Acid-sensitive PEGylated cabazitaxel prodrugs for antitumor therapy
2021-11-06ToLiuHuiZouJingqingMuYuYngXuGuohuLiuXingjieLingShutoGuo
To Liu,Hui Zou,Jingqing Mu,N Yu,Yng Xu,Guohu Liu,Xingjie Ling,Shuto Guo,*
a Key Laboratory of Functional Polymer Materials of Ministry of Education, State Key Laboratory of Medicinal Chemical Biology and Institute of Polymer Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China
b Laboratory of Controllable Nanopharmaceuticals, CAS Center for Excellence in Nanoscience, CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology, Beijing 100190, China
c University of Chinese Academy of Sciences, Beijing 100049, China
1 These authors contributed equally to this work.
ABSTRACT Although the antitumor drug cabazitaxel shows great therapeutic potential, its high toxicity and poor water solubility limit its utility.However,the use of stimuli-responsive prodrugs is a promising strategy for overcoming these limitations.Herein, we report the synthesis of two highly water soluble, acidsensitive PEGylated acyclic-ketal-linked cabazitaxel prodrugs(PKCs)with improved antitumor efficacy.In an acidic tumor microenvironment,the PKCs hydrolyzed rapidly to release the native drug,whereas they were stable in the normal physiological environment.Compared with cabazitaxel injection,the PKCs had much higher maximum tolerated doses; and in an MDA-MB-231 subcutaneous xenograft nude mouse model, the PKCs showed better antitumor efficacy and safety than cabazitaxel injection.The prodrug strategy reported herein could be useful for the development of other water soluble, acidsensitive prodrugs with improved efficacy.
Keywords:Prodrugs Acid-sensitive Antitumor therapy Cabazitaxel PEGylation
Cabazitaxel(CBZ),a semisynthetic second-generation taxane,is used to treat metastatic castration-resistant prostate cancer [1].Because CBZ has lower P-glycoprotein affinity than first-generation taxanes, it demonstrates potent activity against paclitaxeland docetaxel-resistant tumors [2].However, its poor water solubility limits its utility.For intravenous administration,CBZ has been combined with Tween 80 and ethanol (in a formulation designated as Jevtana®) [3-6], but the co-solvent and the surfactant often cause severe side effects, such as hypotension,rashes,and erythema[7].In efforts to improve the water solubility of CBZ and facilitate its intravenous administration, several prodrugs have been synthesized, including amphiphilic polymer-conjugated prodrugs, β-cyclodextrin-PEG-conjugated prodrugs and human serum albumin-conjugated prodrugs [8-12].However, most of the prodrugs use ester linkages, and the transformation of these prodrugs to the native drug in tumors may thus be inefficient[13-15].Therefore,the development of CBZ prodrugs that undergo tumor-microenvironment-responsive transformation would be highly desirable [16-25].
Because of the low pH in tumor microenvironments(~6.8)and endolysosomes (4.5-6.0), acid-sensitive linkages are useful in the design of antitumor prodrugs [26-29].Examples include Besponsa®, an antibody-drug conjugate comprising a hydrazine-containing ozogamicin derivative linked to a ketone-containing antibody via an acid-sensitive hydrazone linkage,has been approved by the US FDA for treatment of acute myelocytic leukemia [30].In addition to hydrazone linkages, acyclic ketal linkages,which can be used for hydroxyl-group-containing drugs,have recently emerged as appealing alternatives for constructing acid-sensitive prodrugs [31-36].Given that PEGylation is often used to increase the solubility of hydrophobic drugs,we envisioned that conjugating hydroxyl-group-containing CBZ with PEG via an acetone-based acyclic ketal linkage would afford water soluble,acid-sensitive CBZ prodrugs.We expected that the resulting prodrugs would efficiently release the native CBZ, the metabolite acetone,and the biocompatible PEG upon acid-catalyzed hydrolysis in tumors and would show improved antitumor efficacy relative to CBZ injection.
