杏仁种皮酚类物质的低共熔溶剂提取及其抗氧化能力
2021-11-26钟海雁
熊 颖,周 波,钟海雁
杏仁种皮酚类物质的低共熔溶剂提取及其抗氧化能力
熊 颖1,2,3,周 波1,2,钟海雁1,2※
(1. 林产可食资源安全与加工利用湖南省重点实验室,长沙 410004;2. 中南林业科技大学食品科学与工程学院,长沙 410004; 3. 湖南省植物园,长沙 410116)
为了探讨低共熔溶剂(Deep Eutectic Solvents, DESs)对杏仁种皮酚类物质的提取率及其提取物抗氧化能力,以4种传统溶剂和5种低共熔溶剂为提取溶剂,比较评价了低共熔溶剂对杏仁种皮酚类物质的提取效果,并采用DPPH、ABTS自由基清除法和氧自由基吸收能力(Oxygen Radical Absorption Capacity, ORAC)分析杏仁种皮低共熔溶剂提取物的抗氧化能力。结果表明,低共熔溶剂对杏仁种皮酚类物质的提取效果更佳,其中氯化胆碱-草酸(DESs2)和氯化胆碱-苹果酸(DESs3)两组低共熔溶剂对杏仁种皮酚类的提取率分别为酸化甲醇的2.18和1.84倍;与酸化甲醇的杏仁种皮提取物相比,DESs2和DESs3杏仁种皮提取物的DPPH自由基清除能力分别提高了26.89%和73.13%,ABTS自由基清除能力分别提高了33.18%和114.81%,氧自由基吸收能力分别提高了14.92和17.73mol/g。HPLC结果表明传统溶剂的杏仁种皮提取液中酚类物质组成较复杂,而氯化胆碱-草酸(DESs2)、氯化胆碱-苹果酸(DESs3)和氯化胆碱-苹果酸-脯氨酸(DESs5)的杏仁种皮提取液中酚类物质组成较为单一,说明低共熔溶剂在提取酚类物质时具有很强的专一性。
酚类化合物;抗氧化能力;低共熔溶剂;杏仁种皮
0 引 言
杏仁种皮又被称为杏仁红衣、杏仁种皮,颜色深黄,是杏仁深加工过程中的副产物,除了少数被用作饲料之外,大部分都被丢弃[1]。杏仁种皮含有的酚类物质种类繁多,如没食子酸、原儿茶酸、绿原酸、没食子酸丙酯等[2-6],酚类物质除了具有清除自由基[7]、抗氧化[8]的功效之外,还具有抗菌[9-11]、抗动脉粥样硬化[12]、调节肠道菌群[13-14]、抗肿瘤[15-16]等功效。
低共熔溶剂(Deep Eutectic Solvents,DESs)是一种绿色溶剂,这类溶剂具有低毒、可降解、环境友好以及提取的高效性和目标性明确的特点。随着低共熔溶剂的不断研究和开发,国内外许多学者开始利用低共熔溶剂提取植物活性成分,如酚酸[17]、黄酮[18]、酚类物质[19]、皂苷[20]等。然而目前还没有关于低共熔溶剂提取杏仁种皮中酚类物质的研究报道。尽管很多DESs应用于活性物质提取的研究均证明了其有效性,但DESs在实际应用中仍存在的一些问题,尤其是DESs固有的低蒸气压使其从提取液中被回收具有一定难度,由此可见DESs作为新型绿色溶剂的全部潜力仍未开发。
本研究比较了杏仁种皮的5种低共熔溶剂和4种传统溶剂提取物的抗氧化能力,并分析了低共熔溶剂和传统溶剂杏仁种皮提取物酚类组成的区别,以期为杏仁种皮的开发利用和低共熔溶剂的高效利用提供新思路。
1 材料与方法
1.1 原料与试剂
杏仁种皮(山杏):河北省张家口市永昌源果仁食品有限公司;1,1-二苯基-2-三硝基苯肼(DPPH)分析纯上海源叶生物科技有限公司;福林酚分析纯合肥博美生物科技有限公司;2,2-偶氮二异丁基脒二盐酸盐(ABAP)标准品美国Sigma公司;没食子酸分析纯天津市科密欧化学试剂有限公司;甲醇色谱纯美国Sigma公司;乙酸色谱纯天津市化学试剂研究所有限公司;2,2-联氮-双(3-乙基苯并噻唑啉-6-磺酸)二铵盐(ABTS)分析纯上海阿拉丁生化科技股份有限公司;没食子酸标准品美国Sigma公司;原儿茶酸标准品美国Sigma公司;3,4-二羟基苯乙酸标准品美国Sigma公司;儿茶素标准品美国Sigma公司;绿原酸标准品美国Sigma公司;没食子酸丙酯标准品美国Sigma公司。
1.2 仪器与设备
数显控温磁力搅拌器HJ-4A江苏金坛市金城国胜实验仪器厂;恒温水浴锅XMTD-7000北京市永光明医疗仪器有限公司;离心机Centrifuge 5418 德国Eppendorf公司;UV1200紫外可见分光光度计上海美普达仪器公司;1200型高效液相色谱仪美国Agilent公司;Agile ZORBAX SB-C18分析型色谱柱4.6 mm×250 mm,5m 美国Agilent公司。
1.3 研究方法
1.3.1 提取前处理
取适量杏仁种皮放入粉碎机中初步磨粉,随后取出放入研钵中,加入适量液氮进一步研磨杏仁种皮粉,过80目筛,处理后的杏仁种皮用密封袋装好封口,于4 ℃保存。
