Effects of the molluscicide candidate PPU06 on alkaline phosphatase in the golden apple snails determined using a near-infrared fluorescent probe
2021-11-06ChngxioxiLiuSuoYngYimuQioYuqingZhoWeisiWngMingxunJiYnqiHeYingZhouLipingDun
Chngxioxi Liu,Suo Yng,Yimu Qio,Yuqing Zho,Weisi Wng,Mingxun Ji,Ynqi He,Ying Zhou,*,Liping Dun,c,**
a College of Chemical Science and Technology, Yunnan University, Kunming 650091, China
b National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention, WHO Collaborating Centre for Malaria, Schistosomiasis, and Filariasis, Key Laboratory of Parasitology and Vector Biology of the Chinese Ministry of Health, Shanghai 200025, China
c Qinghai Provincial People’s Hospital, Xining 810007, China
ABSTRACT We constructed a reaction-based near-infrared fluorescent probe(Niap)to specifically identify alkaline phosphatase (ALP) with fast red fluorescence enhancement.Based on the positive concentrationdependent manner between the fluorescent intensity of the Niap and ALP,probe Niap was used to study the ALP enrichment and variation in golden apple snails (Pomacea canaliculata) exposed to the molluscicide candidate PPU06.After treatment with different concentrations of PPU06 over various times, three organs of the surviving snails, liver, stomach and plantaris, were frozen and sectioned for fluorescent imaging experiments.With increased PPU06 concentration, red fluorescence substantially increased in the liver and reached a maximum within 24 h when the PPU06 concentration was 0.75 mg/L.No obvious changes in the stomach or foot plantaris were found.It showed PPU06 caused liver injury and stimulated the increase of ALP in the liver of P.canaliculata.This study demonstrates a rapid ALP fluorescent identification method that can be used to study the effects of PPU06 on P.canaliculata.It also provides optical evidence that may aid in the discovery of new chemistry for snail control.
Keywords:Fluorescence sensor ALP Golden apple snails (P.canaliculata)Molluscicide candidate Bio-imaging
Alkaline phosphatase (ALP) catalyzes the hydrolysis and transphosphorylation of many monophosphate esters [1-3] and plays an important role in phosphorylation and dephosphorylation of protein metabolism in cells [4-6].The increase of endogenous ALP is closely related to the proliferation and differentiation of osteoblasts and bone mineralization [7-9], with bone diseases,diabetes, liver dysfunction, breast and prostatic cancer [10-12].Fluorescent probes for monitoring ALP in mammals are now available[13-16],but no research has been conducted on mollusks,such as snails, to study ALP changes in response to molluscicides.
Golden apple snails(Pomacea canaliculata)are large freshwater snails native to the Amazon basin in South America [17].In the 1980s, P.canaliculata were introduced into China for food culture but later abandoned because of bad management and poor taste.Due to the lack of natural enemies and strong environmental adaptability [18], P.canaliculata invaded southern China and severely damaged agricultural production there[19].Since it is an intermediate host of the roundworm Angiostrongylus cantonensis,consumption of uncooked P.canaliculata causes parasitic diseases,which are important human health problems [20].Effective molluscicides are needed to reduce agricultural losses and manage parasitic diseases [21].
In 2018, we developed a molluscicide candidate PPU06(Scheme 1)for use against Biomphalaria straminea,the intermediate host of Schistosoma mansoni [22,23].We have now extended the use of PPU06 to P.canaliculata.At a concentration of 1 mg/L,PPU06 resulted in 90%mortality of P.canaliculata in 48 h(Table S1 in Supporting information).PPU06 also exhibited strong effects on the enzyme activities of ALP, ACP and NOS in the snails [22].As fluorescent probes are useful for detecting and examining analytes[24-26], such as cations,anions and enzymes that can have rapid and visual changes of the fluorescence spectral properties[27-30],we aimed to develop an effective ALP probe to determine enzyme levels changes in P.canaliculata following PPU06 treatment to better understand the PPU06 mode of action.

Scheme 1.Structure and working mechanism of Niap.
In this study, we designed and synthesized a new reactionbased NIR fluorescent probe(Niap),using ALP-dependent catalytic dephosphorylation, for the selective detection of the ALP enrichment and variation in P.canaliculata following PPU06 treatment.It showed that as PPU06 increased,the red fluorescence(from NIR-1)significantly increased in the liver and reached a maximum when the PPU06 concentration was 0.75 mg/L.No obvious changes in the stomach or foot plantaris were found.By frozen tissue sections and fluorescent imaging experiments,PPU06 was proven to cause liver injury and stimulate the ALP increase in the liver,but had less effect on other oranges.Probe Niap provided a rapid ALP identification method with optical evidence of the effects of candidate PPU06 in
P.canaliculata.
