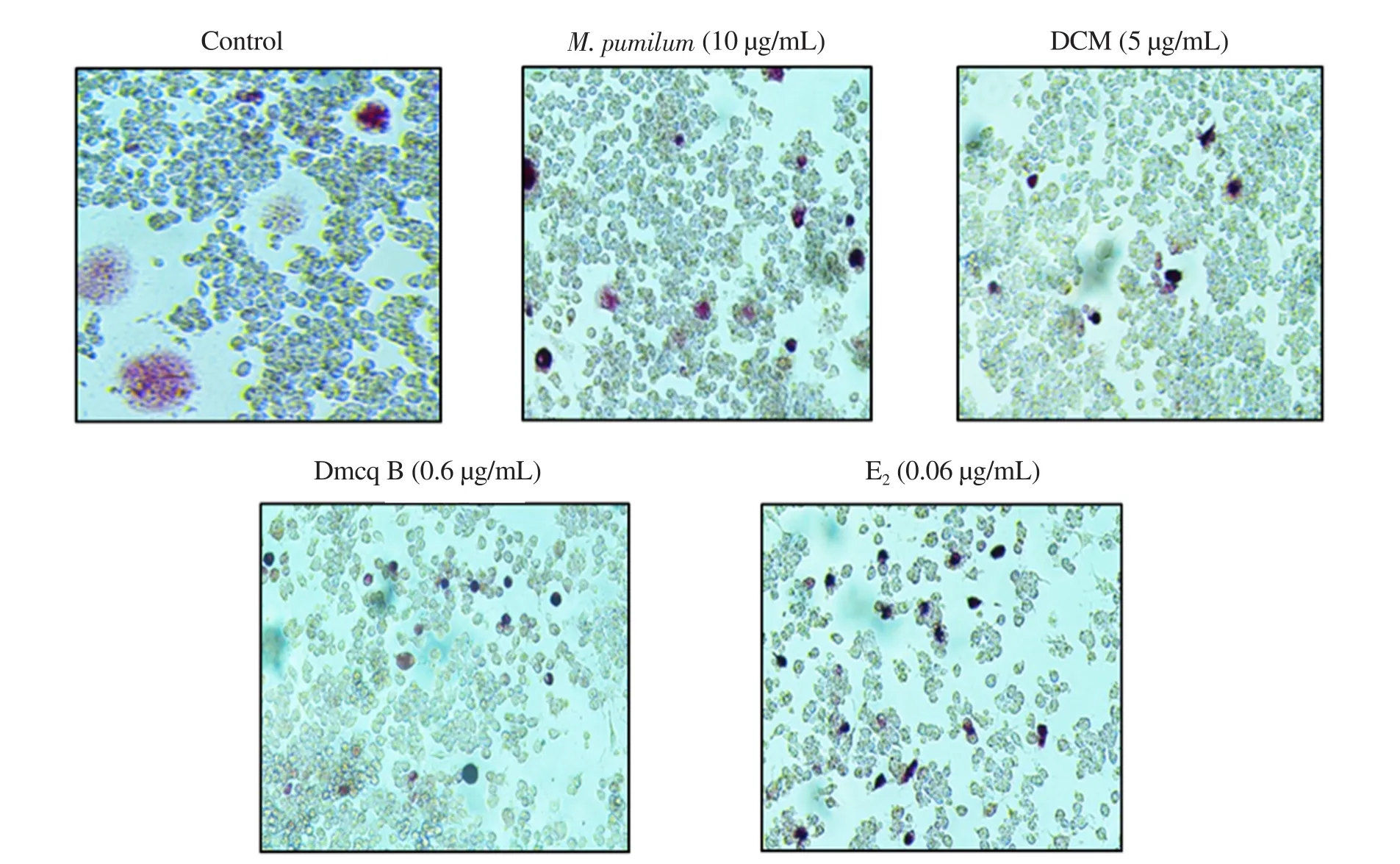
Figure 1. Effects of Marantodes pumilum var. alata (M. pumilum) crude aqueous extract, DCM fraction, Dmcq B, and E2 (positive control) on TRACP staining after 7 days of differentiation. The photomicrographs were captured for all groups using a confocal microscope (magnification ×100). DCM: dichloromethane;Dmcq B: demethylbelamcandaquinone B; E2: estradiol.
