Batroxobin inhibits astrocyte activation following nigrostriatal pathway injury
2021-11-02ZhuoZhangXueBaoDanLi
Zhuo Zhang, Xue Bao, Dan Li
Abstract Batroxobin is a thrombin-like serine protease from the venom of the Bothrops atrox and Bothrops moojeni snake species. Sirtuin 1 (Sirt1) has been shown to play an important role in neuroprotection after traumatic brain injury. However, its underlying mechanism of action remains poorly understood. The purpose of this study was to investigate whether the mechanism by which batroxobin participates in the activation of astrocytes is associated with Sirt1. Mouse models of nigrostriatal pathway injury were established. Immediately after modeling, mice were intraperitoneally administered 39 U/kg batroxobin. Batroxobin significantly reduced the expression of cleaved caspase-3 in both the substantia nigra and striatum, inhibited neuronal apoptosis, and promoted the recovery of rat locomotor function. These changes coincided with a remarkable reduction in astrocyte activation. Batroxobin also reduced Sirt1 expression and extracellular signal-regulated kinase activation in brain tissue. Intraperitoneal administration of the Sirt1-specific inhibitor EX527 (5 mg/kg) 30 minutes prior to injury could inhibit the abovementioned effects. In mouse astrocyte cultures, 1 ng/mL batroxobin attenuated interleukin-1β-induced activation of astrocytes and extracellular signal-regulated kinase. EX527 could also inhibit the effects of batroxobin. These findings suggest that batroxobin inhibits astrocyte activation after nigrostriatal pathway injury through the Sirt1 pathway. This study was approved by the Animal Ethics Committee of China Medical University, China (approval No. CMU2020037) on July 19, 2015.
Key Words: astrocyte; brain; central nervous system; factor; injury; pathway
Introduction
Traumatic brain injury (TBI) initiates primary injury mechanisms that induce significant neuronal loss and neurological dysfunction. Nigrostriatal pathway injury is a severe form of TBI that results from multiple injuries (Kawano et al., 2005). Subsequently, secondary injury events, such as inflammation and glial cell activation, are thought to induce the development of many of the neurologic deficits observed after TBI (Tang et al., 2020).
Batroxobin can decrease the fibrinogen level in blood. Studies have shown that batroxobin exerted protective effects against neuronal apoptosis following lesions of the central nervous system (Fan et al., 2013; Yu et al., 2015; Li et al., 2016a). As a thrombin-like serine protease from the venom of the snake species Bothrops atrox and Bothrops moojeni, batroxobin has been used to facilitate studies of various ischemic disorders,such as stroke and deep-vein thrombosis (Bell, 1997; Guo and Schluesener, 2006). Our previous study showed that batroxobin could attenuate neuronal apoptosis by reducing the inflammatory factors and scar tissue around the lesion site (Li et al., 2016a). However, whether and how batroxobin is involved in the regulation of astrocyte activation after brain injury has not yet been elucidated.
Sirtuins are mammalian homologs of silent mating type information regulation 2 (Sir2) in Saccharomyces cerevisiae and members of the class III histone/lysine deacetylase family (Houtkooper et al., 2012). Sirtuin 1 (Sirt1) is the first homologous gene of this family identified in mammals.The pleiotropic responses of Sirt1 have shown that its pharmacological activation or upregulation could facilitate neuroprotective effects in several models of brain injury and neurodegenerative diseases (Guo et al., 2011; Zhang et al., 2011; Houtkooper et al., 2012). Notably, Sirt1 is widely expressed in the adult brain (Sakamoto et al., 2004)and participates in the maintenance of brain integrity through the regulation of neuronal apoptosis (Chang et al., 2009). However, the mechanisms of Sirt1 that underlie neuroprotection after brain injury, especially those related to glial cell activation, have not been fully investigated.
Thus, the present study aimed to investigate the relationship between batroxobin and Sirt1 in the process of astrocyte activation and determine whether this mechanism is related to Sirt1 expression.
Materials and Methods
Experimental animals
All adult male Kunming mice (8–10 weeks old,n= 60, specific pathogen free level, for thein vivostudy) and neonatal Kunming mice (1–3 days old,n= 27, specific pathogen free level, for thein vitrostudy) were provided by the Animal Department of China Medical University, Shenyang, China(License No. SCXK (Liao) 2018-0004) and were housed on a 12/12-hour light/dark cycle, with a temperature of 22–24°C and humidity of 50 ± 5% with food and water freely available. All procedures were performed with approval of the Institutional Animal Care Committee of China Medical University (approval No. CMU2020037) on July 19, 2015. All efforts were made to minimize animal suffering and reduce the number of animals sacrificed.