Herein, we report the design and synthesis of two PEGylated acetone-based acyclic-ketal-linked CBZ prodrugs (PKCs) with adjustable acid sensitivities (Scheme 1).The PKCs were obtained in high yields by means of acid-catalyzed reactions of CBZ(which has a OH group at the 2′-position) and one of two different 2-alkoxypropenes under mild reaction conditions (Scheme 1).In PKC-s, CBZ was linked directly to PEG1000via a ketal linkage(Scheme 1a), whereas in PKC-f, CBZ was linked, again via a ketal linkage, to PEG1000modified with a short carbon chain(Scheme 1b).We expected that insertion of the short carbon chain would cause the ketal of PKC-f to hydrolyze faster than the ketal of PKC-s[37,38].The structures of the PKCs and the synthetic intermediates were determined by NMR spectroscopy(Figs.S1-S4 in Supporting information).The spectra showed the characteristic signals for CBZ (δH7.0-8.5 and δC125-210), the ketal (δH1.0-1.1 and δC103), and PEG (δH3.3 and δC73) (Fig.1).

Scheme 1.Synthesis of(a)PKC-s and(b)PKC-f.The f and s denote faster and slower hydrolysis, respectively.
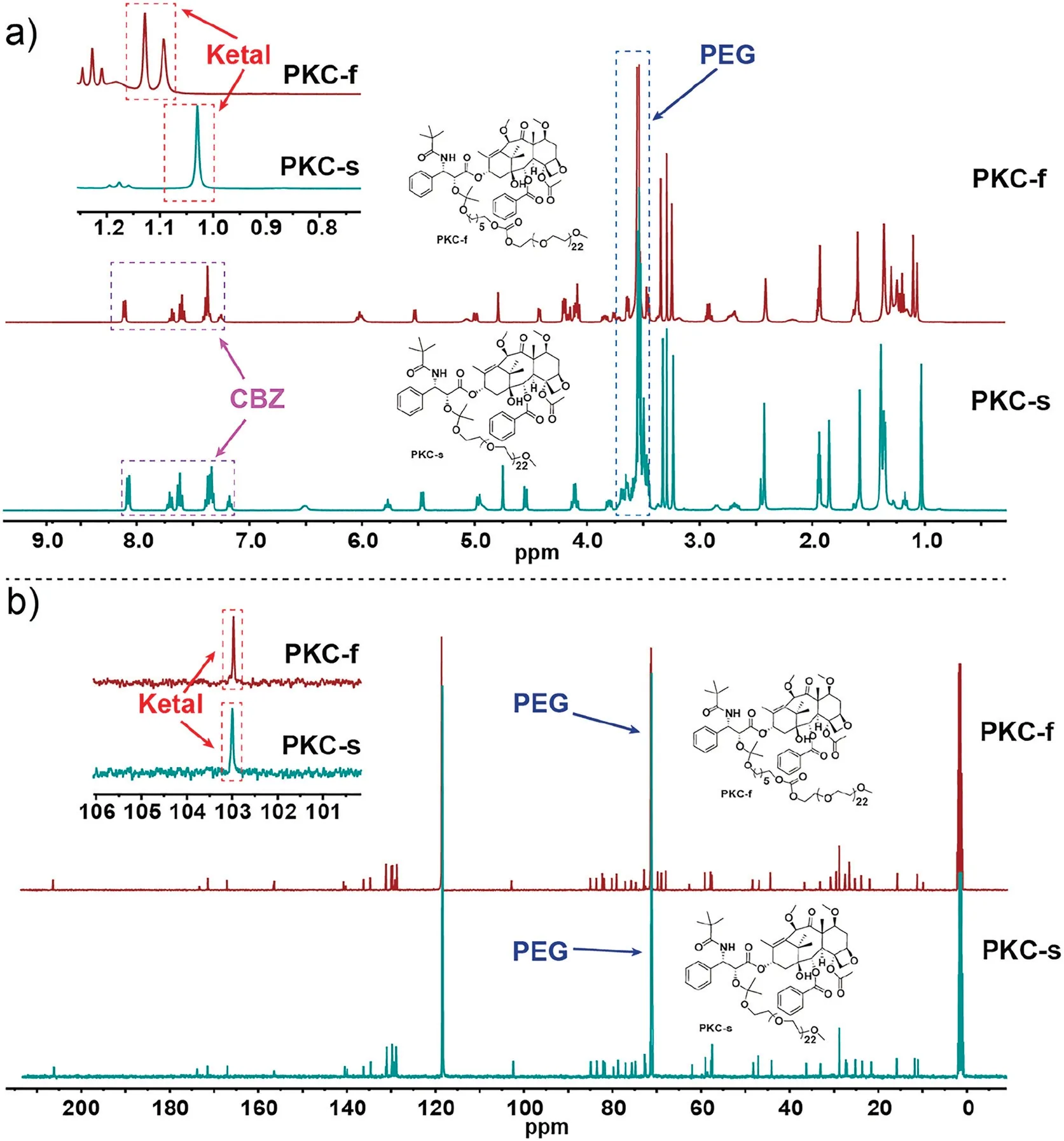
Fig.1.Characteristic chemical shifts in the(a) 1H NMR and(b) 13C NMR spectra of the PKCs.