The preparation of 2,NIR-1 and Niap were described in detail in Supporting information.Intermediate compound 2 was synthesized, from cyclohexanone and 4-diethylamino-2-hydroxybenzaldehyde,which were then reacted with p-hydroxybenzaldehyde to obtain compound NIR-1.At 0°C, the mixture of 230 mg of compound NIR-1 (0.5 mmol) and 35.00 mL of anhydrous pyridine were slowly added with 1.0 mL of POCl3(1 mmol) and reacted at room temperature for 2 h.By introducing the recognition group of phosphoryl trichloride to compound NIR-1,the Niap was prepared with a yield of 76% (Scheme S1 in Supporting information).The chemical structure of the Niap was confirmed by1H NMR,13C NMR spectroscopy and MALDI-ESI mass spectrometry, which were shown in Supporting information.
We first performed fluorescent titration of the Niap(20 μmol/L)in the presence of varying amounts ALP (0-50 U/mL) in a MeOHPBS(v/v=1/9)buffer system(0.01 mol/L,pH 8.0)(Fig.1A).With an increased level of ALP in the test system,the fluorescence intensity at the 655 nm increased nearly 9-fold and reached a maximum at 50 U/mL ALP in a concentration-dependent manner (Fig.S5 in Supporting information).UV titration tests were then performed(Fig.1B).With the addition of ALP, the UV max absorption peak blue-shifted from 585 nm to 573 nm,the strength doubled and the solution color changed from purple to dark blue.Then,fluorescent intensities of the Niap (20 μmol/L) at 655 nm were scored, in the presence of varying concentrations of ALP(0,20,35 and 50 U/mL)as a function of varying time in the MeOH-PBS buffer system(0.01 mol/L,pH 8.0,v/v=1/9).In the mixture of the Niap and ALP,the fluorescence enhancement stopped at 65-70 min, which means that their reaction was completed within 70 min(Fig.S4 in Supporting information).To verify the selectivity of the Niap,related fluorescence intensities were tested after 1000 μmol of each of various analytes,such as ClO-,H2O2,Mg2+,Fe2+,Zn2+,Ca2+,L-Val, L-Trp, L-Tyr, L-Leu, L-Glu, L-Thr, glucose, GSH and ALP, was added to the test system (Fig.1C and Fig.S6 in Supporting information).The results showed that only the addition of ALP led to the enhancement of the fluorescence intensity at 655 nm,indicating good selectivity of the Niap toward ALP.

Fig.1.Fluorescence emission spectrum(A)and UV-vis absorption spectrum(B)of Niap (20 μmol/L) in the presence of varying amounts of ALP (0-50 U/mL) in the MeOH-PBS buffer system(0.01 mol/L,pH 8.0,v/v=1/9).(C)Fluorescence response of Niap(20 μmol/L)in the presence of various analytes(1000 μmol/L)(ClO-,H2O2,Mg2+,Fe2+,Zn2+,Ca2+, L-Val, L-Trp, L-Tyr, L-Leu, L-Glu, L-Thr,glucose,GSH and ALP)in the MeOH-PBS buffer system(0.01 mol/L,pH 8.0)(λex=585 nm).(D)Cytotoxicity of Niap against various cell lines(a:A549,b:HepG2,c:HeLa;d:SW480,e:L02 and f:BEAS-2B.Data were obtained using the standard MTS assay.The cells were pretreated with various concentrations of Niap.(E)Confocal fluorescence images of HepG2 cells and HeLa cells.Group one:cells were incubated with Niap(20 μmol/L)for 30 min;group two:cells were pretreated with Na3VO4(100 μmol/L)for 30 min and then incubated with Niap (20 μmol/L) for another 30 min.Scale bar: 20 μm.
Cytotoxicity of the Niap was evaluated by the MTS method,with four types of cancer cells, including lung carcinoma cells (A549),liver hepatocarcinoma cells (HepG2), cervical cancer cells (HeLa)and human colon cancer cells (SW480), and two types of normal cells of human normal liver cell (L02) and human normal lung epithelial cells(BEAS-2B)(Fig.1D).The experiment indicated that the Niap had low toxicity and might have other biological applications.The Niap was used for bio-imaging ALP in HepG2 cells and HeLa cells.In one group,after 1 h incubation of the Niap at 37°C, a weak red fluorescence was present in Hela cells and a stronger red fluorescence appeared in HepG2 cells, indicating a higher level of ALP in HepG2 cells than that in Hela cells.In another group, we first incubated HepG2 and Hela cells with the ALP inhibitor sodium vanadate (Na3VO4) for 30 min, and then incubated the pre-treated cells with the Niap for 1 h.Comparing the results of the above two groups,due to the inhibitor’s action on reducing the ALP concentration in the cells, the reaction between the probe and ALP weakened, and the red fluorescence was very faint in the second group in both HepG2 and Hela cells (Fig.1E).These findings confirmed the selectivity and successful fluorescence response of the Niap for ALP in cells.
