Variation in Polymerization Degree of C-A-S-H Gels and Its Role in Strength Development of Alkali-activated Slag Binders
2021-12-01LIUQingZHANGJiakangSUYueweiXianjun
LIU Qing, ZHANG Jiakang, SU Yuewei, LÜ Xianjun
(College of Chemical and Biological Engineering, Shandong University of Science and Technology, Qingdao 266590, China)
Abstract: To investigate the variation in the degree of polymerization calcium aluminium silicate hydrate (C-A-S-H) gel and its role in the evolution of the strength of waterglass slag binders, the compressive strength, hydration products, degree of hydration of the slag, and the degree of polymerization of C-A-S-H gels of binders were examined. The experimental results indicate that the pH of the pore solution increased with an increase in the Na2O concentration. However, mortar with an optimum compressive strength value of 81.0 MPa at 28 d was obtained when water glass modulus was 1.5. The main hydration product is a C-A-S-H gel for which the quantity and the degree of polymerization depend strongly on the Na2O concentration; for a given range, both increase with increasing Na2O concentration, thus yielding an enhanced strength. A further increase in the Na2O concentration continuously increases the quantity of C-A-S-H gels while drastically reducing the degree of polymerization. The positive effect of the former is counteracted by the adverse effect of the latter, ultimately, leading to a decreased strength. Furthermore, we reveal that the degree of polymerization for C-A-S-H gels may be affected by pH, through a series of complex chemical reactions.
Key words: Na2O concentration; compressive strength; C-A-S-H gels; degree of hydration; degree of polymerization
1 Introduction
As a by-product of steel production with satisfactory pozzolanic activity, ground-granulated blast furnace slag (GGBFS) is widely used to produce cementitious materials[1,2]. A new kind of environmentally friendly cementitious material - alkali-activated slag (AAS) binders - is characterized by lower energy consumption, lower CO2emission, and lower cost than those of ordinary Portland cement (OPC)[3,4]. It has been shown that AAS binders exhibit excellent mechanical properties and durability[5,6].
Waterglass (Na2O·nSiO2) is the most commonly used substance for slag activation, yielding the highest compressive strength among those of other alkaline activators[3,5,7]. The strength can be modulated by controlling the alkali concentration (%Na2O) and the silica modulus (SiO2/Na2O) of the waterglass. It is widely accepted that a high alkali concentration results in high pH for the pore solution, which significantly affects the hydration process and properties of AAS binders. A high pH commonly leads to a higher degree of hydration of the slag and higher number of hydration products, which are considered to have a positive effect on strength[8,9]. However, a series of studies have reported that the optimum Na2O concentration and silica modulus are 3.5%-5.5% and 1.0-1.5, respectively[10-12]. As the more hydration products are produced, the strength of AAS binders reduces if the alkali concentration is excessive. A probable explanation is that excess free alkali in AAS binders is responsible for the degradation of properties through processes such as carbonization, efflorescence, and embrittlement, which result in degraded strength[4,13].
Poorly crystalline calcium aluminium silicate hydrate (C-A-S-H) gels are the major hydration products of AAS binders and are essential because of their mechanical properties[14,15]. The nature of C-A-S-H gels (degree of polymerization) is significantly affected by the pH in AAS binders, which controls the solubility of active icon, such as Ca2+, Al3+, and Si4+[16]. Consequently, elucidation of the properties of the hydration products under different alkali concentrations is essential. Several studies have examined the influence of Na2O on the degree of hydration of slag and the quantity of hydration products[3,17,18]. However, the effects of Na2O concentration on the degree of polymerization of C-AS-H gels have rarely been explored. The relationship between the C-A-S-H gel structure and the evolution of strength has been thoroughly investigated[14,19,20], revealing that binders containing C-A-S-H gels with a high degree of polymerization tend to exhibit higher strength. However, no detailed systematic study of the role of the degree of polymerization of C-A-S-H gels in the evolution of strength. To accurately predict and improve the strength of AAS binders, the variation in the degree of polymerization of C-A-S-H gels with alkali concentration and its role in hydration must be understood.