综合文献方法和预试验结果[21-23]选用四种传统溶剂(Conventional Solvents,CS)对杏仁种皮酚类物质进行提取:60%甲醇(MeOH)、60%乙醇(EtOH)、酸化甲醇(MeAc,甲醇∶去离子水∶12 mol∶L HCl为70∶29∶1)和去离子水。
低共熔溶剂参考前人研究和预试验结果用相同的氢受体和不同的氢供体来制备[24-26]。将氢供体和氢受体置于锥形瓶中,在80 ℃水浴下不断搅拌至形成稳定无色透明的液体[27],由于低共熔溶剂黏度较大,为探究黏度对提取物的影响,本研究参考文献报道[28]设置了四个不同的浓度梯度(100%、90%、75%和50%,均为质量分数),制备的溶剂组分配比与名称如表1。

表1 低共熔溶剂的组成
1.3.2 提取方法
以1:10的料液比将杏仁种皮粉和溶剂置于10 mL烧杯中,保鲜膜封口,在25 ℃条件下不间断搅拌提取12 h。随后将混合物在4 000 r/min下离心20 min,取上清液并用对应溶剂定容至5 mL。
1.3.3 总酚含量和抗氧化能力测定
总酚的测定采用福林酚法,参照GB/T 8313-2008测定提取液和空白溶剂的吸光度,以提取液和空白溶剂的吸光度之差作为总酚吸光度。吸光度()与没食子酸浓度(,g/mL)的标准曲线为:=0.084 5+0.058 5,2=0.997 9。杏仁种皮总酚含量由公式(1)计算,杏仁种皮的总酚含量以没食子酸当量计(mg/g)。
式中为待测液总酚浓度,g/mL;为提取物定容体积,mL;为稀释因子;为杏仁种皮样品质量,g。
酚类物质的抗氧化能力采用DPPH、ABTS和ORAC(Oxygen Radical Absorbance Capacity)三种方法进行评价,三种评价抗氧化能力的方法均以Trolox为对照品,酚类物质的抗氧化能力以Trolox当量计(mol/g),以提取液和空白溶剂的抗氧化能力之差作为酚类物质的抗氧化能力。DPPH自由基清除能力的测定参考李志晓等[29]的方法,吸光度()和Trolox浓度(,mol/g)的标准曲线为:=−0.038 8+0.872 9,2=0.997 2。ABTS自由基清除能力的检测参考Luo等[30]的方法,吸光度()和Trolox浓度(,mol/g)的标准曲线为:=−0.021 39+0.678 2,2=0.995 4。ORAC测定按照本实验室方法进行[31],杏仁种皮ORAC氧自由基吸收能力以Trolox当量表示(mol/g)。曲线下面积()和Trolox浓度(,mol/g)的线性回归方程为:=0.002 8+ 0.274 3,2=0.997 3。
1.3.4 提取物HPLC分析
取适量1.3.2中的种皮提取液用色谱级甲醇适当稀释得待测液,离心后取2 mL上清液过0.45m滤膜,待测。由于实验室已根据本研究所用杏仁种皮的特性建立了杏仁种皮酚类物质的HPLC分析方法,因此采用本实验室方法设定HPLC参数[32]。
1.3.5 数据处理
数据计算采用Excel 2019进行,数据分析采用SPSS 22.0进行,图片绘制采用Origin 2018,所有数值均为三次平行测定的算术平均值。
2 结果与分析
2.1 提取物总酚含量对比
不同溶剂对杏仁种皮酚类物质提取的影响如图1a所示,图1a表明传统溶剂对杏仁种皮提取率为0.14~0.55 mg/g,而DESs对杏仁种皮的提取率为0.14~1.20 mg/g,且不同DESs对杏仁种皮酚类物质的提取效果也有较大的差别,这可能是因为溶剂组分搭配不同使得溶剂和酚类物质之间作用力有所区别,从而导致对酚类物质的提取效果出现差异[33],总体看来DESs对杏仁种皮酚类物质的提取效果要普遍优于传统溶剂。
此外如图1a所示,DESs对杏仁种皮酚类物质的提取率受溶剂浓度影响较大,且不同种类DESs对酚类物质提取率随着浓度变化而表现出不同的变化趋势。DESs1的提取率随浓度降低而升高,这可能是由于氯化胆碱呈碱性而甘油呈中性导致DESs1呈碱性,植物本身的生理环境呈弱酸性[34],因此高浓度DESs1的酚类提取率较低,但水的加入能冲淡溶剂的碱性,因此50%DESs1的提取率为100%DESs1的5.5倍。随着溶剂浓度从100%降低至50%,DESs2、DESs3和DESs5的杏仁种皮提取率分别减少了0.88、0.71和0.32 mg/g,这是因为这三种溶剂组分中含有机酸,酸性溶剂具有更强的极性,因此更加有利于酚类物质的提取,这三个组别中低浓度溶剂的提取效果较差是因为加水导致溶剂酸度降低从而影响酚类提取率[35]。在一定浓度范围内DESs4溶剂浓度的降低导致总酚提取率有所降低,但当浓度继续降低时总酚提取率会有所增加,这可能是因为水不仅能在一定程度上减弱溶剂与酚类物质之间的作用力还会降低溶剂黏度,因此导致DESs4组别的提取率出现先降后增的趋势[36]。
