Effect of Interacting Abiotic Storage Conditions on Respiration, Dry Matter Losses and Aflatoxin B1 Contamination of Stored Turkey’sShelled Hazelnuts (英文原文)
2021-12-03KalliopiMylonaAngelMedinaNareshMagan
Kalliopi Mylona, Angel Medina, Naresh Magan✉
(Applied Mycology Group, Environment and AgriFood Theme, Cranfield University,Cranfield, Beds. MK43 0AL, U.K.)
Abstract: Hazelnuts are an economically important nut which is consumed world-wide. It is prone to infection by Aspergillus flavus and contamination with aflatoxins. Taking Tukey’s hazelnuts as a research object, the objective of this study were to (a) quantify respiration rates and (b) dry matter losses (DMLs) and(c) aflatoxin B1 (AFB1) contamination of naturally stored shelled hazelnuts at different temperatures and water availabilities. Subsequently, shelled hazelnuts were inoculated with additional A. flavus inoculum prior to storage to examine effects on dry matter losses and on AFB1 contamination. Maximum respiration of hazelnuts and associated mycoflora was under wetter conditions of 0.90~0.95 water activity (aw =12.5%~18% moisture content). This resulted in between approx. 10% DML at 25 and 30 ℃ after 5 days storage. Inoculation and storage of shelled hazelnuts + A. flavus inoculum resulted in similar patterns of respiration with optimal levels at 25~30 ℃ and >0.90 aw. Indeed, AFB1 contamination was highest at the maximum water levels tested of 0.90 aw. Indeed the contamination level exceeded the legislative limits set by the EU for AFB1 contamination of these nuts. Correlation between DMLs and all the AFB1 data in both sets of studies showed that very small changes in DML due to poor drying or storage of ≥0.6% resulted in AFB1 contamination levels exceeding the EU legislative limits. Thus, efficient drying and safe monitored storage is necessary to minimise the risk of AFB1 contamination in this economically important commodity and to avoid exposure of consumers to such toxins.
Key words: Turkey’s hazelnuts; storage; respiration; dry matter losses; aflatoxin B1; Aspergillus flavus;legislative limits
1. INTRODUCTION
Hazelnut (Corylus avelannaL.) is a popular tree nut and predominantly cultivated in Turkey and southern Europe. Turkey is the largest producer of hazelnuts, responsible for approx. 75% of world production[1]. Hazelnuts are considered to an important source of nutrients, especially lipids including monounsaturated fatty acids, tocopherols and phytopherols and phenolic compunds, all considered to have health benefits[2-3].
Harvested hazelnuts have shells that provide a good barrier to fungal contamination and potential for contamination with mycotoxins, especially aflatoxins (AFs). However, slow drying of the hazelnuts, especially natural sun drying can take several weeks providing conducive conditions for fungal colonisation and AFs contamination. This combined with poor storage conditions can exacerbate the contamination with AFs[1]. Thus there is strict legislative limits for the maximum aflatoxin B1(AFB1, a class 1a carcinogen) and total AFs for such tree nuts in many countries including the European Union for human consumption with AFB1and total AFs of 5 and 10 µg/kg respectively[4].
Hazelnuts, like other edible seeds, are generally alive and typically respire at very low levels under safe storage water availability conditions (water activity, aw; <0.70 aw= 7.5%~8% moisture content(m.c.). Under these conditions, while fungal contaminants remain viable they are unable to initiate spoilage or any additional toxin contamination[5-6].However, at harvest the hazelnuts are very moist>25% m.c. During the drying process the m.c. is reduced to safe levels. However, slow or inefficient drying and subsequent storage can allow the contaminant fungal species, especially those of theAspergillussectionFlavi(e.g.,A. flavus) to colonise the nuts resulting in increased contamination with AFs, especially AFB1. Such species are dry loving(xerophilic) and are able to rapidly colonise lipid rich substrates under intermediate moisture conditions because of their ability to produce relevant hydrolytic enzymes[7]. This results in dry matter losses (DMLs),off-odours and a deterioration in nutritional quality[8].
Respiration has been effectively used to measure the metabolic activity in stored commodities,especially cereals[9-14]. Respiration is the aerobic oxidation of carbohydrates, represented by the following equation[15]:
C6H12O6+ 6O2→6CO2+ 6H2O + 677.2 cal (or 2835 kJ)
This includes both respiration of the commodity(cereal, nut) and of the accompanying microorganisms.The relevant contributions of the microorganisms and of the commodity respiration to the total respiration has been a matter of interest for many years. Seitz et al.[16-17]showed that the the fungal contribution to DML increases during storage at a rate dependent on the prevailing m.c., temperature,level of kernel damage and the fungal community contaminating the phyllosphere surfaces of the commodity.