Thus, we examined the activation of slag by waterglass with different Na2O concentrations. The evolution of strength and pH of the pastes were investigated, and the hydration products of binders were analyzed using X-ray diffraction (XRD) and thermogravimetric analysis (TGA). Moreover, the degree of hydration of the slag and the degree of polymerization and microstructure of C-A-S-H gels were analyzed by29Si nuclear magnetic resonance (29Si NMR) spectroscopy and scanning electron microscopy (SEM). Finally, the mechanism of the variation in the degree of polymerization of C-A-S-H gels with various Na2O concentration was investigated.
2 Experimental
2.1 Materials
GGBFS with a specific surface area of 375 m2/kg was supplied by Rizhao Material Co., Ltd., and its chemical composition is presented in Table 1. The broad hump at 20°-35° (2θ) of the XRD pattern of GGBFS indicates a typical amorphous structure (Fig.1). Traces of calcite and akermanite were detected as the major crystalline compounds.
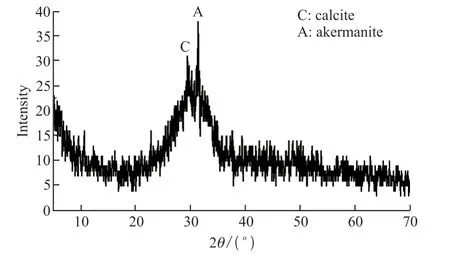
Fig.1 XRD pattern of GGBFS
Waterglass with 8.11% Na2O, 25.89% SiO2, and 66% H2O was used as an alkaline activator in this study. The initial solid content of waterglass was kept at 15% of the total binder mass, and the modulus of the waterglass solution was adjusted by adding reagent-grade NaOH pellets (AR, ≥ 98%). The mixed activator solution was cooled to room temperature (25 ± 1 ℃).
2.2 Experimental procedure
The mix proportions of slag pastes are showed in Table 2. The paste samples are marked as Mx, where M andxrefer to the waterglass and the modulus of waterglass, respectively. The water-to-binder ratio was 0.5 (by mass) for all paste samples. Slag and waterglass solutions with the desired moduli were mixed until the pastes samples were homogeneous. Subsequently, the mixed paste samples were cast into steel molds with dimensions of 40 mm × 40 mm × 160 mm and cured for 24 h. All paste samples were cured at temperature of 20 ± 1 ℃ and relative humidity ≥ 90%. According to the Chinese National Standard GB175-2007 and GB201-2000, the compressive strength and hydration properties characterization were measured after 1 day, 7 days, and 28 days.

Table 1 Chemical composition of the slag (oxides in wt%)
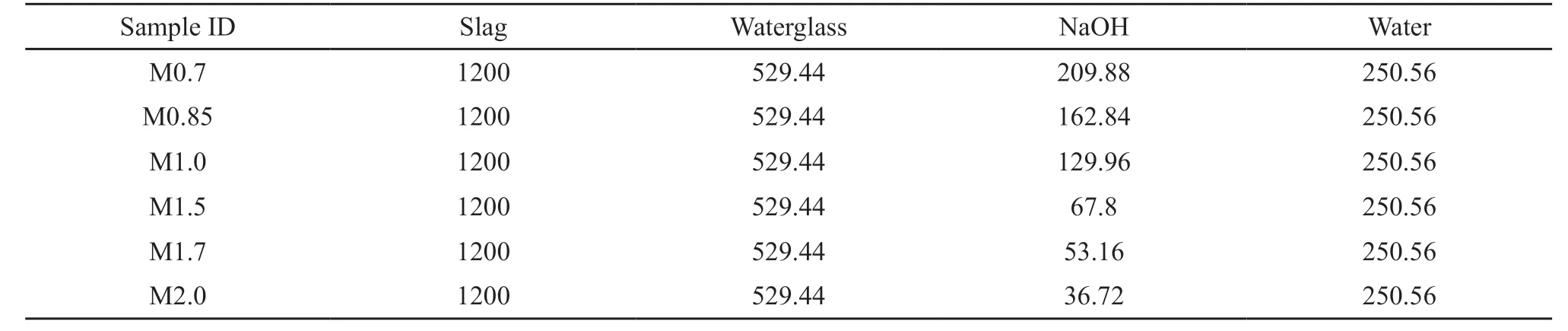
Table 2 Mix composition of the pastes with different moduli/g
At each curing age, the compressive strengths of paste samples were measured using a YES-300B hydraulic compression tester with a load rate of 2400 N/s. Each sample was measured three times to minimize error. A piece of broken paste was milled to a thickness of less than 425 μm and mixed with pure water with a water-to-powder ratio of 1 (by mass). After shaking for 6 h at 25 ℃, a pH indicator (PHS-25PH) was used to measure the pH of an extract. The remaining broken pastes were immersed in absolute ethanol for 72 h to arrest further reaction.The soaked pastes were dried at 50 ℃ to a constant weight. Fragments with large and flat surfaces were characterized by SEM, while other fragments were further pulverized for XRD, TGA, and29Si NMR analyses.