We also measured the physiochemical properties of the PKCs.The drug contents of PKC-f and PKC-s were 39 wt% and 41 wt%,respectively, as determined by HPLC.The critical micelle concentrations of these amphiphilic surfactant-like PKCs were determined by means of a fluorescence probe technique with pyrene as the probe[39].The formation of micelles was confirmed by a marked change in the I377/I383fluorescence intensity ratio(Fig.S5 in Supporting information),and on the basis of this change,the critical micelle concentrations of PKC-f and PKC-s were determined to be 0.23 mg/mL (equivalent to 0.11 mmol/L) and 0.28 mg/mL (equivalent to 0.15 mmol/L), respectively.Finally, the solubilities of the PKCs in water exceeded 400 mg/mL.
Because acid-catalyzed hydrolysis of the PKCs released CBZ(Fig.2a),we were able to study the acid sensitivity of the PKCs by monitoring CBZ release by HPLC(Figs.2b and c).We found that PKC hydrolysis followed pseudo-first-order kinetics, and therefore the hydrolysis half-lives(t1/2)were calculated by means of the formula(ln2)/k,where k is the hydrolysis constant[32,33].The t1/2values of PKC-f at pH 5.0,6.8,and 7.4 were 0.54,24.8,and 83.5 h,respectively(Fig.2d,Fig.S6 in Supporting information);and the corresponding values for PKC-s were 3.2, 126, and 462 h (Fig.2e and Fig.S6).Compared with previously reported cyclic-ketal-based prodrug conjugates[40],our acyclic-ketal-linked PKCs hydrolyzed faster.In addition, the hydrolysis rate of PKC-f was about 6 times that of PKC-s.The electron-withdrawing PEG in PKC-s caused reduced charge density on the ketal bond and thus unfavored hydronium ion addition and hydrolysis; whereas, the short carbon chain in PKC-f increased the distance between the electron-withdrawing PEG and the ketal linkage and therefore caused an increase in the hydrolysis rate [37,38].
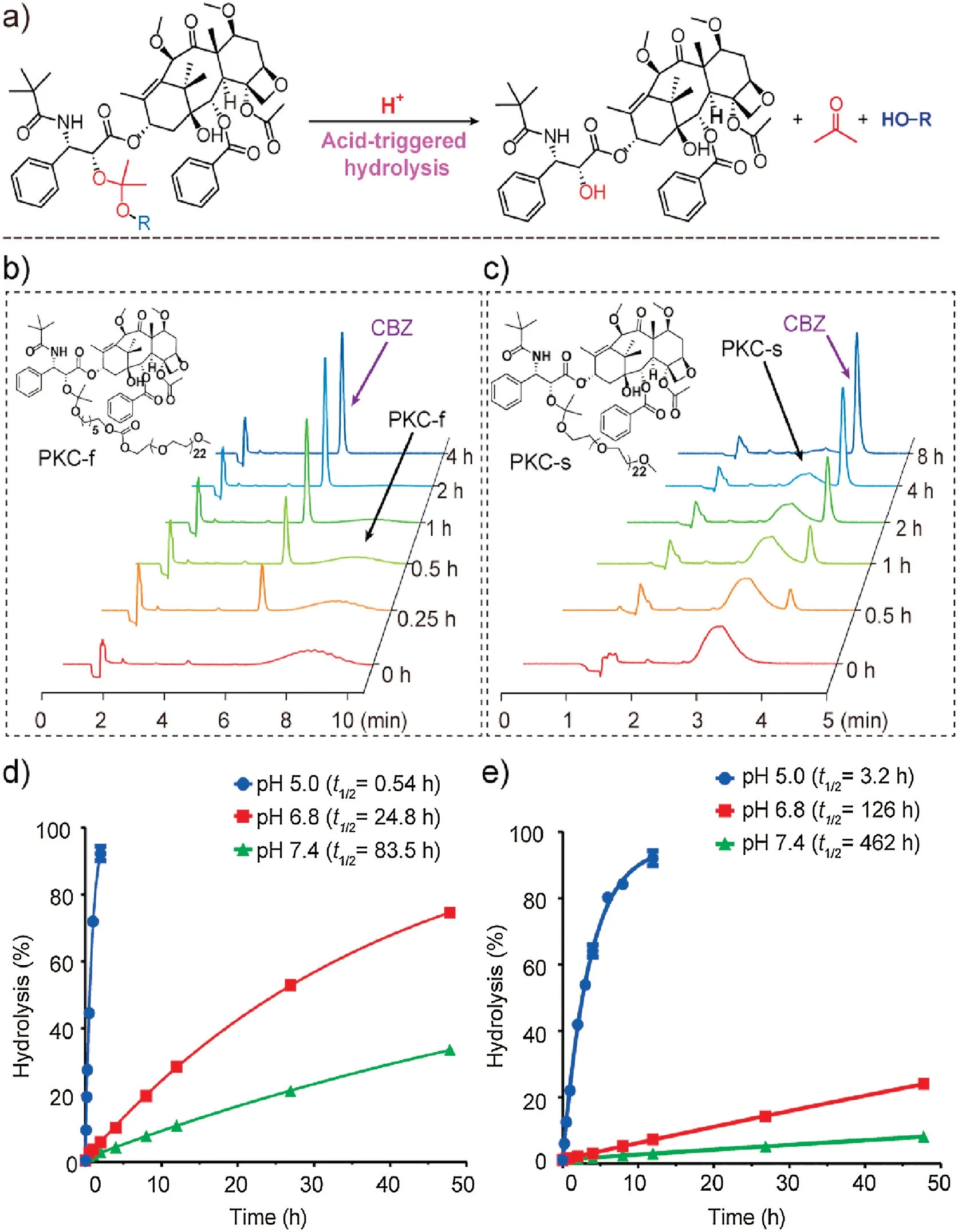