Recent studies have demonstrated that changes in CO2production during storage in a range of commodities (cereals, groundnuts) can be effectively used as an indicator of DML, and this can be linked to potential mycotoxin contamination[11-12,18-20]. These studies utilised the aerobic respiration rates under increasingly conducive interacting temperature x awconditions due to oxidation of carbohydrates/ lipids and the CO2production to calculate quality losses as DML percentages. The respiration rates (R) using Gas Chromatography and the associated DMLs can then be used to establish a “storability risk index” to predict overall quality changes and mycotoxin contamination in stored cereals and nuts[8,21].
DMLs of between 0.04%~2% have been found to indicate impacts on seed quality and risks of mycotoxin contamination relevant to the EU legal maximum limits[11-14,17-20]. A recent study of shelled groundnuts stored at different m.c.s and temperatures quantified the respiration rates and DMLs and related these to the AFB1contamination[20]. This was utilised to evaluate relative risks of different storage conditions affecting AFB1contamination levels exceeding the EU legislative limits. There are no such comparable studies of hazelnuts, although this is an important food commodity, which is used in a wide range of processing food chains from baked goods to chocolate-based snacks and ice creams.
The objectives of this study were to (a)quantify respiration rates and (b) dry matter losses,(c) AFB1contamination of naturally contaminated hazelnuts and those which were inoculated with additionalA. flavusinoculum prior to storage on toxin contamination levels. The relationship between DMLs and AFB1contamination was established for the first time.
2. MATERIALS AND METHODS
2.1 Hazelnuts and water adsorption curves
Raw hazelnuts without shells were supplied by TUBITAK MAM, Marmara Research Centre, Turkey.Known volumes of sterile water (0~2 mL) were added to 5 g sub-samples of naturally contaminated shelled hazelnuts in 40 mL glass Universal bottles.These were sealed, shaken vigorously and stored overnight at 4 ℃ to allow water absorption and equilibration. Samples were then equilibrated at room temperature (T) before the measurement of awvalues of each sub-sample, using the dew point/water activity meter AQUALAB Model Series 4TE(Decagon Instruments; Washington, USA). Water adsorption curves were determined by plotting the amounts of added water (mL) against awvalues measured. These curves were used to determine the exact quantities of water needed to reach the target awlevels for each set of experiments performed.After this, the m.c. of the samples was determined by drying them in an oven at 105 ℃ for 16 h.
ForA. flavusinoculation experiments batches of hazelnuts (500 g× 4) were initially gamma irradiated (12 kGy; Syngergy Health, Swindon, UK)to kill any resident microflora and stored in sealed bags at 4 ℃ until use. At this irradiation dose the nuts retained germinative capacity but were free of fungal contamination[21]. The moisture adsorption curve was also prepared for the irradiated raw hazelnuts.
2.2 Effect of storage regimes of naturally contaminated shelled hazelnuts on dry matter losses
The naturally contaminated shelled hazelnut samples (160 g) were placed in surface sterilized 500 mL Duran flasks. Sterile water was added to obtain the target awvalues of 0.70 (control), 0.85, 0.90 and 0.95 (=5%, 10%, 12.5% and 18% m.c. wet weight basis) by reference to the moisture sorption curve.These were stored at 4 ℃ for 24 h. Four replicates(10 g) per treatment were placed in 40 mL clear glass Volatile Organic Analysis (VOA) vials with sealable PTFE caps containing a silicone septum (23188 Supelco) to allow for later gas sampling from the headspace. Hazelnut samples corresponding to the same awlevels were incubated in 12 L polypropylene environmental chambers together with 2×500 mL beakers of glycerol/water solutions to maintain the target Equilibrium Relative Humidity (ERH) of the atmosphere relative to each awtreatment value. The hazelnut samples were incubated for up to 5 days.
The storage treatments used in this study were in the temperature range of 15~30 ℃ and the awrange was 0.70~0.95. Carbon dioxide (CO2) production were measured every 24 h. The sampling method involved use of Gas Chromatography measure the CO2accumulated in the head space as previously described by Mylona & Magan (2011). The percentage CO2concentration were used to calculate (a) Respiration (R) rate in mg CO2(kg/h),(b) total cumulative production of CO2after 5 days storage and (c) the total DMLs[11].