2.3 Testing methods
An X-ray diffractometer (Rigaku Ultima IV, Cu Kα radiation (λ = 1.5416 Å)) was used to obtain XRD patterns in a scan range of 5°-70° (2θ), scanning speed of 8°/min, and a step size of 0.02°.
TGA was conducted on a Mettler Toledo TGA2 between 30 ℃ and 900 ℃ in a nitrogen atmosphere at a heating rate of 10 ℃/min.
Solid-state29Si NMR spectra were recorded at 119.1 MHz on an Agilent-600 (14.1 T) spectrometer using a probe for 4-mm o.d. zirconia rotors at a spinning speed of 10.0 kHz. The pulse width was set at 6 μs with a relaxation delay of 20 s and 4 300-6 500 scans.
SEM was conducted on a SIGMA300 with an accelerating voltage of 2.0 kV.
3 Results and discussion
3.1 Evolution of strength of pastes
The compressive strength evolution of pastes with different moduli were measured after 1 day, 7 days, and 28 days (Fig.2). Generally, the strength of pastes gradually increases with decreasing modulus from 2.0 to 1.5, however, a further decrease in modulus results in a significant reduction in strength. Specifically, the 28-day strength of sample M1.5 increased from 70.5 MPa to 81.0 MPa, an approximate 15% enhancement over that of sample M2.0 with the lowest Na2O content. As reported in the Refs.[9,12,21], the enhanced strength was attributed to the increase in the pH of the sample and the consequent improvement of the degree hydration of the slag, which resulted in more hydration products and the formation of a more compact microstructure. However, the strength of pastes sharply decreases when the modulus is less than 1.5. For instance, the 28-day strength of sample M0.7 drastically dropped from 81.0 MPa to 65.0 MPa, a decrease of nearly 20% in comparison with sample M1.5. A similar evolution of strength was observed in previous studies, where it was reported that excessive Na2O leads to a significant reduction in strength because of the carbonization, efflorescence, and embrittlement caused by abundant free alkali[3,11,18]. However, most of these studies have not evaluated the role of C-A-S-H gel structures on the evolution of strength.
3.2 pH evolution of pastes
Fig.3 shows the pH of slag binders with different moduli. It has been reported that the pH of the pore solution significantly affects the hydration reaction of slag and the nature of the hydration products[16,22].
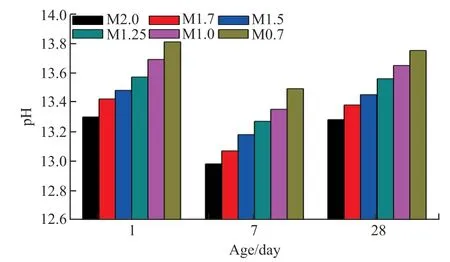
Fig.3 pH variation in slag binders versus modulus
As expected, the pH of pastes continuously increased with the decrease in the modulus, regardless of curing time. Sample M2.0 exhibited the lowest pH levels of 13.30, 12.98, and 13.28 after 1 day, 7 days, and 28 days, respectively. The low pH resulted in a low hydration degree of slag, which was consistent with the low strength of sample M2.0, particularly after 1 day. In contrast, the pH increased to 13.81, 13.49, and 13.75 at the above curing ages when the modulus was 0.7. The increased pH indicates a higher degree of hydration of slag and more hydration products (see XRD and TGA results). However, instead of M0.7, sample M1.5 shows the highest strength after 7 and 28 days of curing (see Fig.2).
