High-yield synthesis of a novel water-soluble macrocycle for selective recognition of naphthalene
2021-11-06ManHuaDingJuanLiaoLinLiTangGuangChuanOuFeiZeng
Man-Hua Ding,Juan Liao,Lin-Li Tang,Guang-Chuan Ou,Fei Zeng*
Department of Biology and Chemistry, Hunan University of Science and Engineering, Key Laboratory Comprehensive Utilization of Dominant Plants Resources in South Hunan, Yongzhou 425199, China
ABSTRACT A novel good water-soluble macrocycle containing two pyridinium moieties was synthesized in high yield.It could form 1:1 complexes with neutral guests containing naphthalene or phenyl units in water.The water-soluble macrocycle can selectively encapsulate naphthalene to form a 1:1 complex over a variety of polycyclic aromatic hydrocarbons.
Keywords:Water-soluble Macrocycle Naphthalene Supramolecular chemistry Recognition
Efficient synthesis of novel macrocyclic hosts with unique structures and good host-guest properties is a permanent and challenging topic in the field of supramolecular chemistry [1-7].During the last decade,considerable effort has been devoted to the development of macrocyclic molecular and a number of new macrocyclic receptors with novel properties have been reported,such as heterocalix[n]aromatics [8-10], pillar[n]arene [11-16],helicarenes [17-19], naphthotubes [20-24], Ex-box and Ex-cage[25-32], and others [33-37].However, most of the reported macrocyclic hosts showed poor solubility and weak host-guest interactions in water.To develop a new macrocyclic molecule with good water solubility and molecular recognition properties not only helps us to understand and mimic the biological processes,but also enriches the toolbox of supramolecular chemists.Water soluble groups including sulfonate [38-40], carboxylate [41-43]and quaternary amine groups[44,45]have been modified onto the macrocyclic hosts to increase their water solubility.These approaches often suffer from long synthetic steps and low yields,which restrict their further application in the complicated supramolecular self-assembly.Undoubtedly, it is important to develop novel water-soluble macrocyclic hosts that could be obtained in high yields and show good host-guest properties in water.Recently, Li and coworkers [46,47] reported the efficient synthesis of water-soluble macrocyclic hosts using dynamic covalent chemistry (DCC) approach.In their method, both macrocycles and [2]catenanes could be obtained in high yields under the thermodynamic control.However,the purification of[2]catenanes need chromatographic.Previously, our group [42,43]prepared a novel water-soluble cylindrical macrotricyclic host,and found that the host could bind two N-methylquinolinium salts to form 1:2 complexes in water.Inspired by these results,we deduced that whether we could find a new strategy to construct novel water-soluble macrocyclic host with significant host-guest properties in high yields.
So far as we know,1,8-bis(4-pyridylethynyl)anthracene,which looks like a molecule“clip”,has been used as donor building block to prepare trigonal prisms[48].Thus,the anthracene-based“clip”could also serve as half part of a macrocycle and a macrocyclic host could be obtained when a suitable building block was introduced to this clip.Herein, we report the efficient synthesis of a novel water soluble macrocyclic host 12+•2Br-and its complexation with neutral guests in water.By the utilization of the anthracene-based“clip” to react with 1,4-bis(bromomethyl)benzene, host 12+•2Brcan be obtain via simple filtration in a high yield of 82%.Moreover,it was found that host 12+•2Br-could form 1:1 complexes with guests 2 and 3 in water solution (Fig.1).Interestingly, we discovered that host 12+•2Br-could only selectively accommodate naphthalene among a variety of polycyclic aromatic hydrocarbons in water.This selective host-guest recognition could be employed for the further removal of naphthalene from sewage.

Fig.1.Structures and the proton designations of host 12+•2Br-, guests 2 and 3.
Synthesis of host 12+•2Br-was outlined in Scheme 1.Compound 4 was first prepared according to the literature procedure[48].By the reaction of 4 and commercially available 1,4-bis(bromomethyl)benzene in acetonitrile at 90°C, host 12+•2Br-could be easily synthesized in 82% yield.Macrocyclic host 12+•2Br-showed moderate solubility in water, and its structure was confirmed by1H NMR,13C NMR, HRMS spectra and crystal structure analysis( Supporting information).

Scheme 1.Synthesis of host 12+•2Br-.