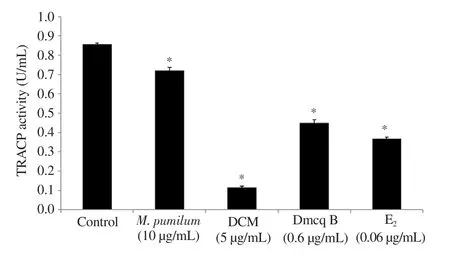
Figure 2. Quantitative analysis of TRACP 5b activity after 7 days of differentiation. The data are presented as mean ± SEM, n=6. *significantly different (P<0.05) compared with the normal control.
3.2. Reduction of the resorbed pits area by M. pumilum var.alata treatments
TRACP-positive giant multinucleated cells induced by RANKL were able to produce numerous resorption pits on calcium phosphate apatite-coated plates (Figure 3). Mature RAW264.7 induced osteoclasts to elicit resorption lacunae and pit formation upon stimulation with RANKL. The resorption pits observed in control cultures covered larger areas, which was decreased by M. pumilum var. alata treatments. The perimeter of resorbed pit areas were significantly reduced by M. pumilum var. alata treatments (Figure 4),indicating that the M. pumilum var. alata exerted an inhibitory effect on mature osteoclast function.
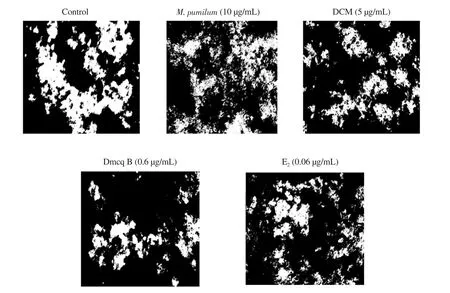
Figure 3. Effects of M. pumilum var. alata crude aqueous extract, DCM fraction, Dmcq B, and E2 (positive control) on resorption activity after 7 days of differentiation. The photomicrographs were captured for all groups using an inverted microscope (magnification ×100). In the normal control group, continuous resorbed pit (called as trench) could be observed. However, for the treatments groups, only series of small pits could be observed. The resorbed pit areas are reduced with all M. pumilum var. alata treatments, comparable to that of E2 treatment group.
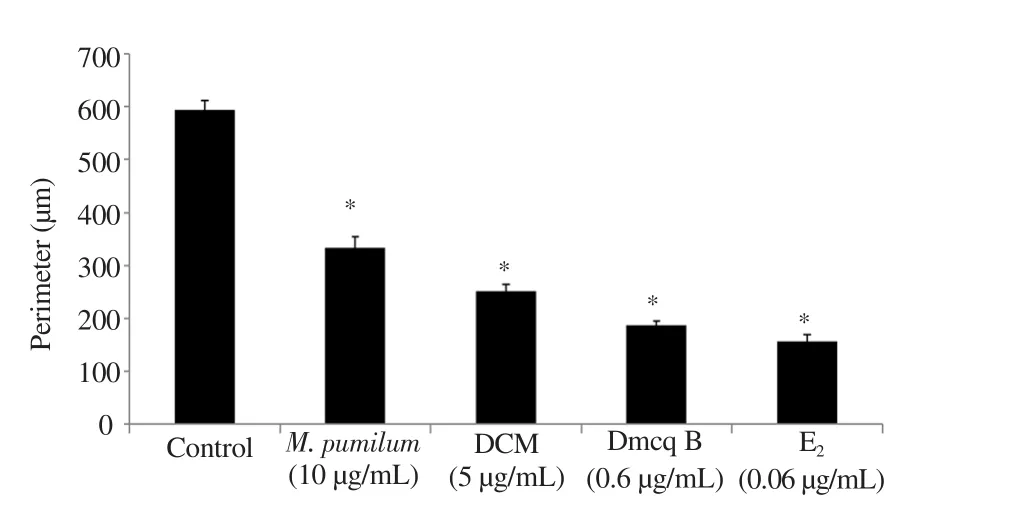
Figure 4. Quantitative of resorbed pit area (perimeter) after 7-day differentiation induction. The data are presented as mean ± SEM, n=6.*significantly different (P<0.05) compared with the normal control.