2.3 Hazelnuts inoculated with A. flavus and stored to measure respiration activity, dry matter losses and aflatoxin B1 contamination during storage
Aspergillus flavusisolate 1217 obtained from hazelnut, with known aflatoxin production was kindly supplied by TUBITAK MAM, Turkey. This strain was cultured on malt extract agar medium at 25 ℃ for 5~7 days. Spore suspensions ofA. flavuswere used as an inoculum for the storage experiments.The surface of the colony was rubbed with a sterile loop and the conidia placed in 10 mls of sterile water containing 0.01% Tween 80. This was shaken vigorously to obtain the spore suspension. The concentration was adjusted by dilution with sterile water to 5×107spores/mL using a haemocytometer.
Shelled hazelnuts in surface sterilized jars(batches of 100 g) were adjusted with sterile water to 0.85, 0.90 and 0.95 aw(=10%, 12.5% and 18% m.c.)by reference to the specific water adsorption curve.The actual amounts of water added were slightly less to allow for the addition of the inoculum. After equilibration overnight at 4 ℃, the treatments were equilibrated at 25 ℃. Then a 0.2 mL aliquot ofA.flavusinoculum was added to the hazelnut treatment samples and thoroughly mixed. Four replicate of each awtreatment were placed in the 40 mL glass Universal bottles and again incubated in the environmental chambers as described previously.The treatments and replicates at each awlevel were incubated separately at 15, 20, 25 and 30 ℃.
Every 24 h the glass Universal bottles for the different treatments and replicates were sealed with PTFE caps containing a silicone septum (23188 Supelco) for 1 hr and then a gas sample taken with a headspace syringe. This was used to inject into the GC for CO2quantification. After sampling the lids were removed and the samples returned to the environmental chambers at each temperature and awlevel. This procedure was repeated daily.
2.4 Dry matter loss calculations
The equation for the aerobic oxidation of carbohydrates/lipids as detailed previously was used.The respiratory quotients were calculated by the ratio of oxygen consumed to carbon dioxide produced, which allowed for the determination of dry matter loss (DML). From this equation, 14.7 g of CO2per kilogram of grain is equivalent to 1% DML. The DMLs were calculated for the different storage treatments of naturally contaminated shelled hazelnuts and those inoculated with additionalA.flavusspores.
The percentage (%) DML vs. time curves were determined by integrating the respiration rate vs.time data. The storage time was divided into 24 h periods and the amount of CO2during each period was calculated by the respiration rate. The sum of the CO2amounts for each period was the total CO2produced during the storage period and used to calculate the DMLs.
2.5 Aflatoxin B1 quantification
At the end of each experiment, the hazelnut treatments/replicates were dried at 60 ℃ for 48 h,milled and stored at 4 ℃ pending further analysis.A sub-sample of 5 g was combined with 10 mls of 70% ethanol and placed on a orbital shaker for 1 hr.This was filtered and a total of 5 mls of the filtrate was applied to an immunoaffinity column (Romer,Tulln, Austria) using a syringe containing 10 mL of deionised water, and the filtrate was passed through the column at a flow rate of 3 mL/min. The column was washed with 20 mL of deionised water at a flow rate of 5 mL/min, then air was passed through the syringe at least three times. The aflatoxins bound to the column were eluted with 1 mL of methanol at a flow rate of 0.5 mL/min. Aflatoxins were collected into an Eppendorf vial (2.5 mL) and transferred to an amber HPLC glass vial.
HPLC analysis: The isocratic mobile phase consisted of water: methanol:acetonitrile (6:3:2);350 mL of 4 M nitric acid and 0.120 g of potassium bromide per litre were added. The flow rate was set at 1 mL/min and the column temperature was 22 ℃.A sample volume of 100 mL was injected in triplicate.Fluorescence detection was set to an excitation wavelength of 360 nm and an emission wavelength of 430 nm. Post-column derivatisation with electrochemically generated bromine by using a PTFE tube(30 cm) was applied to enhance aflatoxin fluorescence intensity. For this purpose an electrochemical cell supplying 100 mA current was positioned between the column and fluorescence detector.
2.6 Statistical analyses
All experiments have been performed in triplicate and carried out twice. Data were analysed with Microsoft Office Excel 2007 and with the package STATISTICA 9 (StatSoft®, Inc. 2010. STATISTICA(data analysis software system), version 9.1.www.statsoft.com). The standard error of the mean has been calculated in all trials and it is denoted with vertical bars in the Figures or included in the Figure legend in 3-D graphs.