Animal surgery
In the present study, nigrostriatal pathway injury was used to model TBI as described previously (Kawano et al., 2005;Yoshioka et al., 2010; Li et al., 2016b). After the mice were anesthetized with 10% chloral hydrate (50 mg/kg), they were placed in a stereotaxic apparatus (TME Technology Co., Ltd.,Chengdu, China) with the incisor bar set at 3 mm below the intra-aural line. The skin superficial to the skull was medially incised, the periosteum was removed from the cranium, and a small oval hole was formed with a dental drill, wherein a 2.0 mm knife was inserted with the orientation of the blade perpendicular to the midline. The knife was fixed in a lineartype space frame, and the tip of the knife was inserted into the right side of the brain (anteroposterior: –1.5 mm;mediolateral: +0.5 mm; dorsoventral: –6.0 mm to the bregma)(Paxions and Watson, 2009). Subsequently, the knife was slowly removed, and hemostatic suturing was performed. In the sham group, only skull exposure was performed.
Experimental protocol
Mice were randomly divided into the following five groups: sham (without injury), batroxobin (treated with batroxobin after injury), vehicle (treated with batroxobin or with phosphate-buffered saline (PBS) after injury), EX527(pretreated with EX527 before injury), and EX527 + batroxobin(pretreated with EX527 before injury and then treated with batroxobin after injury). A dose of 39 U/kg batroxobin (DF-521;Beijing Tobishi Pharmaceutical Co., Beijing, China) or vehicle(0.1 M PBS) was administered via intraperitoneal injection immediately after injury (Li et al., 2016a). In the EX527-treated groups EX527 (Selisistat; 5 mg/kg; Selleck Chemicals, Shanghai,China) was solubilized as described previously (Lv et al., 2015)and intraperitoneally injected 30 minutes before injury. The following procedures were performed at the indicated time points following injury: (1) behavioral testing on days 1, 4, 7,14, and 21 (n= 12 mice/group); (2) immunohistochemistry and western blot analysis on day 1 (n= 6 mice/group).
The mice used for immunohistochemistry and western blot analysis were transcardially perfused with 0.9% saline followed by ice-cold 4% paraformaldehyde in 0.1 M PBS (pH 7.4). After fixation, brains were removed, further fixed in 4%paraformaldehyde overnight, and then immersed in a 30%sucrose solution (prepared with 20 mM PBS, pH 7.4) until they sank to the bottom. The brains were cut in 10 consecutive series of 10 µm-thick horizontal sections, with every 10thslice retained, using a freezing microtome (CM1850UV,Leica, Weztlar, Germany) and stored at –20°C for further immunofluorescence staining. Other fresh brain tissues were used for western blot assays.
Behavioral analysis
Animals were pre-trained for 3 days to obtain baseline values.The behavioral studies were performed blindly and repeated at least twice in three different trials to substantiate the data.
Grip test
The grip test was conducted to objectively quantify the muscular strength of mice as previously described (Khuwaja et al., 2011). The mouse was placed midway on a string between two supports and scored on a scale ranging from 0 to 6 as shown in Additional Table 1. The same operator measured all mice in a group to minimize variability.
Beam walking test
The beam walking test for fine motor coordination was performed according to an established protocol (Chen et al.,2012). Mice were placed along an elevated (50 cm) narrow wooden beam (0.8 cm × 100 cm) and walked to a darkened goal box at the opposite end of the beam. The time (latency)required for the mouse to cross the beam (not exceeding 60 seconds) was measured. The beam and box were cleaned of mouse droppings and wiped with towels soaked with 70%ethanol and then water before the next beam was placed on the apparatus.
Culture and purification of mice astrocytes
Primary astrocytes from neonatal Kunming mice were obtained as described previously (Kong et al., 1996; Li et al., 2017). In brief, the mouse cortex was dissociated with 0.05% trypsin/ethylenediaminetetraacetic acid and cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin. The medium was changed after 3 days and every 3 days thereafter. An astrocyte culture with a purity of greater than 90% was obtained on day 9 by shaking at 250 r/min for 24 hours. On day 9, the astrocytes were activated by exposure to 1 ng/mL interleukin-1β (IL-1β) as previously described (Sticozzi et al., 2013; Li et al., 2017). The astrocytes(1 × 106) were pretreated with 0.1 or 0.26 U/mL batroxobin(Yang et al., 2011), 10 µM EX527 (test concentration data are shown in Additional Figure 1) or dimethyl sulfoxide (as a vehicle) for 30 minutes in serum-free media before stimulation with IL-1β (R & D Systems, Jiangsu, China). Astrocytes in the control group did not receive any treatment.