3.3. Modulation of M. pumilum var. alata treatments towards inflammatory mediators
As shown in Figure 5, only DCM fraction and Dmcq B significantly decreased TNF-α
expression, while all treatments markedly reduced the protein expression. Moreover, for IL-6, both gene and protein expressions were reduced by all M. pumilum var. alata treatments,comparable to Etreatment.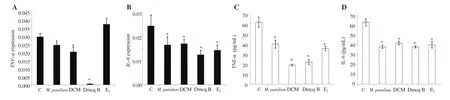
Figure 5. Effects of M. pumilum var. alata crude aqueous extract, DCM fraction, Dmcq B, and E2 (positive control) on the gene and protein expression of proinflammatory cytokines (A and C: TNF-α; B and D: IL-6) after 24-hour treatment. The data are presented as mean ± SEM, n=6. *significantly different(P<0.05) compared with the normal control. C: control.
3.4. Modulation of M. pumilum var. alata treatments towards ER protein expressions
Eas a positive control caused a remarkable increase in ERα and ERβ protein expressions in osteoclasts. M. pumilum var. alata treatments were comparable with Ein terms of the ability to increase ERα and ERβ protein expressions (Figure 6).
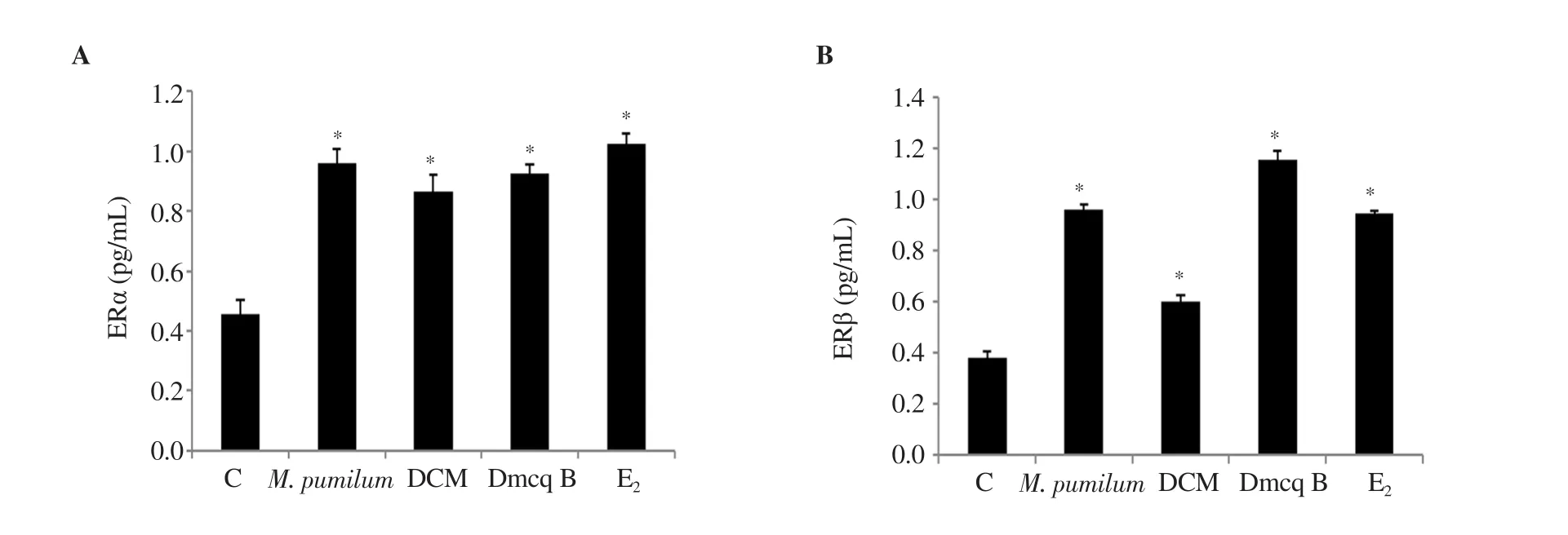
Figure 6. Effects of M. pumilum var. alata crude aqueous extract, DCM fraction, Dmcq B, and E2 (positive control) on (A) ERα and (B) ERβ after 72 hours of treatment. The data are presented as mean ± SEM, n=6. *significantly different (P<0.05) compared with the normal control.