2.6.1 Statistical analysis of DML and toxin analysis data
The Shapiro-Wilk, W test for normality was used to assess the normality of the total DML and toxin data while the homogeneity of the variance within the data was assessed by the Levene’s test.Data not normally distributed were logarithmically transformed in order to stabilise the variance and their normality reassessed by the Shapiro-Wilk W test. Normally distributed data were analysed by one and two-way Analysis of Variance (ANOVA)for determination of the significance of the effect of each factor (awand temperature) and their interaction (aw×T) on the variable. Log-transformed data still not normally distributed were analysed by the Kruskal-Wallis test by ranks.
2.6.2 Correlations between DML and toxin production data
Scatter plots of the DML data against the aflatoxin B1was produced. The Spearman Rank Order Correlations test was used to determine the significance of the correlation between the two variables in each case.
3. RESULTS
3.1 Naturally contaminated hazelnuts
Table 1 shows the effect of a range of water activity (aw) conditions on the mean respiration rate and total DML of naturally contaminated hazelnuts after 120 h incubation at 25 and 30 ℃. This clearly shows that both respiration rates and DMLs increased with increasing water availability at both these temperatures. It is notable that at relatively safe conditions for storage (0.70 aw) very little respiration occurred even after 120 h incubation with the associated DMLs very low at both temperatures. For 0.85 awboth respiration rates and DML started increasing, with the highest values observed in the wettest treatments of 0.95 awat both temperatures. It was notable that respiration rates were more than double that at 25 ℃, when increased to 30 ℃ in the highest water availability treatment.
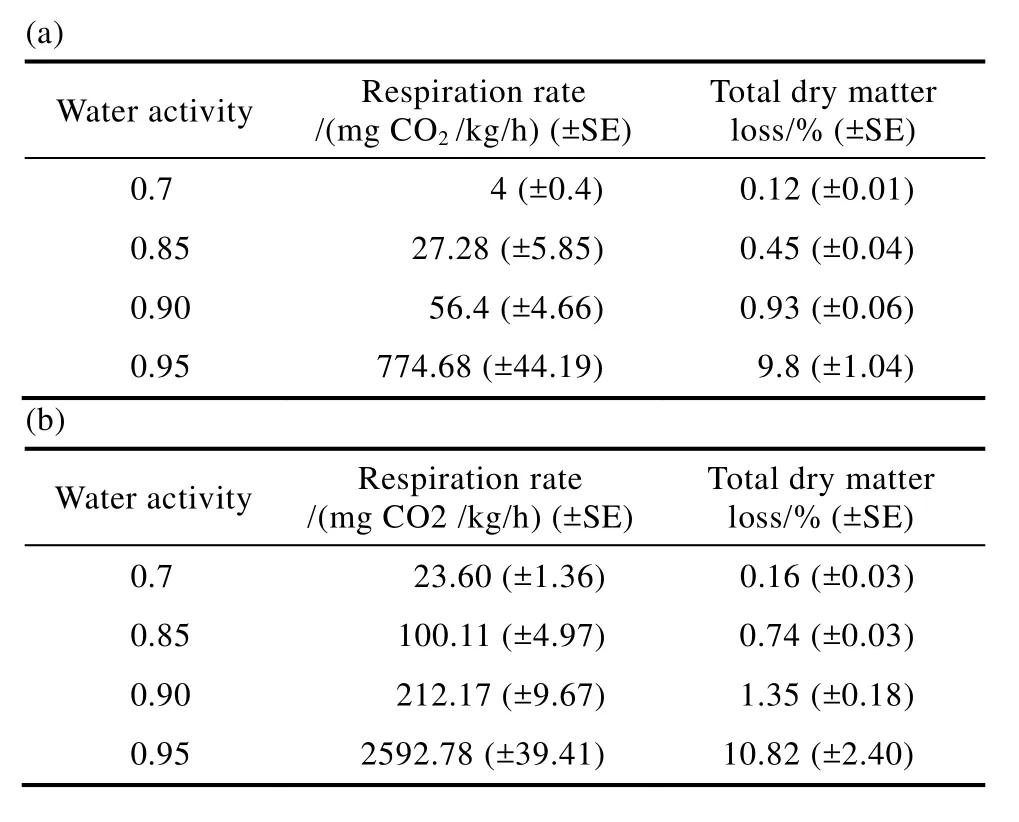
Table 1 Effect of water activity and temperature of storage on accumulated respiration rates and mean total dry matter losses(DML ±SE) of naturally contaminated hazelnuts after 120 h incubation at (a) 25 and (b) 30 ℃.
