Pathological humerus fracture due to anti-interferon-gamma autoantibodies: A case report
2021-11-22ChengHsunYangFengChihKuoChenHsiangLee
Cheng Hsun Yang, Feng-Chih Kuo, Chen-Hsiang Lee
Cheng Hsun Yang, Division of Infection Disease, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung 833, Taiwan
Feng-Chih Kuo, Department of Orthopaedic Surgery, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung 833, Taiwan
Chen-Hsiang Lee, Division of Infection Disease, Department Internal Medicine, Chang Gung University, College Medicine, Chang Gung Memorial Hospital, Kaohsiung Medicine Centre, Kaohsiung 833, Taiwan
Abstract BACKGROUND Various etiologies contribute to pathological fractures, including bone infections. Recently, non-tuberculosis Mycobacterium-related bone infections among patients with anti-interferon-gamma autoantibody-induced adult-onset immunodeficiency has raised concerns in Southeast Asia, with the common presentations including osteomyelitis. However, it also rarely manifests as traumatic fractures, as reported in this case.CASE SUMMARY A diabetic female fractured her humerus after a traumatic accident and received fixation surgery. Abnormal necrotic bone tissue and abscess formation were noted, and she was diagnosed with a pathological fracture due to nontuberculosis Mycobacterium infection. Multiple bone involvement was also revealed in a bone scan. Anti-interferon-gamma autoantibodies were then checked due to an unexplained immunocompromised status and found to be positive. Her humerus fracture and multiple bone infections healed after steroid and anti-non-tuberculosis Mycobacterium medication treatment following fixation surgery.CONCLUSION Comprehensive preoperative evaluations may help identify pathological fractures and guide the treatment course.
Key Words: Immunocompromised status; Neutralizing anti-interferon-gamma autoantibody; Non-tuberculosis mycobacterium; Pathological fracture; Pre-operative evaluations; Case report
INTRODUCTION
Pathological fractures can be secondary to conditions ranging from metabolic diseases to tumors, infections, or neuromuscular pathologies. Unfortunately, those due to infections can be mistaken for malignant tumors[1]. Distinguishing between hematogenous osteomyelitis and bone tumors is difficult when there are no obvious clinical clues, and radiographic changes in osteomyelitis are often mistaken for tumors[2]. Herein, we present a diabetic female diagnosed with a humerus fracture after a traumatic injury. The purpose of this case report is to remind orthopedic surgeons not to overlook the possible diagnosis of a pathological fracture secondary to nontuberculosisMycobacterium(NTM) bone infections due to anti-interferon-gamma autoantibody (AIGA)-induced adult-onset immunodeficiency, a disease with increasing incidence in Southeast Asia in the recent decade, that can present as a traumatic fracture.
In this case, the AIGA presence itself was proposed to have occurred due to genetic susceptibility of human leukocyte antigen (HLA) genes[3] and through molecular mimicry, such as an epitope of interferon-gamma that mimicsAspergillusantigen (Noc-2 protein)[4], a known cause of this type of autoimmunity. The accumulation of environmentalAspergillusantigen exposure might gradually trigger an immune response, producing auto-antibody against human interferon-gamma and thereby resulting in opportunistic infections. Among these infections, NTM has prompted greater concern due to its being an important component of opportunistic infections. In this case report, we aimed to elucidate the NTM bone infection due to AIGAinduced adult-onset immunodeficiency that caused pathologic fracture which was overlooked as traumatic fracture.
CASE PRESENTATION
Chief complaints
A 69-year-old diabetic female presented with right upper arm pain with deformity.
History of present illness
The patient fell from her bed during the night and fractured her right humerus (Figure 1). She was then admitted for surgical fixation (Figure 2).
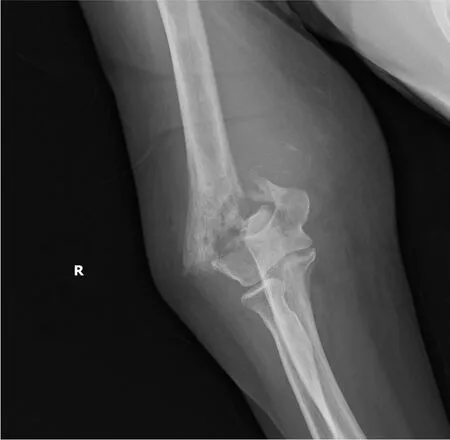
Figure 1 Displaced supracondylar humerus fracture of the right elbow on plain X-ray.