Immunofluorescence staining
Immunofluorescence labeling was performed according to our established protocol (Li et al., 2016a, b, 2017). Briefly, the brain sections and astrocytes were hydrated three times in 0.01 M PBS and blocked with solution containing 5% bovine serum albumin and 0.3% Triton X-100 in PBS for 1 hour at room temperature. Sections were incubated with rabbit anti-Sirt1 antibody (1:100; CST, Shanghai, China) and rabbit antiphosphorylated extracellular signal-regulated kinase (p-ERK)antibody (1:150; Abcam, Shanghai, China) or chick anti-glial fibrillary acidic protein (GFAP) antibody (1:150; Millipore,Burlington, MA, USA) at 4°C overnight. Subsequently, the sections were incubated with the secondary antibodies,including fluorescein isothiocyanate-488 conjugated donkey anti-rabbit IgG (1:100; Abcam) and Cy3 conjugated donkey anti-chick IgG (1:100; Abcam), for 2 hours at 37°C. Then, the sections were incubated with 4’,6-diamidino-2-phenylindole(Zhongshan Company, Shanghai, China) for 5 minutes and mounted on glass slides. The slices were imaged with an FV 3000 laser confocal fluorescence microscope (Olympus, Tokyo,Japan).
Western blot assay
Brain tissues and astrocytes extracted from the lesions were assayed to determine the expression of Srit1, p-ERK, and GFAP.The striatum and substantia nigra of the injured hemisphere were assessed for total caspase-3 and cleaved caspase-3 expression. Western blotting was performed according to our established protocol (Li et al., 2016a, b, 2017). Briefly,tissues and cells were harvested in radioimmunoprecipitation assay buffer. The protein concentration was determined using a bicinchoninic acid protein assay. Proteins (50 µg)were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes (Millipore, Cuyahoga Heights, OH, USA), and blocked using 5% bovine serum albumin. Then, the membrane was probed with primary antibodies (listed in Table 1).Subsequently, the membranes were incubated with goat anti-rabbit IgG conjugated to horseradish peroxidase (1:5000) for 90 minutes at 37°C, and immunoreactive bands were detected by enhanced chemiluminescence (Bio-Rad, Hercules, CA,USA). The relative intensity of each protein was determined by normalizing its intensity to that of glyceraldehyde 3-phosphate dehydrogenase, and the results were quantified using ImageJ 5.0 software (Wright Cell Imaging Facility, Toronto, Canada).
Statistical analysis
All data are presented as the mean ± standard error of the mean (SEM). One-way analysis of variance followed by Bonferronipost hocanalysis was performed to determine significant differences among multiple groups using GraphPad Prism5 (GraphPad Software, San Diego, CA, USA). Differences with aPvalue < 0.05 were considered statistically significant.
Results
Batroxobin improves the neurobehavioral function of mice with nigrostriatal pathway injury
First, we determined whether the improved neurobehavioral recovery with batroxobin reflects a reduction in neuronal death in the striatum and substantia nigra. The expression levels of caspase-3 (32 kDa) and cleaved caspase-3 (17 kDa),a typical effector caspase in apoptosis, were significantly decreased following batroxobin treatment on day 1 post-injury(P< 0.01 for the substantia nigra and striatum; Figure 1A and B). To ascertain the neuroprotective effects of batroxobin, we evaluated motor and neurological functions. The behavioral results showed that nigrostriatal injury significantly induced neural apoptosis and dysfunction compared with the sham group. Treatment with batroxobin significantly reduced the latency of beam crossing on days 1–21 after brain injury(from day 1 to 14,P< 0.05; for day 21,P< 0.01; Figure 1C).Additionally, mice treated with batroxobin exhibited better grip strength at 4, 7, 14, and 21 days after injury (from day 4 to 14,P< 0.05; for day 21,P< 0.01; Figure 1D). Moreover, the results showed that EX527, an inhibitor of Sirt1, significantly reversed the protective effects of batroxobin (Figure 1).