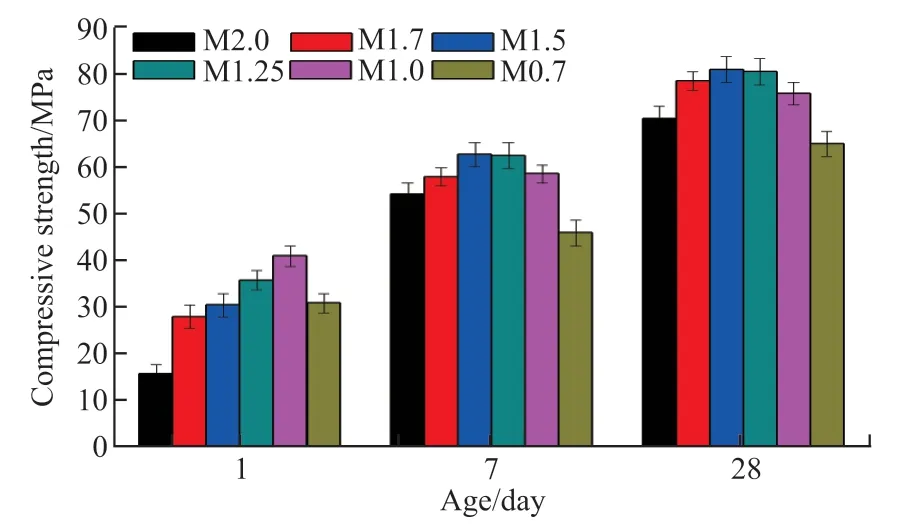
Fig.2 Compressive strength evolution of pastes with different moduli
3.3 Hydration products of pastes
The results of the XRD and TG/DTG of paste samples with different moduli are shown at Fig.4. Fig.4(a) shows the XRD analyses of 1-day and 28-day hydration products of pastes with varied moduli. For all pastes, the strongest diffraction peaks at 2θ= 29.5° corresponding to C-A-S-H gels are identified. The amorphous hump can be explained by the minimally crystalline structures of the C-A-S-H gels[17,23]. For sample M2.0 with the lowest Na2O concentration, a low peak intensity is observed, indicating a limited quantity of C-A-S-H gels. Compared with that of sample M2.0, the C-A-S-H gels peak for sample M0.7 is significantly enhanced for both, 1-day and 28-day samples. The enhanced intensity is strongly associated with the increase in the pH of the pore solution, indicating an increasing quantity of C-A-S-H gels with increasing Na2O concentration. There are several studies reporting that a higher pH produces a higher degree of hydration of slag and thus forms the more hydration products[8,9].as a remanent of a crystalline phase in slag The diffraction peak of akermanite (2θ= 31.29°), and is observed in all pastes, indicating its low reactivity. The presence of a small amount of calcite may be related to the carbonation of pastes during the curing period.
The 28-day TG/DTG curves for pastes with different moduli are presented in Fig.4(b). For each sample, there is a major drop in the DTG signal that recovers by the time the temperature is up to 250 ℃ is observed, corresponding to the dehydration of C-A-S-H gels[8,17,24]. There is a rapid increase in the weight loss of the C-A-S-H gels with increasing Na2O concentration. This indicates that increased pH has contributed to the increase in the quantity of C-A-S-H gels. The weight losses of samples M2.0, M1.5, and M0.7 are 16.3%, 17.3%, and 21.0%, respectively. This increased weight loss is consistent with the XRD results.

Fig.4 (a) XRD patterns and (b) TG/DTG curves of slag pastes with different moduli (S: C-A-S-H gels, C: calcite, A: akermanite)
This combination of techniques definitively demonstrates that as the concentration of Na2O is increased, more C-A-S-H gels are formed. This behavior has been widely described in previous studies[8,9], where AAS binders containing higher Na2O concentration tend to exhibit a higher degree of hydration and more hydration products, having a positive effect on the strength of the material. However, most studies do not address the variation in the degree of polymerization of C-A-S-H gels with alkali concentration and its effect on the material strength.