Fig.2.Hydrolysis of PKCs.(a)Hydrolysis reaction of PKCs.HPLC chromatograms of(b)PKC-f and(c)PKC-s at pH 5.0 at 37°C.Hydrolysis curves of(d)PKC-f and(e)PKC-s at pH 5.0, 6.8, and 7.4 at 37°C.Data are means±SDs (n=4).
We evaluated the cytotoxicity of the CBZ formulations in an MDA-MB-231 human breast cancer cell line.The IC50values of free CBZ, PKC-f, PKC-s, and CBZ injection (prepared according to the method used for the commercial formulation Jevtana®) were 1.6,12.0, 21.3, and 1.1 nmol/L, respectively (Fig.3a).Compared with free CBZ,the PKCs had higher IC50values,probably because of their relatively low cellular uptake and because they hydrolyzed slowly under the neutral cell culture conditions, as has been reported previously for an acid-sensitive ketal glycoside prodrug [41].Moreover,PKC-f,which hydrolyzed faster than PKC-s,had a lower IC50than PKC-s.
Taxanes are known to kill tumor cells by inhibiting the depolymerization of microtubules to tubulin[42].To test the ability of CBZ formulations to inhibit microtubule depolymerization, we incubated MDA-MB-231 cells with the CBZ formulations at 500 nmol/L for 12 h,and we then stained α-tubulin with anti-α-tubulin and cell nuclei blue with DAPI (4′,6-diamidino-2-phenylindole).After washing and fixation, cells were visualized with a confocal laser scanning microscope (Fig.3b).When the cells were treated with PBS, the microtubule fibers extended to fill the entire cytoplasm.In contrast,the microtubules of cells treated with PKCs or free CBZ were tangled and densely aggregated around the nuclei.The proportions of cells with aggregated microtubules were calculated [43] to be 100%, 100%, 59%, and 2.1% for cells treated with CBZ, PKC-f, PKC-s, and PBS, respectively (Fig.3c).These observations indicate that the PKCs substantially hindered microtubule depolymerization and led to tumor cell apoptosis, which suggests that they might have antitumor efficacy in vivo.