In order to avoid interference from external factors in the tests,we chose snails with similar sizes and weights as the experimental samples.Each snail was fed individually in a separate 500 mL beaker.Based on lab test data(Table S1 in Supporting information),we used 0.75 mg/L as an effective concentration to study the growth and death of P.canaliculata with different exposure times.Ten test snails were incubated with or without 0.75 mg/L of PPU06 for 24 h.Following treatment, three organs of the surviving P.canaliculata, including liver and stomach related to metabolism and plantaris related to muscles, were dissected and frozen to produce tissue sections.
Na3VO4was also applied to some test groups to confirm that the fluorescent signals in the test snails were coming from ALP.The tissues in the pre-treated control group (without PPU06) were directly incubated with the Niap (2×10-4mol/L) for 30 min, and some tissues from pre-treated test groups were incubated first with Na3VO4(1×10-2mol/L) for 10 min and later with the Niap(2×10-4mol/L) for 30 min.In Fig.2A (b2), a significant red fluorescence appeared in the liver of snails exposed to 0.75 mg/L of PPU06.Due to Na3VO4, the concentration of ALP decreased, and therefore the fluorescence was quenched in Fig.2A (c2).This confirmed that the red fluorescence change in liver tissue originated from ALP level changes.Little fluorescence change was observed in stomach and foot plantaris tissues,indicating that there was no obvious ALP change in these two tissues.It was consistent with our test results by alkaline phosphatase assay kit(Fig.S8 in Supporting information).
To test the concentration effects of PPU06 on different organs,we incubated 20 snails in four groups of five,at four concentrations of PPU06 including 0, 0.25, 0.5 and 0.75 mg/L for 24 h.After the treatment,the surviving snails were used to conduct anatomy and fluorescence imaging experiments.After frozen tissue sections,different tissues were incubated with the Niap at a concentration of 2×10-4mol/L for 30 min, and the fluorescent changes were studied with a confocal microscope.In the control group,there was weak red fluorescence in the liver, but with increasing PPU06 concentration, the red fluorescence increased in the liver and reached a maximum at 0.75 mg/L PPU06.However, there was no intensity change in the stomach and foot plantaris in either the control group or the test group (Fig.2B).We performed the same processes with the same concentrations used above but with longer different incubation times(48 h and 72 h)(Figs.S9 and S10 in Supporting information).Similar results were observed.The ALP level increased significantly in the liver but not in the stomach or foot plantaris.

Fig.2.(a)The slices of different organs of P.canaliculata,which were incubated with a PPU06 concentration of 0.75 mg/L for 24 h,were incubated with/without 1×10-2 mol/L inhibitor Na3VO4 for 10 min and then treated with a concentration of 2×10-4 mol/L probe Niap for 30 min.The section thickness was 20 μm.(a1-c2)Liver;(d1-f2)Stomach;(g1-i2) Foot plantaris.Scale bar: 200 μm.(b) After incubation for 24 h at different PPU06 concentrations, the liver slices, stomach slices and foot plantaris slices from P.canaliculata were incubated with a concentration of 2×10-4 mol/L probe Niap for 30 min.The section thickness was 20 μm.(a1-c2)Control;(d1-f2)PPU06 concentration was 0.25 mg/L; (g1-i2) PPU06 concentration was 0.5 mg/L; (j1-l2) PPU06 concentration was 0.75 mg/L.Scale bar: 200 μm.
PPU06 mainly appears to affect the snail liver, and there is a positive concentration-dependent relationship within the effective concentration range.We studied the time effects of PPU06 in the liver of P.canaliculata by fluorescence changes.We incubated 20 snails in four groups of five with PPU06 doses of 0,0.25,0.5 and 0.75 mg/L.After 24 h, 48 h and 72 h of incubation, the surviving snails in the four groups were used to conduct anatomy and fluorescence imaging experiments.
Sections of different frozentissues were incubated with the Niap at a concentration of 2×10-4mol/L for 30 min, and the fluorescence changes were examined using a confocal microscope.The increase of ALP in snail liver was greatest in the 0.75 mg/L concentration and 24 h incubation time.However, in both the stomach and foot plantaris,no obvious fluorescence changes were observed within 72 h (Figs.S11 and S12 in Supporting information).These experiments showed that the effects of the candidate to the liver reached a maximum within 24 h at 0.75 mg/L of PPU06 and weakened gradually by 72 h(Fig.3A).Overdoses of acetaminophen was reported to cause acute liver injury, and these are accompanied by an increase in the ALP level[31].We tested snails with acetaminophen and found that red fluorescence increased in livers as the concentration of the acetaminophen increased(Fig.S13 in Supporting information), which was similar with that in the case of PPU06.Based on these results, PPU06 appears to cause liver injury and stimulates an increase of ALP in the liver.However, in the tests with niclosamide, the only molluscacide recommended by WHO currently,no obvious fluorescence change was found in all the tested slices (Fig.S14 in Supporting information).