4. Discussion
Estrogen is a critical regulator of bone remodeling in terms of promotion of osteoblast proliferation and differentiation, as well as inhibition of activation of osteoclast formation and its survival.Estrogen primarily acts by regulating gene transcription via ERs(ERα and ERβ). When estrogen level fluctuates at menopause,bone remodeling turns up imbalanced, which is due to excessive bone resorption and retardation of bone formation. Osteoclasts are enumerated as essential bone cells governing bone-resorbing activities. M. pumilum is a medicinal herb in Malaysia with phytoestrogenic effects that protect bone from osteoporosis as evidenced by many in vivo studies. A recent study showed that the active compound of M. pumilum var. alata, Dmcq B exerted osteoanabolic effects in terms of the ability to enhance alkaline phosphatase activity and collagen synthesis during the early phase of osteoblast differentiation. It also modulated osteoblastogenesisrelated genes and accelerated calcium deposits during the late phase of mineralization. Dmcq B was also able to reduce the ratio of RANKL relative to osteoprotegerin[22]. However, the effects of Dmcq B on osteoclasts’ activity or differentiation have yet to be clarified.
Therefore, this present study was done to investigate whether or not Dmcq B would affect the differentiation of RAW264.7 cells at similar doses to produce the osteoanabolic effect. First, the TRACP-positive multinucleated cell and pit resorbed area formations were examined to apprehend the effects of crude aqueous extract of M.pumilum var. alata and Dmcq B on bone resorption. M. pumilum var. alata crude aqueous extract and Dmcq B treatments, as well as Etreatment, suppressed RANKL-stimulated TRACP-positive giant multinucleated cells, and accordingly, the cells abated in size, in terms of reduction of cell fusion and multinucleation. In line with this, there have been reports stating that the size of the osteoclast is associated with resorption activity. The larger the size of the osteoclasts, the more they resorb bone matrix[21]. This finding is supported by the study of Lv et al.[3], which showed that the rate of TRACP 5b activity is also correlated with the size of osteoclast. Beyond the modest effects of M. pumilum var. alata treatments on osteoclasts formation and TRACP 5b activity, they also showed their anti-resorbing action by lowering resorptive pit areas. Therefore, these results indicated that M. pumilum var.alata and its phytoestrogen compound, Dmcq B, could suppress osteoclastogenesis.