2.2 提取物抗氧化能力对比
从图1b~图1d可以看出各组提取物的抗氧化能力均存在较大的区别,且各组提取物的DPPH、ABTS和ORAC(氧自由基清除能力)均与总酚含量变化的趋势基本一致,这说明总酚含量对提取物的抗氧化能力有较大影响。传统溶剂提取物的三种自由基清除能力由高到低依次是酸化甲醇、乙醇、甲醇、去离子水,DESs2和DESs3组的杏仁种皮提取物由于总酚含量较高,因此具有较强的抗氧化能力。通过对比可知相同组别提取物的抗氧化能力与其总酚含量变化趋势基本一致,而不同组别提取物抗氧化能力与其总酚含量变化趋势无明显规律可循,这可能是由于提取物中酚类物质种类和含量复杂程度不一,酚类之间或酚类与其他物质之间存在协同作用从而影响到对自由基的清除效果[37-38],因此推测相同组别提取物酚类组成比较类似,但提取量存在一定的区别,不同种类DESs提取物中酚类化合物的组成和提取量均存在一定差异。
2.3 HPLC结果分析
杏仁种皮中天然活性成分种类十分丰富,为进一步讨论传统溶剂和低共熔溶剂对杏仁种皮酚类物质提取的针对性,本研究综合文献报道[2-6]和本实验室条件,采用HPLC对照5种标准品初步分析了杏仁种皮的四种传统溶剂和五种DESs提取物中酚类物质组成的差异。5种标准品的保留时间和标准曲线等信息如表2所示。
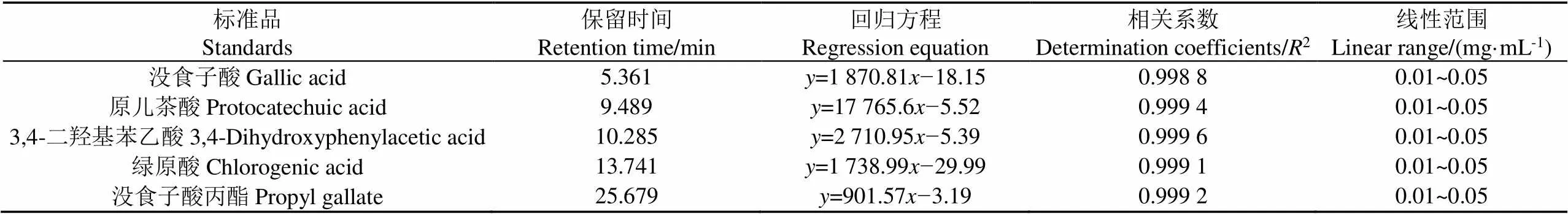
表2 各标准品的保留时间、回归方程和决定系数
部分样品HPLC对比图如图2所示,从图2a可以看出不同传统溶剂提取液所含的物质种类十分相似,甲醇、乙醇和酸化甲醇的杏仁种皮提取液中主要含有没食子酸、绿原酸和保留时间为10.134和11.916 min的物质,去离子水的杏仁种皮提取液中主要含有绿原酸、保留时间为24.403 min的化合物以及没食子酸丙酯,保留时间为10.134、11.916和24.403 min的物质被分别命名为2号、3号和4号物质,前人的研究也表明传统溶剂能对杏仁种皮中的没食子酸、绿原酸和没食子酸丙酯进行有效提取[2,5],但由于杏仁产地或品种的不同,提取物中具体酚类组成也有所区别。从图2b可以看出不同种类DESs提取物的组成区别较大,DESs1的种皮提取液中酚类化合物的组成与传统溶剂类似,DESs2和DESs3的种皮提取液中主要含有保留时间为7.314 min 的物质,该物质被命名为1号物质,DESs4提取物主要含有3号物质、3,4-二羟基苯乙酸和绿原酸,DESs5提取物中主要含有大量的原儿茶酸。
根据HPLC图谱可以得知各组提取物中主要含有没食子酸、原儿茶酸、3,4-二羟基苯乙酸、绿原酸、没食子酸丙酯和四种未知物。有标样酚类物质的含量对比结果如图3所示,图3表明传统溶剂对杏仁种皮中的没食子酸、绿原酸、没食子酸丙酯均有一定的提取效果。DESs1组对杏仁种皮酚类的提取效果与传统溶剂类似,且没食子酸的提取量受DESs1浓度影响较大,浓度为90%的DESs1对没食子酸具有较好的提取效果,这可能是因为DESs1在该浓度下的黏度与溶剂极性更有利于没食子酸的溶出和扩散。DESs2组的杏仁种皮提取物中几乎不含以上五种已知酚,仅在100%DESs2提取物中有少量没食子酸的存在。DESs3组别对杏仁种皮酚类的提取效果与DESs2类似,但100%DESs3对原儿茶酸有一定的提取作用。DESs4的杏仁种皮提取物中五种已知酚类的组成与传统溶剂类似,但各提取物含量受溶剂浓度影响较大。DESs5提取物中仅含有原儿茶酸且提取量受溶剂浓度影响较大,DESs5在不同浓度下对原儿茶酸的提取量介于0.19~0.47 mg/g,这可能是因为DESs5为多元DESs,且该溶剂的氢供体均为极性较强的有机酸,而提取物中的目标产物原儿茶酸拥有较强的分子极性,因此推测极性较强的溶剂更有利于杏仁种皮中原儿茶酸的提取。
未鉴定酚类物质的峰面积对比结果如图4所示。图4表明传统溶剂和DESs1对2号和3号物质的提取效果较好,对4号物质也有一定的提取效果,这在一定程度上表明了传统溶剂和DESs1对杏仁种皮酚类物质的针对性提取效果较差,提取物的抗氧化能力受多种酚类物质共同作用的影响。DESs2组杏仁种皮提取物主要含1号物质,这说明DESs2的组分对该物质的提取有较强的针对性,有报道表明DESs2的葡萄皮提取物中主要含各种黄酮苷[26],因此推测DESs2可能与各类黄酮苷的作用力较强,由此可知1号物质可能为某种黄酮苷。DESs3组别提取物主要含有1号物质和少量3号物质,且这两种酚类物质提取效果均受溶剂浓度影响很大,其中1号物质随着DESs3浓度降低而减少,而3号物质在溶剂浓度为75%时突然出现,这表明3号物质的提取对溶剂的极性有一定要求。DESs4提取物中未知酚的组成与传统溶剂相比更单一,4号物质仅在溶剂浓度为50%时才出现,这说明DESs对酚类的针对性是可调节的。DESs5提取物中不含任何未知酚,由此可见DESs5对原儿茶酸的提取有较强的针对性。