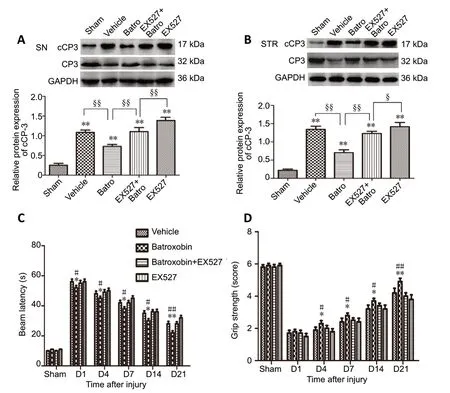
Figure 1 |Effect of batroxobin on the neurobehavioral function of mice with nigrostriatal pathway injury.

Table 1 |Antibodies used in western blot assays
Batroxobin inhibits astrocyte activation around the lesion site in mice with nigrostriatal pathway injuryOur previous study showed that batroxobin could reduce inflammatory factor expression, which is closely related to glial activation, around the lesion site (Li et al., 2016a). Therefore,we assessed the effects of batroxobin on the morphology,including improvements in cell body hypertrophy and short protuberances, and GFAP protein expression of astrocytes.The results showed that batroxobin attenuated astrocyte activation around the lesion site with regard to both the morphology and GFAP protein expression. After application of EX527, the beneficial effects of batroxobin were significantly reversed (P< 0.05; Figure 2).
Batroxobin regulates Sirt1 and p-ERK expression in the brain
As Sirt1 may play a neuroprotective role after brain injury, we assayed the effect of batroxobin on Sirt1 expression. Western blot analysis showed that Sirt1 expression was significantly decreased on day 1 post-injury (P< 0.05; Figure 3A). Because Sirt1 can interfere with ERK activation during astrocyte activation (Li et al., 2017), the effect of batroxobin on ERK activation was determined next. The results showed that batroxobin significantly reduced p-ERK expression (P< 0.01;Figure 3B). However, EX527 could significantly alter the effect of batroxobin (P< 0.05; Figure 3).
Batroxobin inhibits IL-1β-induced astrocyte activation via Sirt1
We further assessed the effect of batroxobin on IL-1β-induced astrocyte activation in primary cultured mouse astrocytes.In the primary cells, after a 24 hours of stimulation with IL-1β, GFAP expression was significantly increased, but cotreatment with 0.1 or 0.2 U/mL batroxobin and IL-1β for 1 day significantly reduced GFAP expression (P< 0.05; Figure 4A and B). Additionally, immunofluorescent histochemical staining showed that the morphology of activated astrocytes was improved after batroxobin treatment. EX527 significantly altered the effects of batroxobin on IL-1β-induced astrocyte activation (Figure 5).
Batroxobin regulates Sirt1 and ERK expression in vitro
To explore the mechanism of batroxobin involvement in IL-1β-induced astrocytic activation, Sirt1 and p-ERK expression levels were detected. As shown in Figure 4A, C and D, IL-1β stimulation significantly decreased Sirt1 expression and increased ERK activation (allP< 0.05), and these results were significantly transformed by co-treatment with 0.1 or 0.2 U/mL of batroxobin for 1 day (allP< 0.05). EX527 significantly reversed the effects of batroxobin on Sirt1 expression and ERK activation.
Immunofluorescence double labeling was performed to detect p-ERK localization. The results showed that cells were clearly GFAP-positive but p-ERK-negative in the control group.Few cells were identified that co-expressed p-ERK and GFAP.After IL-1β stimulation, some astrocytes began to demonstrate strong p-ERK immunoreactivity, mainly located in the nucleus.Significantly weaker p-ERK immunoreactivity was observed in astrocytes after batroxobin treatment. Compared with batroxobin treatment alone, cells that were treated with both EX527 and batroxobin showed markedly increased the GFAP and p-ERK expression (Figure 5).
Discussion
Our present data show that batroxobin facilitated recovery of neurobehavioral function, protected against neuronal damage and apoptosis in both the striatum and substantia nigra, and attenuated astrocyte activationin vivoandvitro. Moreover,Sirt1 expression was involved in all of the effects exerted by batroxobin, and the mechanism of which may be related to increased Sirt1 expression and decreased ERK activation. One of the main limitations of this study was that Sirt1 activity was not examined after TBI. However, our study determined the relationship between batroxobin and Sirt1 in astrocyte activation after injury, which provides a novel theoretical basis for the treatment of patients with TBI.