Firstly, the binding properties of host 12+•2Br-toward neutral guests 2 and 3 were investigated by1H NMR spectra in water solution.Unlike the previous results that reported by our group[43],after electron-poor host 12+•2Br-(4.0 mmol/L)and electronrich guest 2 with 1: molar ratio were mixed in water,no obviously color change was observed.The similar phenomenon was also observed for the aqueous solution between host 12+•2Br-and guests 3.These results led us to doubt that whether host 12+•2Brcould form of complexes with guests 2 and 3.Consequently,the1H NMR experiments were carried out to further investigate the complexation between host 12+•2Br-and guest 2 in water.As shown in Fig.2,the1H NMR spectrum of a 1:1 mixture of 12+•2Brand 2 in D2O showed a great difference with those for free host 12+•2Br-and free guest 2.Upfield shifts of the resonances of protons H1, H2and H3corresponding to guest 2 were observed,which indicated that the naphthalene unit of 2 experienced a shielded magnetic environment in the aromatic cavity of 12+•2Br-.Moreover, the signal of protons Hfand Hgin host 12+•2Br-also shifted upfield, implying that the electron-poor pyridine unit of 12+•2Br-was in shielded magnetic environment and a new complex 1•2 could be formed.Meanwhile, by increasing the amount of guest 2,the spectrum of complex 1•2 showed only one set of resonances, which indicated that the complexation and decomplexation between host 12+•2Br-and guest 2 were a fast exchange process on the NMR time scale at room temperature.The formation of complex 1•2 was also supported by 2D NOESY spectral experiment.As shown in the Fig.S4(Supporting information),the clear correlation signals between protons H1-Hg, H2-Hgand the protons of crown ether units of host 12+•2Br-were observed,which was also consistent with the formation of complex 1•2.Moreover,1H NMR titrations and nonlinear fitting were then performed to quantitatively estimate the 1:1 binding manner between host 12+•2Br-and guest 2.Consequently,it was found that 1:1 complex 1•2 were formed by the mole ratio plot.The binding constant(Ka)of the complex 2⊂12+•2Br-was calculated to be 184±4 L/mol by the Scatchard plot[49].By the counteranion exchange of 12+•2Br-,we also prepared oil-soluble 12+•2PF6-and investigated its complexation with 2 in MeCN.As shown in Fig.S3 (Supporting information),the1H NMR spectrum of the 1:1 mixture of 12+•2PF6-and 2 was essentially the sum of the two components, indicating that no obvious complexation between 12+•2PF6-and 2 occurred and the major driving force for the formation of 2⊂12+•2Br-in water could be hydrophobic interactions.In addition, the binding constant of the complex 3⊂12+•2Br-was measured to be around 64±2 L/mol in D2O at 298 K.Compared to guest 2 containing a naphthalene unit, guest 3 has a smaller phenyl hydrophobic moiety,which accounted for its lower binding constant within host 12+•2Br-.
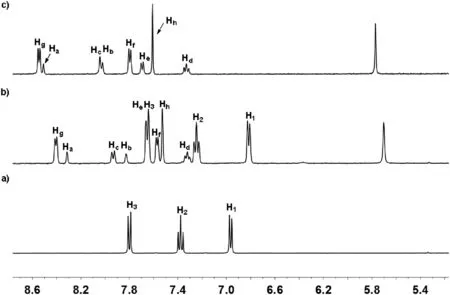
Fig.2.Partial1H NMR spectra(400 MHz,D2O,295 K)of(a)free guest 2,(b)12+•2Br- and 1.0 equiv.of 2, (c) free host 12+•2Br-.[12+•2Br-]0=4.0 mmol/L.
The attempts to obtain the single crystal of 2⊂12+•2Br-and 12+•2Br-were unsuccessful.Fortunately,a yellow single crystal of 12+•2PF6-was obtained by slow vapor diffusion of ether to a solution of 12+•2PF6-in CH3CN, providing unambiguous evidence for the formation of 12+•2Br-. As shown in Fig.3a, the two pyridinium residues orientate in a face-to-face nanner and the distance between two N and C atoms of pyridinium residues are measured to be 5.812 Å (h) and 5.647 Å (g), indicating the moderate-sized cavity of host 12+•2PF6-. Interestingly, it was found that PF6-ion showed not only multiple CH•••F hydrogen bonds but also anion•••π interactions with pyridinium rings with the distance of 2.989 (d), 3.062 (e) and 3.159 (f), respectively.Moreover, π•••π interaction between two anthracene groups of host 12+•2PF6-with a distance of 3.846 Å (i) was also observed(Fig.3b).Because of these multiple noncovalent interactions, the macrocyclic molecule 12+•2PF6-could self-assemble to form a 1D tubular channel with PF6-ions inside the channels.