图3和图4均表明不同溶剂对提取物组成有较大的影响,其中DESs2、DESs3和DESs5在对杏仁种皮中的酚类进行提取时表现出了较强的专一性,这种专一性的出现往往与DESs的结构有关[39]。由于目前关于各种DESs具体形成机理的研究较少,根据Abbott等[40]提出的理论,低共熔溶剂的形成可简单描述为阳离子和阴离子与氢供体作用形成阳离子与配位阴离子。图5为本研究使用的五种DESs形成过程简图,各类DESs的氢供体与氯离子形成配位阴离子,而胆碱阳离子分子链较长,使得配位阴离子与酚类物质结合能力更强,这也是DESs对特定的某些酚类物质表现出高提取率的重要原因[41]。
DESs1和DESs4组由于氢供体为多元醇,处于不同位点的游离—OH基团对杏仁种皮中的酚类物质均有一定的作用力,因此导致这两组提取物的酚类物质组成比较复杂,前人的研究结果也印证了这一规律[42-44]。DESs2和DESs3的氢供体为多元有机酸,其中DESs2的氢供体为草酸,草酸高度对称的分子结构使得配位阴离子对酚类物质的作用力较为集中,导致DESs2提取的目标产物为某一类物质,因此DESs2的杏仁种皮提取物酚类组成比较单一[26],DESs3的氢供体虽然也是多元有机酸,但分子结构不对称且有一个游离—OH基团,目标产物与溶剂作用位点较多,这在一定程度上决定了DESs3的杏仁种皮提取物比DESs2更复杂[45-46]。DESs5为多元DESs,目前对于多元DESs理化性质的研究较少,在本研究中DESs5的氢供体均为极性较强的有机酸,提取物中目标产物原儿茶酸的分子极性较强,因此推测当多元DESs中含有机酸时,提取物中的酚类极性较强的可能性较大,此外也有文献报道表明当DESs中含脯氨酸时,提取物的酚类组成更加单一[47],这与本研究中出现的现象是类似的。
氯化胆碱-草酸(DESs2)和氯化胆碱-苹果酸(DESs3)两组低共熔溶剂对杏仁种皮酚类的提取率分别为酸化甲醇的2.18和1.84倍;与酸化甲醇的杏仁种皮提取物相比,DESs2和DESs3杏仁种皮提取物的DPPH自由基清除能力分别提高了26.89%和73.13%,ABTS自由基清除能力分别提高了33.18%和114.81%,氧自由基吸收能力分别提高了14.92和17.73mol/g。
3 结 论
本研究采用低共熔溶剂和传统溶剂进行杏仁种皮酚类物质的提取,主要从总酚得率、抗氧化能力和提取物组成三个方面进行了对比。不同溶剂对杏仁种皮酚类物质提取率和提取物的抗氧化能力有较大影响,低共熔溶剂对杏仁种皮酚类物质的提取与传统溶剂相比具有明显的优势,其中氯化胆碱-草酸(DESs2)和氯化胆碱-苹果酸(DESs3)两组低共熔溶剂的杏仁种皮酚类提取率分别为酸化甲醇的2.18和1.84倍,提取物的DPPH自由基清除能力分别提高了13.32和36.22mol/g,ABTS自由基清除能力分别提高了9.59和33.18mol/g,氧自由基吸收能力分别提高了17.73和14.92mol/g。同组低共熔溶剂在不同浓度下种皮提取物抗氧化能力与提取物的总酚含量呈正相关,不同种类低共熔溶剂杏仁种皮提取物的抗氧化能力与总酚含量之间无明显规律,低共熔溶剂杏仁种皮提取物与传统溶剂相比普遍具有更高的总酚含量和更强的抗氧化能力。
通过HPLC分析,DESs2、DESs3和DESs5由于组分中含有机酸,因此杏仁种皮提取物酚类组成与传统溶剂相比表现出很强的单一性,其中DESs5的杏仁种皮提取物中原儿茶酸含量高达0.19~0.47 mg/g,DESs1和DESs4由于组分中含多元醇,因此杏仁种皮提取物中酚类组成较复杂。与传统溶剂相比低共熔溶剂均表现出了良好的杏仁种皮酚类物质提取的优势。低共熔溶剂种类影响提取物酚类的组成,而溶剂浓度影响提取物中各酚类物质的含量。因此低共熔溶剂可以作为杏仁种皮酚类物质提取的绿色高效溶剂。
由于本研究是初步对比了低共熔溶剂与传统溶剂对杏仁种皮酚类物质的提取效果,还没有对未知峰进行物质鉴定,因此下一步还应对未知物质进行鉴定,对低共熔溶剂与不同酚类的作用机制进行研究,从而进一步说明低共熔溶剂的组成对杏仁种皮中酚类物质提取的影响。
[1] 吴东栋,张清安,范学辉,等. 苦杏仁种皮中生物活性成分的研究进展[J]. 食品与发酵工业,2019,45(7):288-293.