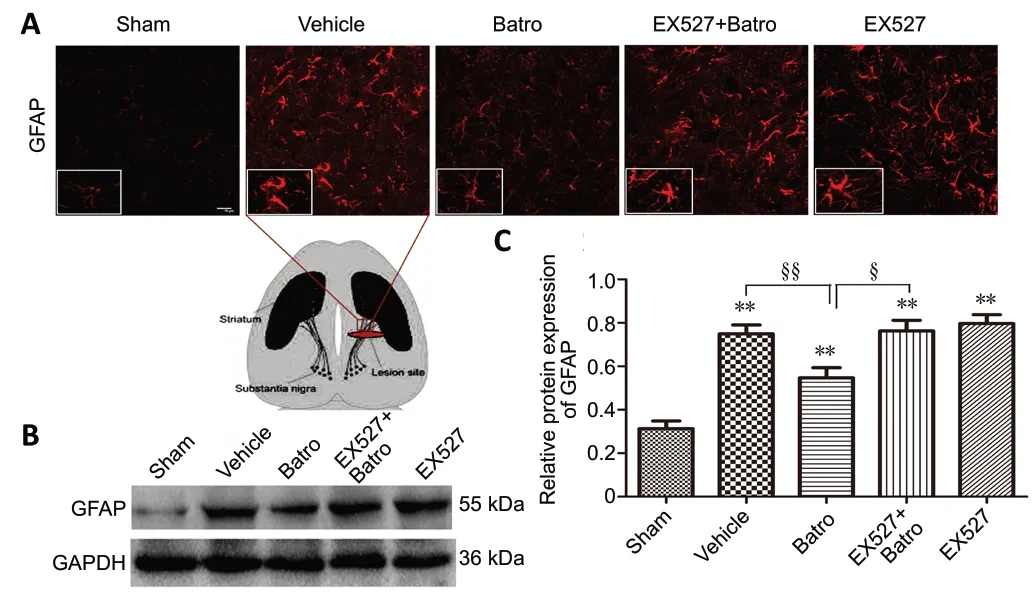
Figure 2 |Batroxobin attenuates astrocyte activation around the lesion site after nigrostriatal pathway injury.

Figure 3 |Batroxobin regulates Sirt1 and p-ERK expression in the brain after nigrostriatal pathway injury.
Batroxobin is a safe drug that is widely used to protect neurons in various brain injury models (Guo and Schluesener, 2006; Li et al., 2016a). It was investigated to explore the mechanism of neuroprotection in nigrostriatal pathway injury. Toxic substances in the blood could invade and deposit at the lesion site, further causing damage to neurons after injury (Franke and Illes, 2006; Fidler, 2011; Tovar-y-Romo et al., 2016).One of the primary concerns during batroxobin treatment is the possibility of inducing bleeding at the lesion site. Our previous study also showed that batroxobin could reduce the inflammatory factors around the lesion site to protect against brain injury (Li et al., 2016a). Glial cells, especially microglial cells and astrocytes, are major mediators of posttraumatic neuroinflammation. Activated resident microglial cells trigger neuroinflammation, which is then maintained and often amplified by activated astrocytes. This, in turn, exposes neurons to neuroinflammation, which can cause neuronal cell death (Leonoudakis et al., 2017). Under activation, glial cells transform their morphology and increase the expression of selective markers of various cell types, such as GFAP (Guo and Schluesener, 2006; Herber et al., 2006) and ionized calcium binding adaptor molecule 1 (Romero-Sandoval et al., 2008).Therefore, clear beneficial effects can be achieved if glial cell activation is controlled for defined periods of time or in a regulated manner.

Figure 4 |Batroxobin inhibits IL-1β-induced astrocyte activation via Sir1.

Figure 5 |Batroxobin regulates p-ERK expression in IL-1β-induced astrocytes.
Batroxobin causes the conversion of fibrinogen into fibrin derivatives, which are rapidly degraded through a secondary fibrinolytic process and eliminated. Several studies have also found a relationship between ERK activation and fibrinogen(Garcia et al., 2005; Hodgkinson et al., 2008; Taherian et al., 2015). For example, Hodgkinson et al. (2008) indicated that fibrinogen stimulated ERK phosphorylation, promoting inflammatory protein expression; Garcia et al. (2005) also found that ERK activation was essential for fibrinogen receptor activation. Thein vitroresults of the present study clearly showed that ERK activation in mouse reactive astrocytes was regulated following interaction with batroxobin and subsequently involved Sirt1 expression. However, in the group treated with EX527, batroxobin failed to produce any attenuation of ERK activation, suggesting that Sirt1 mediated batroxobin-induced inhibition of ERK activation. Thus, we conclude that batroxobin-regulated astrocyte activation may be related to ERK-induced activation and the Sirt1 pathway.