3.4 29Si NMR analysis of C-A-S-H
NMR is the most suitable characterization method for the amorphous structures of C-A-S-H gels[25,26]. Usually, Qn(mAl) is used to represent the different chemical environments of Si in C-A-S-H gels, where Q refers to the Si in the [SiO4]4-unit,nrefers to the number of bridging oxygens linked to other Si or Al atoms for each Si in [SiO4]4-unit, andmrefers to the number of [AlO4]5-units connected to the [SiO4]4-unit[27,28]. The29Si NMR analyses of slag and hydrated pastes after 28 days of curing are presented in Fig.5.
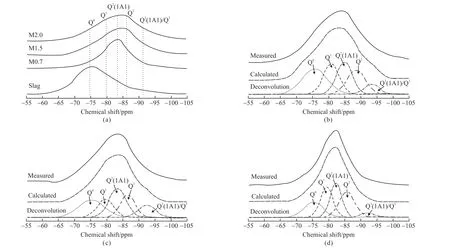
Fig.5 (a) 29Si NMR spectra of slag and hydrated pastes after 28 days of curing, and the deconvolution of 29Si NMR spectra of (b) sample M2.0, (c) sample M1.5, and (d) sample M0.7
As shown in Fig.5(a), only a broad resonance at -74.5 ppm attributed to the Q0site is identified in the29Si NMR spectrum of unreacted slag. This observation is supported by other studies[6,27]. Compared with unreacted slag, the Q1unit at -79 ppm, the Q2(1Al) unit at -83 ppm, and the Q2unit at -86 ppm are identified in all hydrated samples, implying that C-A-S-H gels have formed[29,30]. Further, Q3(1Al) and Q3sites at around -92 ppm are found in all samples. This has been supported in the studies by Puertaset aland Burciaga-Diazet al, where waterglass is used as the activator[30,31]. The presence of Q3(1Al) and Q3units indicates the production of a branched chain[26]. Based on the tobermorite-type model, these branches suggest the formation of linkages between different linear chains, forming highly crosslinked structures of C-A-S-H gels. Moreover, the linkage locations are limited to the bridging sites[32].
In order to clearly distinguish these sites and calculate their relative quantity, the deconvolution of the29Si NMR spectra of slag pastes was carried out. As shown in Figs.5(b)-5(d), the content of each site distinctly varies with modulus, implying that the structure of C-A-S-H gels is significantly altered by the different alkali concentrations. The detailed quantification of the different Qn(mAl) sites is shown in Table 4. The degree of hydration (HD) of slag, the mean chain length (MCL), and Al/Si ratio of C-A-S-H gels were calculated from the quantification of the Qnsites, and the calculation formulas are as follows[33,34]:
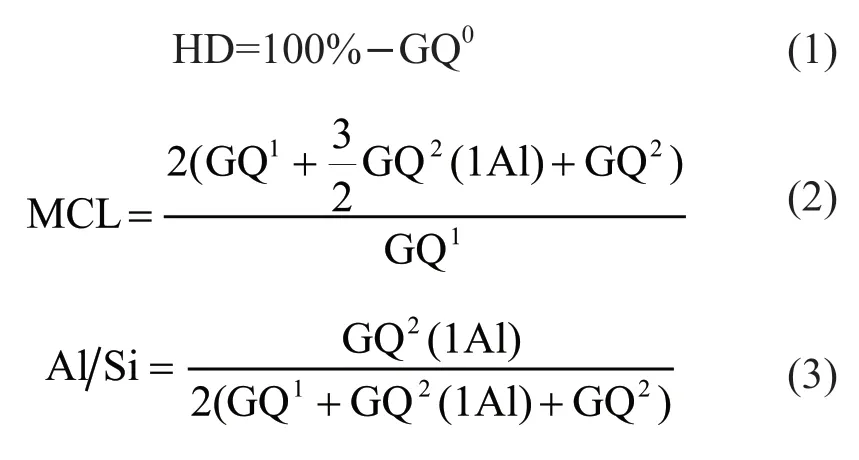
As shown in Table 3, a higher concentration of Na2O yielded a higher HD, indicating the presence of more C-A-S-H gels. The glassy structures of slag can be effectively destroyed under higher pH condition[8,9]. It has been reported that the slag HD can reach up to 80% when waterglass is used as an activator[6,25]. For sample M2.0 with the lowest Na2O concentration, the slag HD is as low as 70.32% while those of sample M1.5 and M0.7 are 74.73% and 81.00%, respectively. The increasing trend of slag HD is consistent with the results of pH evolution, XRD, and TGA. The positive effect of higher slag HD on the strength of AAS binders has been widely accepted; however, an apparent reduction in strength is observed in this study (see section 3.1). Thus, it is debatable to illustrate the evolution of strength based solely on the HD of slag.