Wu Dongdong, Zhang Qing’an, Fan Xuehui, et al. Bioactive components of apricot kernel skin[J]. Food and Fermentation Industries, 2019, 45(7): 288-293. (in Chinese with English abstract)
[2] 陆彩瑞. 长柄扁桃和山杏种皮多酚的提取、成分鉴定及生物活性研究[D]. 西安:西北大学,2018.
Lu Cairui. Study on the Extraction, Chemical Composition and Biological Activity of Polyphenols fromPall and Apricot (L. Lam) seed coat[D]. Xi'an: Northwest University, 2018. (in Chinese with English abstract)
[3] Bolling B W, Dolnikowski Q, Blumberg J B, et al. Polyphenol content and antioxidant ability of California almonds depend on cultivar harvest year[J]. Food Chemistry, 2010, 122(3): 819-825.
[4] Monagas M, Garrido I, Lebrón-Aguilar R, et al. Almond ((Mill.)DA Webb) kernel coats as a potential source of bioactive polyphenol[J]. Journal of Agricultural and Food Chemistry, 2007, 55(21): 8498-8507.
[5] 侯双瑞. 烘焙对杏仁油氧化稳定性影响的研究[D]. 长沙:中南林业科技大学,2018.
Hou Shuangrui. Study on the Influence of Roasting on Oxidative Stability of Apricot Kernel Oil[D]. Changsha: Central South University of Forestry and Technology, 2018. (in Chinese with English abstract)
[6] Smeriglio A, Mandalmi Q, Bisignano C, et al. Polyphenolic content and biological properties of Avola almond (Mill. DA Webb) skin and its industrial byproducts[J]. Industrial Crops & Products, 2016, 83: 283-293.
[7] 赵强,刘乐,杨洁,等. 响应面法优化藜麦糠中多酚超声提取工艺及其抗氧化活性[J]. 中国粮油学报,2020,35(7):143-149.
Zhao Qiang, Liu Le, Yang Jie, et al. Optimization of ultrasonic-assisted extraction of polyphenols fromWilld. bran by response surface methodology and its antioxidant ability[J]. Journal of the Chinese Cereals and Oils Association, 2020, 35(7): 143-149. (in Chinese with English abstract)
[8] 张怡一,徐茜,陈琳,等. 薏米中多酚化合物的分离纯化及抗氧化活性分析[J]. 食品科学,2017,38(13):26-33.
Zhang Yiyi, Xu Qian, Chen Lin, et al. Isolation and purification of polyphenols from adlay and their antioxidant ability[J]. Food Science, 2017, 38(13): 26-33. (in Chinese with English abstract)
[9] 戴航宇,滕春莹,张荣涛. 桦褐孔菌多酚抑菌活性分析研究[J]. 中国酿造,2020,39(11):109-115.
Dai Hangyu, Teng Chunying, Zhang Rongtao. Antibacterial ability of polyphenols from[J]. China Brewing, 2020, 39(11): 109-115. (in Chinese with English abstract)
[10] 王亚萍,费学谦,陆宽宽,等. 油茶籽饼粕中甲醇提取物抑制黄曲霉菌效果及成分分析[J]. 农业工程学报,2019,35(11):322-329.
Wang Yaping, Fei Xueqian, Lu Kuankuan, et al. Inhibitory effect ofand component analysis of methanol extraction from camellia seed cake[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019, 35(11): 322-329. (in Chinese with English abstract)
[11] 葛达娥,魏照辉,图尔荪阿依·图尔贡,等. 丁香酚对蓝莓链格孢霉的抑制作用[J]. 食品科学,2020,41(19):68-73.