3.2 Hazelnuts inoculated with A. flavus; effects of storage under different water activity x temperature conditions on respiration, dry matter losses and aflatoxin B1 contamination
Figure 1 shows the temporal effect of awat 25 ℃ on the mean respiration rate of stored hazelnuts inoculated withA. flavus.This colonisation was most rapid at 0.95 aw. This data at 15~30 ℃ was used to calculate the DMLs of hazelnuts with additionalA. flavusinoculum. Figure 2 shows the impact of colonisation byA. flavuson the DMLs of stored hazelnuts under the interacting conditions of awx temperature. Respiration rates were very low at 15~20 ℃, regardless of storage aw. Only at 25 and 30 ℃, and ≥0.90 awwere there significant DMLs.

Fig.1 Temporal changes in respiration of stored hazelnuts inoculated with A. flavus and stored at 25 ℃ and three different water activities (SEmax = 80.41).
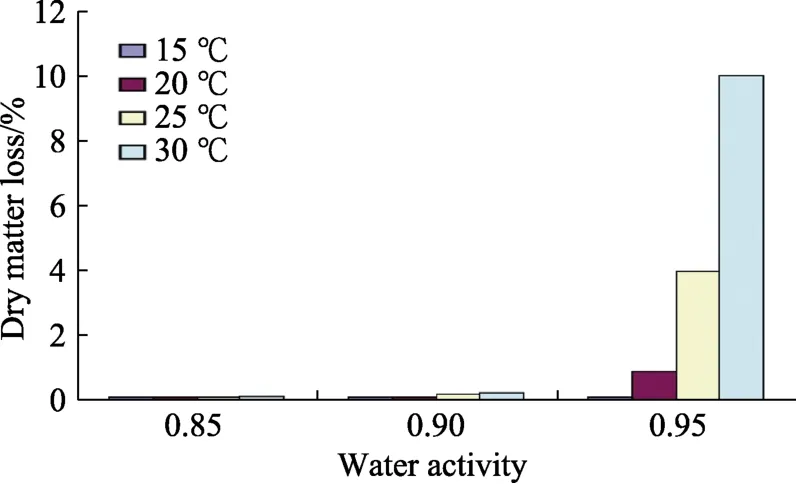
Fig.2 Dry matter losses of stored hazelnuts inoculated with A. flavus in relation to storage temperature x water activity (SEmax = 0.9).
Figure 3 shows the impact of inoculation of hazelnuts withA. flavuson the contamination levels with AFB1at different awlevels at 15~30 ℃. This clearly shows that there are very high amounts of AFB1produced at 0.90 awat 20~30 ℃, with significantly less toxin contamination at 0.85 aw. At 15~20 ℃ no AFB1was produced at 0.80 and 0.85 aw. Where AFB1was produced the levels were all above the EU legislative limits of 5 µg/kg.
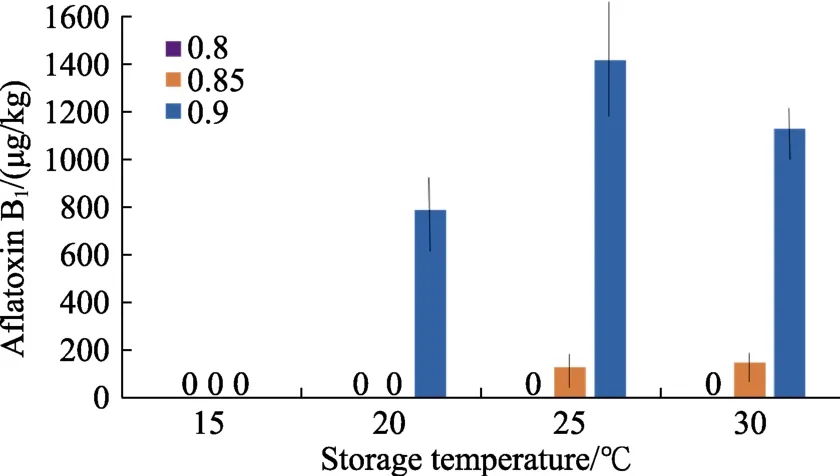
Fig.3 Effect of storage temperature and water activity on contamination of hazelnuts inoculated with A. flavus after 10 days. Bars represent Standard Error of the means.