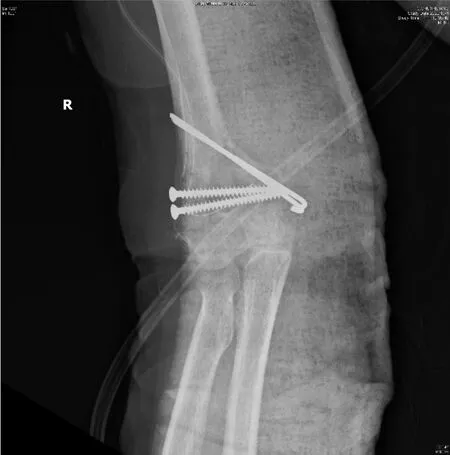
Figure 2 Displaced supracondylar humerus fracture status post-open reduction and internal fixation with screws and Kirschner wires.
History of past illness
The patient had diabetes mellitus type 2 with a hemoglobin A1c level of 5.8, under treatment with glipizide 1# twice daily.
Personal and family history
The patient had no hereditary malignancies or bone diseases and denied smoking cigarettes or drinking alcohol.
Physical examination
A painful right elbow deformity, tenderness, and limited range of motion were noted. After the fracture fixation surgery, the patient’s family found a non-healing wound with abscess formation that had developed on her chest wall near the sternum. Bilateral axillary lymphadenopathy was also noted.
Laboratory examinations
Unlike an acute traumatic fracture, necrotic bone tissues were noted and sequestrated intraoperatively (Figure 3). The specimen consisted of more than 10 tissue fragments, measuring up to 1.9 cm × 1.2 cm × 0.5 cm. Representative sections were taken, showing bone and fibrosynovial tissue with necrosis, granulation tissue proliferation, and marked acute and chronic inflammatory cell infiltration. A malignancy-related pathological fracture was suspected. Sternum abscess excision and pathology revealed skin tissue with subcutaneous necrosis, fat necrosis, and dense acute and chronic inflammatory cell infiltration, without malignancy.Mycobacterium intracellularewas culturedviapus drainage. A lymph node excision biopsy was also performed, and the pathology revealed necrotizing granulation without malignancy. An immunosuppression status was suspected due to opportunistic infections. The patient had a normal lymphocyte subpopulation and tested negative for human immunodeficiency virus (HIV). Neutralizing (n)AIGAs were then considered. Serologically, high titers of AIGAs (1:106) were observed.
Imaging examinations
Plain X-ray revealed a displaced supracondylar humerus fracture of the right elbow (Figure 1). A bone scan revealed increased uptake over the skull, spine, bilateral pelvis, femur, tibia, tarsal bones, scapulae, clavicles, humeri, right forearm, sternum, and rib cage, indicating probable multiple metastases (Figure 4).
FINAL DIAGNOSIS
Disseminated (d)NTM infection with lymphadenopathy and multiple bone lesions causing a right humerus pathological fracture and sternum abscess formation. nAIGAinduced adult-onset immunodeficiency.
TREATMENT
The patient was treated with open reduction and internal fixation with cancellous screws and Kirschner wires (Figure 2) for the fracture. She was administered cortisone (4 mg) 1# orally twice daily for the autoantibodies, and rifampicin, ethambutol, and clarithromycin for the dNTM infection.