Ge Da’e, Wei Zhaohui, Tursunay·Turgun, et al. Inhibitory effect of eugenol onsp. isolated from blueberry[J] Food Science, 2020, 41(19): 68-73. (in Chinese with English abstract)
[12] 何忠梅,李成恩,段翠翠,等. 短梗五加果多酚预防大鼠动脉粥样硬化作用[J]. 食品科学,2018,39(1):200-206.
He Zhongmei, Li Cheng’en, Duan Cuicui, et al. Preventive effect of polyphenols isolated fromfruits on atherosclerosis in rats[J]. Food Science, 2018, 39(1): 200-206. (in Chinese with English abstract)
[13] 刘冬敏,黄建安,刘仲华. 肠道微生物与茶及茶多酚的相互作用在调节肥胖及并发症中的作用[J]. 天然产物研究与开发,2018,30(9):1640-1648.
Liu Dongmin, Huang Jian’an, Liu Zhonghua. The regulation effect of interaction between gut microbiota and tea polyphenols in obesity and comorbidity[J]. Natural Product Research and Development, 2018, 30(9): 1640-1648. (in Chinese with English abstract)
[14] 徐卓,项想,尚尔鑫,等. 丹参茎叶总酚酸对2型糖尿病肾病小鼠肠道菌群和短链脂肪酸的调节作用[J/OL]. 药学学报:1-26 [2020-11-29]. https://doi.org/10.16438/j.0513-4870.2020-1259.
Xu Zhuo, Xiang Xiang, Shang Erxin, et al. Regulatory effect of total phenolic acid from the stems and leaves ofon intestinal microflora and short-chain fatty acids in type 2 diabetic nephropathy mice[J/OL]. Acta Pharmaceutica Sinica: 1-26 [2020-11-29]. https://doi.org/10.16438/j.0513-4870.2020-1259. (in Chinese with English abstract)
[15] 孔德栋,赵悦伶,王岳飞,等. 茶多酚对肿瘤免疫逃逸的抑制机制研究进展[J]. 浙江大学学报:农业与生命科学版,2018,44(5):539-548.
Kong Dedong, Zhao Yueling, Wang Yuefei, et al. Review on inhibition mechanism of tea polyphenols against tumor immune escape[J]. Journal of Zhejiang University: Agriculture and Life Sciences, 2018, 44(5): 539-548. (in Chinese with English abstract)
[16] 钟上勇,李星毅,王艳红,等. 酚类天然产物Buxifoximes A的合成及生物活性[J]. 四川大学学报:自然科学版,2020,57(2):348-351.
Zhong Shangyong, Li Xingyi, Wang Yanhong, et al. Total synthesis and biological evaluation of phenolic natural product Buxifoximes A[J]. Journal of Sichuan University: Natural Science Edition, 2020, 57(2): 348-351. (in Chinese with English abstract)
[17] Park H E, Tang B, Row K H. Application of deep eutectic solvents as additives in ultrasonic extraction of two phenolic acids from[J]. Analytical Letters, 2014, 47(9): 1476-1484.
[18] 熊苏慧,唐洁,李诗卉,等. 一种新型天然低共熔溶剂用于玉竹总黄酮的绿色提取[J]. 中草药,2018,49(10):2378-2386.
Xiong Suhui, Tang Jie, Li Shihui, et al. A new type of natural eutectic solvent for green extraction of total flavonoids from[J]. Chinese Traditional and Herbal Drugs, 2018, 49(10): 2378-2386. (in Chinese with English abstract)
[19] Stefou I, Grigorakis S, Loupassaki S. Development of sodium propionate-based deep eutectic solvents for polyphenol extraction from onion solid wastes[J]. Clean Technologies and Environmental Policy, 2019, 21(8): 1563-1574.
[20] Riberio B D, Coelho M A Z, Marrycho I M. Extraction of saponins from sisal () and jua () with cholinium-based ionic liquid and deep eutectic solvents [J]. European Food Research and Technology, 2013, 237: 965-975.
[21] 杜凯,马养民,郭林新. 杏仁皮单宁提取工艺优化及其DPPH自由基清除活性[J]. 食品工业科技,2019,40(21):174-178.
Du Kai, Ma yangmin, Guo Linxin. Optimization of extraction process of tannins from almond skin and its DPPH radical scavenging activity[J]. Science and Technology of Food Industry, 2019, 40(21): 174-178. (in Chinese with English abstract)
[22] 姜丽巍,白娟,张海婷,等. 山杏仁皮多酚提取及其抑菌和抗氧化性研究[J]. 现代食品,2019(9):182-185,189.
Jiang Liwei, Bai Juan, Zhang Haiting, et al. Study on extraction of polyphenols from almond peel and their antibacterial and antioxidant activities[J]. Modern Food, 2019(9): 182-185, 189. (in Chinese with English abstract)
[23] Bisignano C, Mandalari G, Smeriglio A, et al. Almond skin extracts abrogate HSV-1 replication by blocking virus binding to the cell[J]. Viruses, 2017, 9(7):178. https://doi.org/10.3390/v9070178.
[24] Saha S K, Dey S, Chakraborty R. Effect of choline chloride-oxalic acid based deep eutectic solvent on the ultrasonic assisted extraction of polyphenols from Aegle marmelos[J/OL]. Journal of Molecular Liquids, 2019, 287:110956. http://doi.org/10.1016/j.molliq.2019.110956.