Figure 4 has taken all the data from the hazelnut treatments in terms of DMLs and the contamination with AFB1and examined the correlation of these to criteria in the context of the EU legislative limits.This showed a significant positive correlation between AFB1and DMLs. The dotted red lines indicate the confidence limits. This shows that at around 0.8%~1.0% DML there is a significant risk of exceeding the EU legislative limits for maximum contamination with AFB1in nuts.

Fig.4 Correlation between aflatoxin B1 and Dry Matter Losses(%) for the data sets from the storage experiments with Aspergillus flavus stored under all combinations of temperature x water activity examined. The red dotted lines indicate the 95% confidence limits and the black dotted line indicates the EU maximum toxin limits in nuts.
4. DISCUSSION
This study has shown that stored naturally contaminated shelled hazelnuts under different temperature x water availability conditions significantly influenced the relative respiration rate and the total CO2produced. The CO2production levels (approx.775 and 2 600 mg CO2/kg/h) were significantly higher at 0.95 aw(=18% m.c.) at both 25 and 30 ℃respectively. Previously, Garcia-Cela et al.[20]showed that for shelled peanuts optimum respiration activity occurred at 30~35 ℃ with a total accumulated CO2levels at 30 ℃ being about 2 080 mg CO2/kg/h), These nuts are both high in lipids and the respiration of the shelled nuts and the associated natural mycobiota may influence the overall respiratory activity. In other cereal commodities, the total respiratory activity was also found to be optimum at ≥0.95 aWin paddy and brown rice and maize, especially when inoculated withA. flavusat 30~ 35 ℃[14,19].
The total accumulated CO2was used to quantify the relative DMLs under the different storage conditions. This showed that at 0.95 awapprox. 10% occurred at 25~30 ℃. Garcia-Cela et al.[20]found that at 30~35 ℃ and 0.95 awthe DMLs were about 14%~15% in shelled peanuts. Studies of maize showed that under these conditions up to 17% DML occurred. In contrast, in paddy and brown rice the calculated DMls were 3.5% and 20% respectively when these cereals were inoculated withA. flavus[19].The unprocessed paddy rice was probably protected by the outer protective layers, reducing the potential ingress of fungi when compared to the processed brown rice.
In the present study additional inoculum ofA.flavusresulted in maximum respiration activity and DMls at 0.95 awand 20~30 ℃. This equated to quality losses of around 10% at 30 ℃ and 0.95 aw.Additional inoculum did not result in significantly higher DMLs in stored hazelnuts. This contrasts with wheat, maize or shelled peanuts where the DMLs were much higher when inoculated with mycotoxigenic fungi[12,14].
The production of AFB1was found to be significantly higher at 0.90 awthan at 0.85 or 0.80 aw20~30 ℃. This also showed that at <0.85 awthe production of this carcinogenic mycotoxin represented a significantly lower risk, especially at 15~20 ℃.The plotting of all the data for DMLs and that for AFB1production under the different awx temperature conditions provided a valuable information of the relative risks of toxin contamination exceeding the legislative limits. This clearly showed that at 0.6%~1% DML, contamination of shelled hazelnuts would exceed the recommended maximum contamination levels. This certainly suggests that very small losses in dry matter would result in AFB1contamination above the legislative limits resulting in potential rejection of such lots.
This also suggests that there is a low tolerance to losses due to fungal spoilage in stored shelled hazelnuts, especially when there is inefficient drying regimes. Hazelnuts are lipid rich and thus very hygroscopic. Thus, they can often reabsorb moisture from the environment subsequent to drying during postharvest storage causing colonisation by mycotoxigenic spoilage fungi. In previous studies with cereals,mycotoxin contamination of about 1% resulted in type A and B trichothecenes, zearalenone (both in wheat) and fumonisins toxin levels (maize) exceeded the EU legislative limits[11-14,22]. For fatty acid rich nuts such as peanuts 0.56% was the level at which AFB1contamination levels would exceed the legislative limits[20]. Thus, the tolerances may be much lower in nuts than for cereals. It may be better,provided the hazelnuts are effectively dried to a safe m.c. to do this in shell to protect the nut flesh from becoming colonised and contaminated with mycotoxins. However, care is needed as sometime the internal space between the nut and the shell can provide a microenvironment for xerophilic fungi such asA. flavusto grow, especially where the shell has been damaged due to pests or during harvesting and drying phases[23]. This was found to occur with brazil nuts resulting increased AFB1contamination in in shell nuts[24]. Perhaps this type of information can be effectively utilised from a risk management perspective to develop different categories of low,intermediate, and high risks, relevant to human consumption or for animal feed use[13].