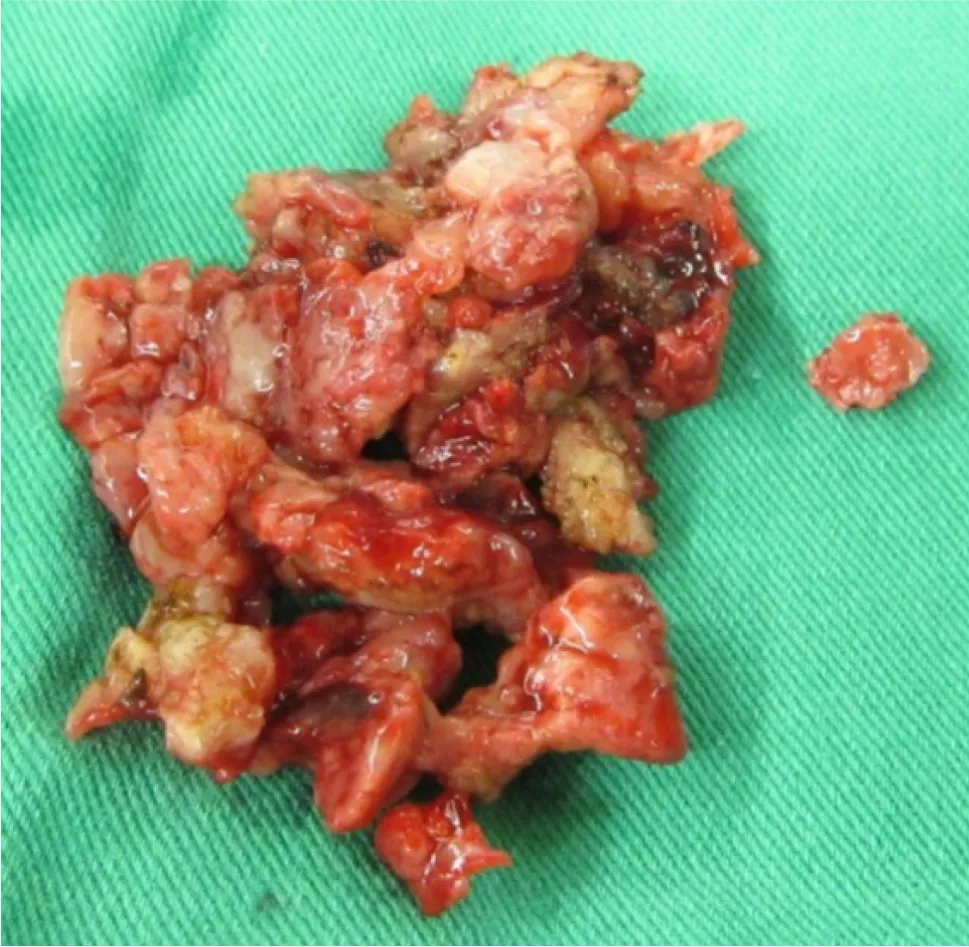
Figure 3 Intraoperative finding of unusual necrotic bone tissues, status post-sequestrectomy.

Figure 4 Bone scan revealed multiple uptake.
OUTCOME AND FOLLOW-UP
After a 1-mo treatment course, a physical examination showed that the humerus fracture had healed, and the sternum infection had also gradually healed along with the other infected bones. The patient will continue to receive regular treatment during future outpatient visits.
DISCUSSION
Etiologies of pathological fractures include bone infection, malignancy, endocrinopathy, osteomalacia, drug-related, rheumatological diseases, and inflammatory bowel disease[5]. While the underlying mechanism of a pathological fracture is important, as a general rule a pathological fracture is caused by minor trauma that typically would not cause the type of fracture observed. In bone infection-induced pathological fractures, the infection must be treated first, and the fractures can only heal once a favorable metabolic environment has been restored[1].
Various pathogens can cause bone infections, among which NTM is a rare but noteworthy cause, as is the present case. The incidence of NTM infection has increased in recent years, likely due to advances in diagnostic technology. In addition, the rate of dNTM infection in HIV carriers has fallen from 16% in 1996 to less than 1% today due to the introduction of effective anti-viral therapy for HIV and macrolide prophylaxis[6]. The cause of other non-HIV-related immunocompromising conditions in patients with dNTM infection is thus of concern, most notably nAIGA-induced adult-onset immunodeficiency. nAIGAs are detected in 88% of Asian adults with multiple opportunistic infections without HIV infection[7]. nAIGA-induced adult-onset immunodeficiency is an autoimmune disease associated with HLA-DQB1/DRB1 and depleted interferon-gamma/interleukin 12-mediated cellular immunity, with increased susceptibility to opportunistic NTM infections frequently involving the lungs, lymphadenopathy, cutaneous, and the musculoskeletal system, as in our case.