[25] Luo Q, Zhang J R, Li H B, et al. Green Extraction of Antioxidant Polyphenols from Green Tea (Camellia sinensis)[J/OL]. Antioxidants. 2020, 9(9): 785. https://doi.org/10.3390/antiox9090785.
[26] Bubalo C M, C´urko N, Tomaševic M, et al. Green extraction of grape skin phenolics by using deep eutectic solvents[J]. Food Chemistry, 2016, 200(1): 159-166.
[27] Dai Y T, Witkamp G J, Verpoorte R, et al. Tailoring prop erties of natural deep eutectic solvents with water to facilitate their applications[J]. Food Chemistry, 2015, 187(15): 14-19.
[28] 尚宪超,谭家能,杜咏梅,等. 超声辅助深共熔溶剂提取两种烟草多酚的方法研究[J]. 中国烟草科学,2017,38(6):55-60.
Shang Xianchao, Tan Jianeng, Du Yongmei, et al. Green extraction of two target tobacco phenolic compounds using deep eutectic solvents[J]. Chinese Tobacco Science, 2017, 38(6): 55-60. (in Chinese with English abstract)
[29] 李志晓,金青哲,叶小飞,等. 精炼过程中油茶籽油活性成分和抗氧化性的变化[J]. 中国油脂,2015,40(8):1-5.
Li Zhixiao, Jin Qingzhe, Ye Xiaofei, et al. Changes of bioactive constituents and antioxidant ability of oil-tea camellia seed oil during refining[J]. China Oils and Fats, 2015, 40(8): 1-5. (in Chinese with English abstract)
[30] Luo W, Zhao M, Yang B, et al. Identification of bioactive compounds inL. fruit and their free radical scavenging activities[J]. Food Chemistry, 2009, 114(2): 499-504.
[31] 陈佩云,钟海雁. 热回流和超声条件下不同溶剂对油茶粕多酚提取的影响[J]. 食品与机械,2016,32(4):172-175,186.
Chen Peiyun, Zhong Haiyan. Effects of different solvents on extraction of phenolics from oil-tea meal under hot reflux and ultrasonic assistances[J]. Food & Machinery, 2016, 32(4): 172-175, 186. (in Chinese with English abstract)
[32] 孙亚娟. 不同烘焙温度对带种皮压榨杏仁油品质特性的影响[D]. 长沙:中南林业科技大学,2017.
Sun Yajuan. Influence of Different Roasting Temperature on Quality Characteristics of Oil Pressed With Skins Ofapricot Kernel[D]. Changsha: Central South University of Forestry and Technology, 2017. (in Chinese with English abstract)
[33] García A, Rodríguez-Juan E, Rodriguéz-Gutierrez G, et al. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs)[J]. Food Chemistry, 2016, 197(15): 554-561.
[34] Dai Y T, van Sprosen J, Witkamp G J, et al. Natural deep eutectic solvents as new potential media for green technology[J]. Analytical Chimica Acta, 2013(766): 61-68.
[35] Oliveria F S, Pereiro A B, Rebelo L P N, et al. Deep eutectic solvents as extraction media for azeotropic mixtures[J]. Green Chemistry, 2013, 15: 1326-1341.
[36] 黄皓,王珍妮,李莉,等. 甘油水溶液提取米糠多酚绿色工艺优化及多酚种类鉴定[J]. 农业工程学报,2019,35(4):305-312.
Huang Hao, Wang Zhenni, Li Li, et al. Optimization of green extraction process and identification of polyphenols variety from rice bran using glycerol/water system[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019, 35(4): 305-312. (in Chinese with English abstract)
[37] 羿月同,李莎莎,樊梓鸾,等. 红豆越橘花青素与金银花多酚协同抗氧化活性[J/OL]. 精细化工:1-8 [2021-03-19]. https://doi.org/10.13550/j.jxhg.20200980.
Yi Yuetong, Li Shasha, Fan Ziluan, et al. Synergism antioxidation of lingonberry anthocyanin and Lonicera japonica polyphenols[J/OL]. Fine Chemicals: 1-8 [2021-03-19]. https://doi.org/10.13550/j.jxhg.20200980. (in Chinese with English abstract)
[38] Su S W, Wang R C, Guo S T, et al. Walnut phenolic compounds: Binding with proteins and antioxidant activities[J]. Transactions of the Chinese Society of Agricultural Engineering, 2016, 32(22): 309-314.
[39] Florindo C, Oliveira F S, Rebelo L P N, et al. Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(10): 2416-2425.
[40] Abbott A P, Harris R C, Ryder K S. Application of hole theory to define ionic liquids by their transport properties[J]. The Journal of Physical Chemistry B, 2007, 111: 4910-4913.
[41] Zhang Y, Li Z Y, Wang H Y, et al. Efficient separation of phenolic compounds from model oil by the formation of choline derivative-based deep eutectic solvents[J]. Separation and Purification Technology, 2016, 163: 310-318.
[42] Peng X, Duan M H, Yao X H, et al. Green extraction of five target phenolic acids fromwith deep eutectic solvent[J]. Separation and Purification Technology, 2016, 157: 249-257.
[43] Wei Z, Qi X, Li T, et al. Application of natural deep eutectic solvents for extraction and determination of phenolics inleaves by ultra performance liquid chromatography[J]. Separation and Purification Technology, 2015, 149: 237-244.