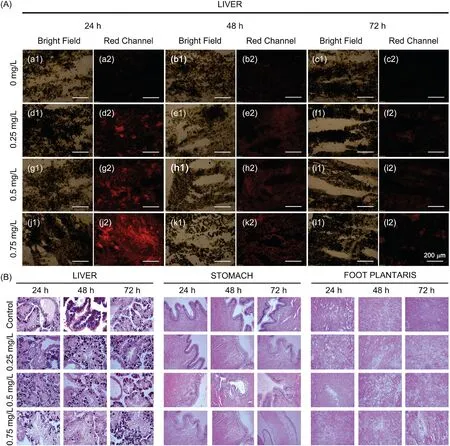
Fig.3.(a)After the different incubation time treatment,the liver slices from P.canaliculata were incubated with a concentration of 2×10-4 mol/L probe Niap for 30 min.The section thickness was 20 μm.(a1-c2) Control; (d1-f2) PPU06 concentration was 0.25 mg/L; (g1-i2) PPU06 concentration was 0.5 mg/L; (j1-l2) PPU06 concentration was 0.75 mg/L.Scale bar:200 μm.(b)After incubation with different PPU06 concentrations for different days,histopathologic slides with H&E staining from the liver,stomach and foot plantaris of P.canaliculata.Slice thickness is 4 μm.
To examine the effect of PPU06 on other organs,we performed H&E staining experiments with three organs of P.canaliculata,after different incubation times and PPU06 concentrations.An Olympus CX31 optical microscope was used to observe the morphological structures,characteristics and pathological changes in each tissue group (Fig.3B).
There were substantial differences between the control group and treatment groups.The livers of the test groups showed obvious degeneration and necrosis with the hepatic acinus disorganized.Hydropic degeneration was observed in the gastric mucosal cell layer.There was edema in the interstitium of the plantar muscle,with patchy areas of myolysis and necrosis.The lesions caused by different PPU06 doses and treatment times showed positive gradient changes.The damage was milder at 0.25 mg/L and 0.5 mg/L, but greater at 0.75 mg/L.The damage worsened with increased time,from 24 h to 72 h.These experiments demonstrated that PPU06 affected the liver, stomach and foot plantaris and showed that the main morphological damage occurred in the liver and foot plantaris.
We constructed a NIR fluorescent probe (Niap) to rapidly and specifically identify ALP,with red fluorescence enhancement,and trace the enrichment and variation of ALP in P.canaliculata under the molluscicide candidate PPU06 treatment.After snails exposure to different concentrations and treatment times of PPU06, we made frozen tissue samples of live, stomach and foot plantaris of living P.canaliculata and used these for fluorescence imaging experiments.As the PPU06 concentration increased, the red fluorescence in the liver enhanced in a concentration-dependent manner.It reached a maximum when the PPU06 concentrationwas 0.75 mg/L, and then weakened and disappeared within 72 h.However, no obvious fluorescent changes were found in the stomach and foot plantaris, which indicated that PPU06 mainly damaged,and stimulated an increase of ALP,in the liver.The Niap revealed the changes of ALP affected by PPU06 and provided a rapid optical identification method for studying of the effects of PPU06.This is the first application of ALP sensor in snails to study ALP changes in response to molluscicides, and this research promotes the mode of action study of PPU06 and may help in the design of other molluscicides.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China(Nos.21672185,22067019,82072309).Liping Duan thanks the support of National Key R&D Program of China(No.2017YFC1200600).
Appendix A.Supplementary data
Supplementary material related to this article can be found,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.12.029.
杂志排行
Chinese Chemical Letters的其它文章
- Molecular recognition triggered aptazyme cascade for ultrasensitive detection of exosomes in clinical serum samples
- Synthesis of sponge-like TiO2 with surface-phase junctions for enhanced visible-light photocatalytic performance
- Zn-based metal organic framework derivative with uniform metal sites and hierarchical pores for efficient adsorption of formaldehyde
- High active amorphous Co(OH)2 nanocages as peroxymonosulfate activator for boosting acetaminophen degradation and DFT calculation
- A lysosomal polarity-specific two-photon fluorescent probe for visualization of autophagy
- Revealing HOCl burst from endoplasmic reticulum in cisplatin-treated cells via a ratiometric fluorescent probe