However, an acute and accurate diagnosis of musculoskeletal NTM infection is often difficult because of the indolent clinical course and difficulty in isolating pathogens[8]. A recent prospective case-control study in Taiwan[9] reported an average 1.6 year delay in the diagnosis of nAIGA-related dNTM due to protean manifestations mimicking other systemic illnesses, including mycobacterial tuberculosis, malignancy, and connective tissue diseases. Multivariate analysis revealed that slow-growing NTM species (i.e., Mycobacterium intracellulare) in nAIGA cases were associated with multiple bone involvement. These findings are comparable with our case. Osteomyelitis and bone marrow infections have also been associated with bone manifestations of dNTM in nAIGA patients in previous studies. In contrast, a pathological fracture was the initial presentation in our case. It is important to note that NTM osteomyelitis often arises from previous trauma or surgical sites among patients with weak immunity and dNTM infection, and osteomyelitis is also considered to be a presentation of immune reconstruction inflammatory syndrome[8].
Our case also impressed as a traumatic fracture initially according to the patient’s injury history but was later found to have an NTM bone infection-related pathological fracture mimicking metastatic malignancy. Interestingly, in addition to granulomatous disease-induced bone loss, nAIGA can also induce an increase in osteoclast formation and bone resorptionviaRANKL-induced activation of the NF-kB pathway, thereby worsening NTM infection-induced bone erosion[10]. We therefore suggest that nAIGA should be considered an independent risk factor for pathological fractures.
As relatively few reports on NTM-related pathological fractures are available, our case provides valuable insight into the comparison with malignancy-related pathological fractures. Risk factors associated with the prognosis of malignancyrelated pathological fractures include primary cancer type, spinal involvement, Eastern Cooperative Oncology Group status, and whether the patient received chemotherapy/radiotherapy. The median survival is 4.1 mo. Surgical treatment for pathological fractures includes intramedullary nails, plate fixation, and arthroplasty.
A retrospective cohort study[11] found that fracture site, method of fixation, and use of cement augmentation did not have a statistically significant impact on survival post-fracture. Another case-control study[12] found that cement fixation, in addition to open reduction and internal fixation, could result in immediate stabilization and thus better pain control without impairing the range of motion. In our case, the orthopedic doctor discussed the risks and benefits of different treatment methods with the patient. She chose to receive open reduction and internal fixation and subsequently underwent cancellous screw with Kirschner wire fixation for the humerus fracture. However, if we had noticed her sternum abscess lesion prior to surgery, we may have chosen a more intense fixation method under the suspicion/evidence of a pathological fracture. A more complete physical examination with preoperative planning may be helpful in such cases. In addition, further studies are warranted to investigate the preferred method of surgery, prognostic factors, and median survival of bone infection (such as NTM)-related fractures.
CONCLUSION
As T lymphocyte dysfunction predisposes patients to dNTM infection, we advise screening for nAIGA in HIV-negative Asian patients with NTM bone infection-related pathological fractures, especially when combined with other unexplained opportunistic infection history such as zoster, salmonellosis, histoplasmosis, and aspergillosis[7]. The histopathologic features of NTM bone infection show a spectrum of inflammatory changes, including granulomatous lesions with or without caseation[13]. With regards to treatment, it is necessary to differentiate nAIGA-related dNTM bone disease from Pott’s disease, monoclonal gammopathies, and other malignancies sharing similar features. Efficacy and drug resistance should be carefully evaluated for long-term anti-NTM treatment, and it is important that this is accompanied by surgical debridement and fracture treatment[8].
The immunosuppressants used for nAIGA include corticosteroids and cyclophosphamide, while rituximab has also been reported to be effective[7,14]. nAIGA titers have been reported to decrease over time even without immunosuppressive treatment. However, rates of chronic opportunistic infections and death remain high[10]. Screening for nAIGA in dNTM-related pathological fractures is recommended to allow for appropriate treatment, thereby decreasing morbidity. A careful preoperative survey is important in potential pathological fracture cases.
杂志排行
World Journal of Orthopedics的其它文章
- Revisiting Pauwels' classification of femoral neck fractures
- Paraspinal strength and electromyographic fatigue in patients with sub-acute back pain and controls: Reliability, clinical applicability and between-group differences
- Preseason elimination impact on anterior cruciate ligament injury in the national football league
- Risk of methicillin-resistant staphylococcus aureus prosthetic joint infection in elective total hip and knee arthroplasty following eradication therapy
- Reliability of a simple fluoroscopic image to assess leg length discrepancy during direct anterior approach total hip arthroplasty
- Anthropometric method for estimating component sizes in total hip arthroplasty