[44] Qi X L, Peng X, Huang Y Y, et al. Green and efficient extraction of bioactive flavonoids fromL. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment[J]. Industrial Crops & Products, 2015, 70: 142-148.
[45] Wei Z F, Wang X Q, Peng X, et al. Fast and green extraction and separation of main bioactive flavonoids from[J]. Industrial Crops & Products, 2015, 63: 175-181.
[46] Li J, Han Z G, Zou Y P, et al. Efficient extraction of major catechins inleaves using green choline chloride-based deep eutectic solvents[J]. RSC Advances, 2015, 5(114): 93937-93944.
[47] Nam M W, Zhao J, Lee M S,et al. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: application to flavonoid extraction from[J]. Green Chemistry, 2015, 17(3): 1718-1727.
Antioxidant capacity of phenols from apricot kernel coat extracted with deep eutectic solvent
Xiong Ying1,2,3, Zhou Bo1,2, Zhong Haiyan1,2※
(1.,410004,;2.,,410004,;3.,410116,)
A new green solvent (Deep Eutectic Solvents, DESs) was used to investigate the antioxidant ability and phenolic composition of apricot kernel coat extracts. In this study, four kinds of conventional solvents and five kinds of deep eutectic solvents were also selected with different concentrations, thereby determining the extraction efficiency of apricot kernel coat polyphenols. DPPH and ABTS free radical scavenging ability and Oxygen Radical Absorption Capacity (ORAC) were used to evaluate the antioxidant ability of apricot kernel coat extracts. Furthermore, a High-Performance Liquid Chromatography (HPLC) was utilized to compare the phenolic composition of apricot kernel coat extracts by conventional and deep eutectic solvents. The standards were also set as gallic acid, protocatechuic acid, 3,4-dihydroxy phenylacetic acid, chlorogenic acid, and propyl gallate. The results showed that the acidified methanol among the conventional solvents presented the best extraction efficiency, where the extraction yield of phenols was 0.55 mg/g. The deep eutectic solvents showed a great difference in the extraction efficiency among different formulations and concentrations. However, the deep eutectic solvents presented a better extraction efficiency, compared with conventional solvents, especially that choline chloride - oxalic acid (DESs2) and choline chloride - malic acid (DESs3) presented a better extraction efficiency for the phenolic compounds from apricot kernel coat. The extraction yields of apricot kernel coat phenolic compounds by DESs2 and DESs3 were 2.20 and 1.85 times that of acidified methanol, respectively. Extracts by acidified methanol showed the best antioxidant ability among the conventional solvents, whereas, the antioxidant ability of apricot kernel coat extracts by deep eutectic solvents was also better than that of conventional solvents. The extracts by DESs3 showed a better DPPH and ABTS radical scavenging ability, followed by DESs2. Specifically, the DPPH and ABTS radical scavenging abilities of apricot kernel coat extracts by DESs3 were 1.73 and 2.15 times higher than that of acidified methanol. The apricot kernel coat extracts by DESs2 and DESs3 showed better oxygen radical absorption capacity, increasing by 14.92 and 17.73mol/g, respectively, compared with the acidified methanol. The results of HPLC showed that there were at least nine kinds of phenols, including gallic acid, protocatechuic acid, 3,4-dihydroxy phenylacetic acid, chlorogenic acid, propyl gallate, and four phenolic compounds with no standards in the extracts from the apricot kernel coat. The phenolic composition of extracts by conventional solvents was complex, and the extraction yields of each phenolic compound were relatively low. There were eight kinds of phenols in the extracts by conventional solvents, while there was a much simpler phenolic composition of extracts by deep eutectic solvents. Extracts by DESs2 and DESs3 contained a large quantity of phenolic components with a retention time of 7.314 min, while this component was not found in extracts by conventional solvents. There was only a large amount of protocatechuic acid in the extracts by choline chloride - malic acid - proline (DESs5), where the extraction yield of protocatechuic acid was 0.19-0.47 mg/g under different concentrations. Consequently, DESs can be expected to serve as an efficient solvent for the effective extraction of phenolic compounds from apricot kernel coats.
phenolic compounds; antioxidant ability; deep eutectic solvents; apricot kernel coat
熊颖,周波,钟海雁. 杏仁种皮酚类物质的低共熔溶剂提取及其抗氧化能力[J]. 农业工程学报,2021,37(15):289-298.doi:10.11975/j.issn.1002-6819.2021.15.034 http://www.tcsae.org
Xiong Ying, Zhou Bo, Zhong Haiyan. Antioxidant capacity of phenols from apricot kernel coat extracted with deep eutectic solvent[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2021, 37(15): 289-298. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2021.15.034 http://www.tcsae.org
2021-03-21
2021-05-06
湖南省教育厅科学研究重点项目(18A154);湖南省创新平台(2019TP1029)
熊颖,研究方向为林产可食资源高值化利用。Email:yuuei6666@163.com
钟海雁,教授,研究方向为木本粮油加工利用及林产可食资源高值化利用。Email:zhonghaiyan631210@126.com
10.11975/j.issn.1002-6819.2021.15.034
TS209
A
1002-6819(2021)-15-0289-10