Next, we evaluated the safety of the PKCs by carrying out hemolysis assays (Fig.3d and Fig.S7 in Supporting information)and determining maximum tolerated doses(MTDs)in Balb/c mice(Fig.S8 in Supporting information).We found that PKCs exhibited negligible hemolysis(<3%)at concentrations ranging from 1 mg/kg to 40 mg/kg (equiv.dose of CBZ), whereas CBZ injection caused substantial hemolysis at concentrations of 5 mg/kg and higher.The MTDs of PKC-f and PKC-s in Balb/c mice were 10 and 60 mg/kg(equiv.dose of CBZ), respectively.In contrast, the value for CBZ injection was only 3 mg/kg, and this formulation exhibited acute toxicity to mice.The above-described results indicate that the PKCs had a wider dosing window than CBZ injection and could be safely administered at higher doses.
To study the antitumor efficacy of the PKCs, we established an MDA-MB-231 subcutaneous xenograft nude mouse model.When administered at their MTDs determined in Balb/c mice, we found that CBZ formulations were well tolerated in tumor-bearing Balb/c nude mice.PKC-f and PKC-s displayed antitumor efficacy superior to that of CBZ injection (Fig.4a).After 12 days of treatment, the tumor volumes in mice that received PBS or CBZ injection were approximately 2-4 times the volumes in mice that received the PKCs.The better antitumor efficacy of the PKCs relative to that of CBZ injection was attributed to their better safety and higher injection doses.Moreover, there was no significant difference between the body weights of the mice treated with the CBZ formulations and those treated with PBS (Fig.4b).We also determined survival curves for each treatment group (Fig.4c).After 21 days,the survival rate among mice treated with PKC-f was 80%, whereas the rates among mice treated with PKC-s, CBZ injection,and PBS were 40%,40%,and 20%,respectively.At the end of the treatment period, tumors and organs were collected,fixed,sectioned, and stained with hematoxylin-eosin or Ki-67 (a cell proliferation marker) for histological and immunohistochemical analyses.The proliferative activity of tumor cells stained with Ki-67 is indicated by a brown color;and compared with CBZ injectiontreated mice,PKC-treated mice showed much smaller brown areas,indicating greater antitumor efficacy (Fig.4d).Notably, hepatic damage was observed in liver sections from mice treated with CBZ injection, whereas no obvious damage was found in the livers of the PKC-treated mice (Fig.4d, Fig.S9 in Supporting information).Blood chemistry tests (CRE, BUN, ALT, and AST) to evaluate liver and kidney function showed no obvious abnormalities(Fig.S10 in Supporting information).All animal protocols and studies were approved by the Institutional Animal Care and Use Committee of Nankai University.