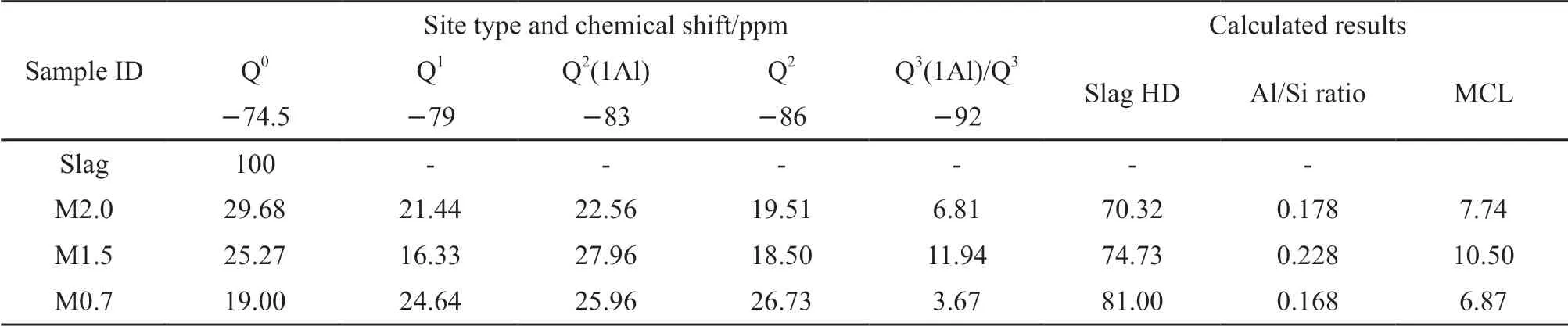
Table 3 Quantification of the deconvolution of different Qn sites in the 29Si NMR spectra along with calculated results for HD, MCL, and Al/Si of slag pastes with different moduli cured for 28 days
As previously reported[30,31], the Q3(1Al) and Q3units were formed in slag pastes activated by waterglass. In this study, it is difficult to clearly distinguish the two because of the overlap and low concentrations. The formation of Q3(1Al) and Q3units implies that these C-A-S-H gels are three-dimensional structures. Sample M1.5 has the highest percentage of Q3(1Al)/Q3(11.94%), implying a highly cross-linked structure of C-A-S-H gels. This proportion sharply reduces to 6.81% for sample M2.0 and 3.67% for sample M0.7, respectively. This reduction is probably related to the decrease in the MCL of the C-A-S-H gels.
The MCL refers to the average number of tetrahedra between two empty bridging sites, reflecting the degree of polymerization of the C-A-S-H gels[35]. The MCL reaches a maximum value of 10.5 when the modulus is 1.5. Several studies have shown that the MCL can be over 12.0 in waterglass-activated slag systems[25,36]. C-A-S-H gels with a high degree of polymerization may partly contribute to the maximum 28-day strength of sample M1.5. The MCL of sample M2.0 with the lowest alkali dosage is only 7.74. This is related to the low concentration of dissolved [SiO4]4-and [AlO4]5-units in the pore solution caused by the lower slag HD. However, sample M0.7 with the highest alkali dosage shows the lowest MCL (6.87). Consequently, the formation of a long MCL is likely to be controlled by the alkali concentration (details to be presented in section3.6). Moreover, it is worth noting that the MCL of C-A-S-H gels with high Al/Si ratio is likely to be large. This trend was also described by previous studies, demonstrating that Al can fill the empty bridging sites, giving rise to longer MCL for C-A-S-H gels[37,38].