Of note, estrogen also exhibited its anti-resorbing action by regulating inflammatory mediators that are synergically responsible for initiating bone resorption. As the most potent cytokine stimulators of bone resorption in vitro, TNF-α and IL-6 possessed the ability to directly augment the differentiation of committed osteoclast precursors[23,24]leading to the activation of mature osteoclast to resorb bone by several actions, which either aids the activity of RANKL in osteoclastogenesis activation process[25], directly stimulates osteoclast formation from human or mouse monocytes[26],or activates osteoclast formation independent of RANKL signaling[27]. Furthermore, the production of these cytokines is accelerated with a decrease in estrogen levels in the body. Therefore,in a state of post-menopausal women, the increase of these cytokines causes an increase in bone resorption processes leading to an imbalance of bone remodeling and osteoporosis[12,28]. In addition, physiologically, TNF-α and IL-6 are capable of inhibiting the synthesis of collagen, alkaline phosphatase, and osteocalcin in osteoblast cells, hence inhibiting osteoblastogenesis[29]. Thus,TNF-α and IL-6 would play a central role in postmenopausal bone loss directly promoting osteoclast formation and its survival. In the present study, all M. pumilum var. alata treatments were at par with Ewhich was able to significantly reduce the secretion of TNF-α and IL-6 only at the protein level. In terms of gene expression, only the IL-6 gene showed a similar trend with its protein expression.This might be due to post-transcription regulation in TNF-α
gene expression that occurred during the experiment. These results reasonably speculated that M. pumilum var. alata crude aqueous extract and Dmcq B may further protect against post-menopausal bone loss through the modulation of pro-inflammatory cytokines expression produced by osteoclast precursor.Consistently, the present study showed that all M. pumilum var. alata treatments could dampen RANKL-induced osteoclast differentiation probably by modulating ER signaling pathway. It was also found that all M. pumilum treatments, as well as E, increased the protein expressions of ERα and ERβ. The detection for both ER in RANKL-induced osteoclast cells would be expected to facilitate estrogen response[30]. The classical genomic activities of estrogen hormonal binding with its receptor (particularly ERα or ERβ)subsequently bind to estrogen response elements in target genes function for gene activation or repression[31,32]. The antiosteoporotic actions regulated by estrogen will ultimately result in a suppression of osteoclast-mediated bone resorption[32,33]. This is in line with the finding of this study showing that M. pumilum var. alata extract and its compound, Dmcq B, significantly induced ERα and ERβ protein expressions. These results suggested that M. pumilum and Dmcq B possess phytoestrogen property[34].
E, an exogenous estrogen with phenolic and hydroxyl groups,is one of the most efficacious estrogens for binding ERs[35]. A previous study has shown that phenolic rings in certain active natural compounds are directly associated with estrogenic activity[36]. In addition, phytoestrogens such as genistein, coumestrol, apigenin,and kaempferol[37,38], which showed the ability to bind to ERs and estrogen-like activity, have the effect of reducing bone loss[39,40].The phenolic compound from Dmcq B contains two phenolic rings, which may exhibit chemical structural characteristics of compounds with estrogen-like activity. Moreover, the role of ER in the regulation of E-induced anti-osteoclastic action has been reported[41]. These results suggest that all M. pumilum var. alata treatments possess pharmacological value in modulating osteoclast differentiation in terms of its activation, formation, and survival possibly through an estrogen-like mechanism. Therefore, in the future, further development is warranted to improve Dmcq B efficacy on osteoporosis treatment especially in in vivo study and extend the study to human trials.
In conclusion, the crude aqueous extract of M. pumilum var. alata and its active compound, Dmcq B, blunted osteoclastogenesis and reduced bone resorbing-activity potentially through its antiinflammatory and phytoestrogenic properties.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This study is supported by NKEA Research Grant Scheme (NRGS)(NH1113D018-2).
Authors’ contributions
HAH performed the osteoclast lab experiments and the data analysis as well as drafted the manuscript. NAA performed the plant extraction. JAJ, ANS and KH developed the theoretical formalism and design of the study. NAM and INM were involved in the data interpretation. NSS was involved in the osteoclast lab experiment.ANS supervised the project and revised the article.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Chemical constituents and biological activities of essential oils of Amomum genus(Zingiberaceae)
- Salsola imbricata Forssk. ameliorates acetic acid-induced inflammatory bowel disease by modulating dysregulated antioxidant enzyme system and cytokine signaling pathways in mice
- An in vitro and in silico study of anti-dermatophytic activity of gossypol from fruits of Thespesia populnea (L.) Sol. ex Correa
- Ethyl acetate extract of Smilax glabra Roxb roots and its major active compound astilbin promote osteoblastogenesis in vitro by upregulating bone cell differentiationassociated genes