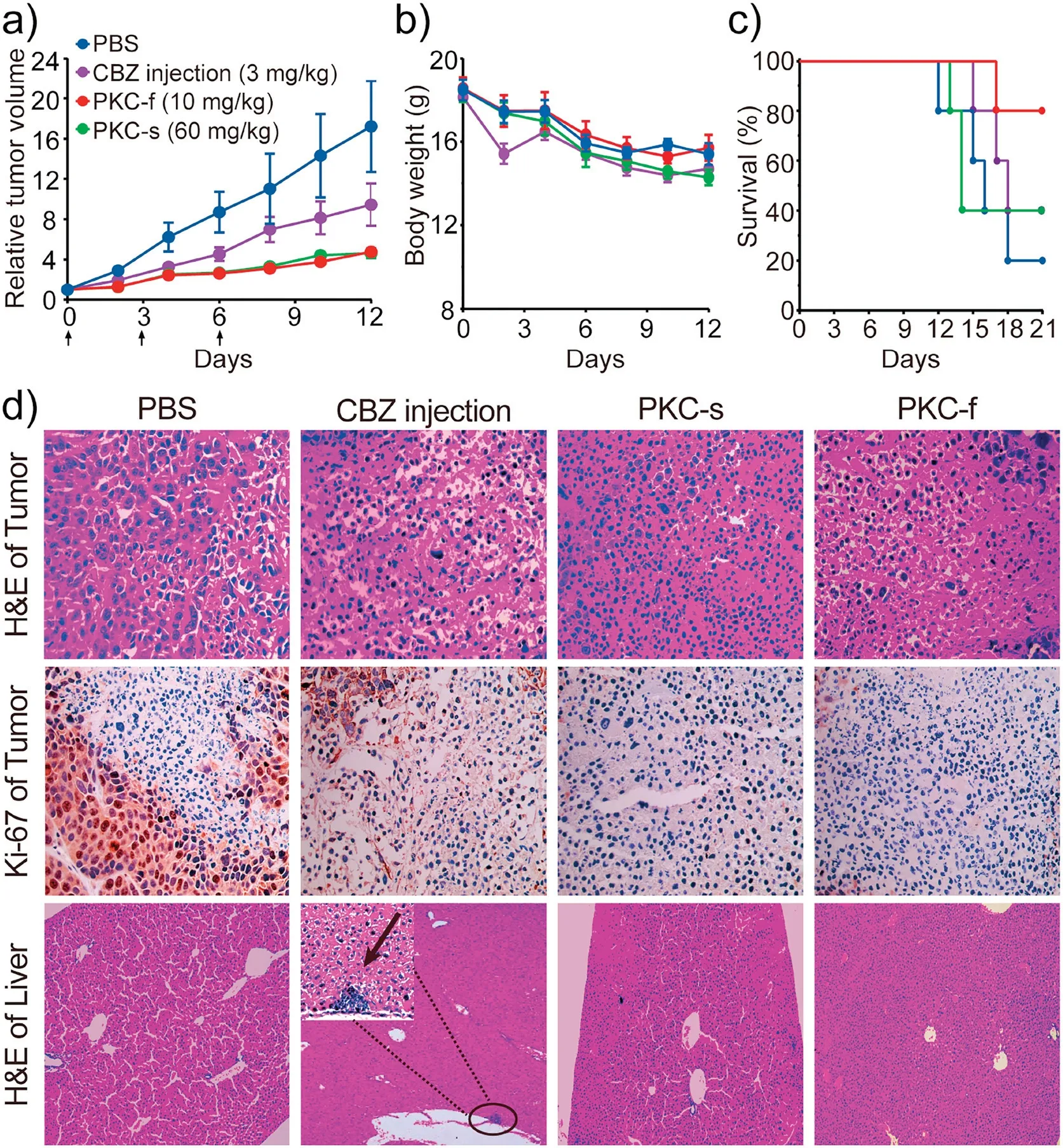
Fig.4.Evaluation of antitumor efficacy.(a)Tumor growth profiles of mice treated with CBZ formulations or with PBS.Black arrows indicate the days on which mice received treatments via tail vein injection.(b) Body weight changes.(c) Survival curves.(d) Hematoxylin-eosin staining and Ki-67 immunohistochemistry staining(brown) of tumor sections and hematoxylin-eosin staining of liver sections.The black arrow indicates liver injury.Tumor, 400× magnification; liver, 100×magnification; inset, 400× magnification.
In summary, we synthesized two highly water soluble, acidsensitive PEGylated acyclic-ketal-linked CBZ prodrugs (PKCs) and evaluated their antitumor efficacy.The PKCs showed adjustable acid sensitivity and were readily hydrolyzed in mildly acidic tumor microenvironments to release CBZ.Compared with CBZ injection,the PKCs showed minimal hemolysis and higher MTDs(3-20 fold)in Balb/c mice.In vivo antitumor studies indicated that the PKCs were more effective than CBZ injection at inhibiting tumor growth.The strategy reported herein may stimulate the synthesis of other acid-sensitive prodrugs and facilitate the development of new chemotherapeutics.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.51773098), the Natural Science Foundation of Tianjin of China(No.18JCYBJC28300),and the Fundamental Research Funds for Central Universities (China).
Appendix A.Supplementary data
Supplementary material related to this article can be found,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.12.008.
杂志排行
Chinese Chemical Letters的其它文章
- Molecular recognition triggered aptazyme cascade for ultrasensitive detection of exosomes in clinical serum samples
- Synthesis of sponge-like TiO2 with surface-phase junctions for enhanced visible-light photocatalytic performance
- Zn-based metal organic framework derivative with uniform metal sites and hierarchical pores for efficient adsorption of formaldehyde
- High active amorphous Co(OH)2 nanocages as peroxymonosulfate activator for boosting acetaminophen degradation and DFT calculation
- Effects of the molluscicide candidate PPU06 on alkaline phosphatase in the golden apple snails determined using a near-infrared fluorescent probe
- A lysosomal polarity-specific two-photon fluorescent probe for visualization of autophagy