More remarkably, the evolution of MCL is in line with the development of strength. A few studies have reported that AAS binders including C-A-S-H gels with higher MCL tend to show higher strength, but, without detailed data and analysis[14,19,20]. We have demonstrated that the quantity of C-A-S-H gels increases with increasing Na2O concentration while there is an evident reduction in the degree of polymerization when the Na2O concentration exceeds a certain level. The evolution of strength depends strongly on the quantity and degree of polymerization in C-A-S-H gels. For a given range, both increase with Na2O concentration, cooperatively enhancing strength. However, when the Na2O concentration is excessive, the positive effect of the former is counteracted by the adverse effect of the latter, resulting in decreased strength. More information about the microstructure of C-A-S-H gels and the variation mechanism of polymerization degree are presented in the following section.
3.5 Microstructure analysis of C-A-S-H gels
Magnified images of C-A-S-H gels are shown in Fig.6. There is a strong possibility that the microstructures of C-A-S-H gels are significantly affected by the degree of polymerization. The C-A-S-H gels in samples M2.0 and M0.7 exhibit a coarse microstructure. Numerous fine particles with varied sizes loosely gather together and eventually form an amorphous reticulated structure with a large number of disconnected pores. This porous structure is associated with a low degree of polymerization, adversely affecting the strength of the structure. With increasing degree of polymerization, the number of pores is distinctly reduced as are the sizes, forming the more compact and smoother microstructure of sample M1.5. It is this dense structure that yields the highest compressive strength. The SEM images once again reveal that the degree of polymerization can significantly affect the microstructure of C-A-S-H gels and thus their final strength.

Fig.6 SEM images of C-A-S-H gels in slag pastes cured for 28 days: (a) M2.0, (b) M1.5, (c) M0.7
3.6 Variation mechanism of the degree of polymerization of C-A-S-H gels
3.6.1 Dissolution and monomer formation
Under the attack of an alkaline solution, active ions such as Si4+and Al3+are released into the pore solution because of the destruction of the vitreous phase of slag. The dissolved Si4+and Al3+ions mainly exist in the form of Si(OH)4and Al(OH)4-in the pore solution. These species continue to react with OH-to form [OSi(OH)3]-, [(OH)2SiO2]2-, and [(OH)SiO3]3-monomers[39,40]via the chemical reactions shown in Eq.(4) through Eq.(6):
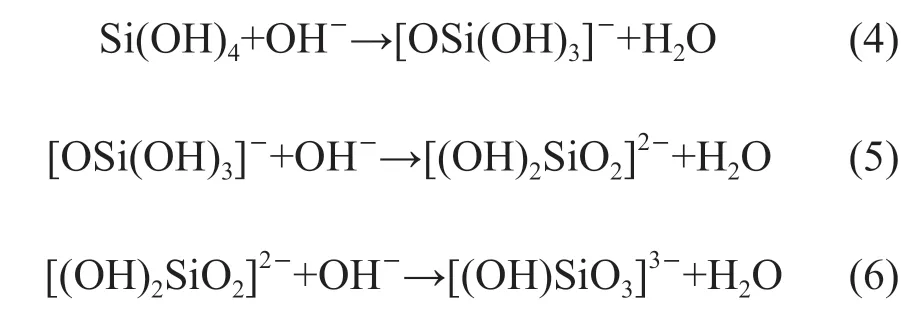
An increase in the OH-concentration promotes these chemical reactions[41]. Therefore, it is readily apparent why the concentration of each monomer will be significantly impacted by changes in the alkali concentration. Although the specific species involved in this process cannot be verified because of the complexity of the chemical reactions, it is reasonable to assume that the concentrations of [(OH)2SiO2]2-and [(OH)SiO3]3-monomers tend to be higher in the presence of higher OH-concentrations. Consequently, sample M0.7 may contain more [(OH)2SiO2]2-and [(OH)SiO3]3-monomers because it has the highest pH in the pore solution than those of samples M2.0 and M1.5. 3.6.2 Formation of C-A-S-H gels