Fig.3.Crystal structure of 12+•2PF6- (a).Crystal structure of self-assembly 12+•2PF6- side view (b), top view (c).Dashed lines denote the noncovalent interactions between the two components.CH•••F hydrogen bond distances (Å):a=2.461, b=2.404, c=2.557.PF6- ions and hydrogen atoms not involved in the noncovalent interactions are omitted for clarity.
Inspired by the formation of complex 1•2 in water, we further investigated the capability of 12+•2Br-to accommodate a variety of polycyclic aromatic hydrocarbons in water.As shown in Fig. 4a,1H NMR spectroscopy revealed that 12+•2Br-can encapsulate naphthalene to formation of 1:1 complex.However,the formation of 1:1 complexes between 12+•2Br-and anthracene,phenanthrene,triphenylene,pyrene and perylene were not observed (Figs.4b-f).These observations could be explained by the fact that host 12+•2Br-had a small cavity and could only recognize small polycyclic aromatic hydrocarbons such as naphthalene.However,because of the extremely poor solubility of naphthalene in water,the corresponding binding constants of host 12+•2Br-and naphthalene could not be determined.The highly selective binding behavior of host 12+•2Br-toward naphthalene could be further used for the separation of naphthalene from a variety of polycyclic aromatic hydrocarbons.
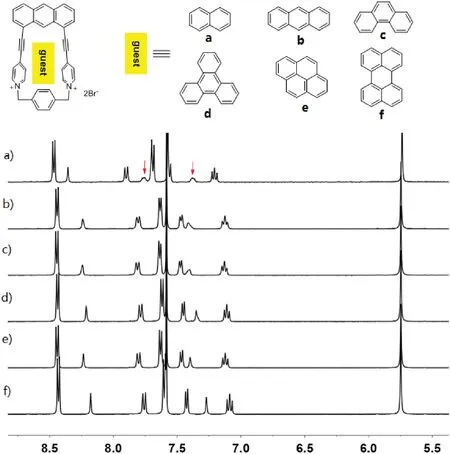
Fig.4.Partial 1H NMR spectra(400 MHz,D2O,298 K)of 12+•2Br-after addition of a variety of water-insoluble guests including (a) naphthalene, (b) anthracene, (c)phenanthrene, (d) triphenylene, (e) pyrene and (f) perylene.All the spectra were recorded after sonicating the corresponding suspensions for no less than 24 h to allow the host-guest complexation to reach the equilibrium.The resonance signals of the guests are labelled with red arrows.
In conclusion, by taking advantage of anthracene-based “clip”structure,we developed an efficient approach to construct a watersoluble macrocycle and studied its binding ability toward neutral guests containing naphthalene or phenyl units in water.The formation of 1:1 complexes between host 12+•2Br-and 2 or 3 were confirmed by the1H NMR titrations experiment.Additionally, we demonstrated that the major driving force for the formation of 2⊂12+•2Br-or 3⊂12+•2Br-in water might be hydrophobic interactions.We further investigated its ability to host a variety of polycyclic aromatic hydrocarbons in aqueous solution.It was found that host 12+•2Br-could selectively encapsulate of naphthalene to formation of 1:1 complexes over a variety of polycyclic aromatic hydrocarbons.This highly selective accommodation hydrophobic guest in water could be explained by the fact that host 12+•2Br-had a relatively small hydrophobic cavity.The application of this novel water-soluble host for the separation of naphthalene from a variety of polycyclic aromatic hydrocarbons and removal of naphthalene from sewage, are underway in our laboratory.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
The authors are grateful for the financial support from the National Natural Science Foundation of China(Nos.21602055 and 51772091); Natural Science Foundation of Hunan Province (No.2017JJ3094) and Research Foundation of Education Bureau of Hunan Province (No.18C1072).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.11.019.
杂志排行
Chinese Chemical Letters的其它文章
- Molecular recognition triggered aptazyme cascade for ultrasensitive detection of exosomes in clinical serum samples
- Synthesis of sponge-like TiO2 with surface-phase junctions for enhanced visible-light photocatalytic performance
- Zn-based metal organic framework derivative with uniform metal sites and hierarchical pores for efficient adsorption of formaldehyde
- High active amorphous Co(OH)2 nanocages as peroxymonosulfate activator for boosting acetaminophen degradation and DFT calculation
- Effects of the molluscicide candidate PPU06 on alkaline phosphatase in the golden apple snails determined using a near-infrared fluorescent probe
- A lysosomal polarity-specific two-photon fluorescent probe for visualization of autophagy