Originally known as a histone deacetylase, recent studies have identified Sirt1 as a regulatory factor of neuronal survival and death (Della-Morte et al., 2009; Hinojosa-Godinez et al., 2019).Moreover, an increasing number of studies have suggested that Sirt1 plays a major role in several neuroprotective processes. Overexpression of Sirt1 increased the survival of SH-SY5Y cells exposed to nitric oxidein vitro(Chong and Maiese, 2008). Increased expression of Sirt1 is considered a common mechanism underlying protective effects, and specific inhibitors of Sirt1, such as sirtinol and EX527, can abolish this neuroprotection (Chong and Maiese, 2008; Lv et al., 2015), thereby suggesting that Sirt1 plays a key role in regulating the neuroprotective mechanism. Furthermore,the present study demonstrated that batroxobin significantly alleviated neuronal apoptosis in both the substantia nigra and striatum at the acute stage of nigrostriatal pathway injury.Although few studies have examined the interactions between Sirt1 and batroxobin, some connections exist. TBI triggers neurodegeneration and a series of neurologic impairments,which also are associated with overproduction of neuroinflammatory cytokines, further injuring the remaining neurons and activating glial cells through positive feedback(Block and Hong, 2005; Barreto et al., 2011). Batroxobin could reduce post-traumatic neuroinflammation and attenuate scartissue formation after brain injury in our previous study. Sirt1 was also confirmed to reduce inflammation, apoptosis, and even the activation of astrocytes (Li et al., 2016a, 2017).
Taken together, our results show that batroxobin inhibited neuronal apoptosis and improved the recovery of neural function in association with Sirt1 expression. The underlying mechanism may be that batroxobin-regulated astrocyte activation does not directly depend on ERK activation but has an absolute requirement for Sirt1 expression. Consistent with these findings, we suggest that Sirt1 expression, induced by batroxobin, plays a key role in the protective mechanism against the nigrostriatal pathway injury.
On the basis of the overall beneficial effects observed following batroxobin treatment, our findings revealed that batroxobin may be a potential therapeutic agent for TBI through regulation of neurodegeneration, neurologic impairment, and astrocyte activation. However, these protective effects were abolished by EX527 treatment,suggesting that the neuroprotective benefits of batroxobin are associated with Sirt1 expression.
Acknowledgments:The authors would like to thank Professor Jie Lv from China Medical University, China for assistance in data analysis.
Author contributions:Study conception and design, manuscript writingand revision: DL; experimental implementation: ZZ, XB; data analysis: DL,ZZ. All authors read and approved the final manuscript.
Conflicts of interest:The authors declare that they have no competing interests.
Financial support:This study was supported by the National Natural Science Foundation of China, No. 81801239 (to DL), China Postdoctoral Science Foundation, No. 201 9M651165 (to DL) and Doctoral Start-up Foundation of Liaoning Province of China, No. 20180540041 (to DL).The funders had no roles in the study design, conduction of experiment,data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement:The study was approved by the Institutional Animal Care and Use Committee of China Medical University(approval No. CMU2020037) on July 19, 2015.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Table 1:The evaluation standard for grip test.
Additional Figure 1:Effect of EX527 on Sirt1 expression in vivo and vitro.
杂志排行
中国神经再生研究(英文版)的其它文章
- The use of hydrogel-delivered extracellular vesicles in recovery of motor function in stroke: a testable experimental hypothesis for clinical translation including behavioral and neuroimaging assessment approaches
- Advances in human stem cell therapies: pre-clinical studies and the outlook for central nervous system regeneration
- MicroRNAs in laser-induced choroidal neovascularization in mice and rats: their expression and potential therapeutic targets
- The emerging role of probiotics in neurodegenerative diseases: new hope for Parkinson’s disease?
- The phenotypic convergence between microglia and peripheral macrophages during development and neuroinflammation paves the way for new therapeutic perspectives
- Modeling subcortical ischemic white matter injury in rodents: unmet need for a breakthrough in translational research