Under highly alkaline conditions, Al(OH)4-, [OSi(OH)3]-, [(OH)2SiO2]2-, and [(OH)SiO3]3-monomers are produced. Polycondensation between aluminate and silicate monomers takes place via the attraction between hydroxyl groups, producing a significant amount of oligomers (i e, dimers and trimers)[42-44]. It has been reported that polycondensation rapidly occurs among Si(OH)4,species in alkaline solution[45,46]. These oligomers continuously react with other species to form larger polymers. Subsequently, these long aluminosilicate chains combine with Ca2+via physical electrostatic interactions to form C-A-S-H gels with a high degree of polymerization. In contrast, the polycondensation reactions between [(OH)2SiO2]2-and [(OH)SiO3]3-are less like take place because of their of electrostatic repulsion[45]. Although these two monomers with a high negative surface charge may react with Si(OH)4, Al(OH)4-, and [OSi(OH)3]-, the end products are limited to shorter chain oligomers rather than long polymers[46], which produces a C-A-S-H structure with a low degree of polymerization. The polycondensation reactions among these species are also supported by their Gibbs free energies[47,48].
Therefore, it is possible that a substantial quantity of species with a high negative surface charge exists in the sample M0.7, inhibiting the formation of long aluminosilicate chains because of electrostatic repulsion, thereby producing C-A-S-H gels with a low degree of polymerization. On the other hand, the C-A-S-H gels with a low degree of polymerization are formed in sample M2.0 because of the lower concentration of monomers caused by reduced pH. With regard of to sample M1.5, the moderate concentration of each monomer may produce the highest degree of polymerization in the C-A-S-H gels that are formed.
4 Conclusions
This study demonstrates the variation in the degree of polymerization of C-A-S-H gels and its role in the evolution of strength of slag binders activated by waterglass with different Na2O concentrations. The following conclusions are drawn:
a) The compressive strength of waterglass-activated binders is significantly affected by the modulus (Na2O concentration). Although the pH of the pore solution continuously increased with increasing Na2O concentration, the strength of the mortar reached a maximum when the modulus was 1.5, and subsequently reduced drastically.
b) XRD and TG analyses confirmed that C-A-S-H gels were the dominant hydration products, and that increasing the Na2O concentration increased the quantity of the C-A-S-H gels, which was consistent with what would be expected from the associated increase in pH.
c)29Si NMR analysis proved that a high Na2O concentration improved the degree of hydration of slag, and yielded more C-A-S-H gels. However, excessive Na2O concentration adversely affected the degree of polymerization of the C-A-S-H gels, which resulted in a reduction in strength.
d) SEM images revealed that C-A-S-H gels with a low polymerization degree exhibited a coarse microstructure with several disconnected pores. In contrast, a more compact and smoother microstructure was observed when the degree of polymerization was high, resulting in improved strength.
e) Based on the set of proposed chemical reactions for the formation of C-A-S-H gels, monomers with a high negative surface charge, such as [(OH)2SiO2]2-and [(OH)SiO3]3-, were preferentially produced in the case of high Na2O concentration. Because of electrostatic repulsion, these monomers could only form short-chain oligomers, resulting in C-A-S-H gels with a low degree of polymerization.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Synthesis of 1,1,3,3,5,5-hexamethyl-1,5-bis[(3-ethyl-3-methoxyoxetane)propyl]trisiloxane and Research on Its UV-curing Performance
- Preparation of Gallic Acid-chitosan Copolymer and Its Antibacterial Activity against Staphylococcus Aureus and Escherichia Coli
- Cadmium-containing Wastewater Induced by SRB: Effect of Multiple Ions and Mineralization Process
- Microstructure Transition and Grain Refinement Mechanism of Undercooled Alloys
- Improving Intergranular Corrosion Resistance in Inconel 625 via Grain Boundary Character Distribution Optimization
- Effect of Rolling Process on Microstructure and Wear Properties of High Carbon Equivalent Gray Cast Iron
