The phenotypic convergence between microglia and peripheral macrophages during development and neuroinflammation paves the way for new therapeutic perspectives
2021-11-02FrancescaGrassivaroGianvitoMartinoCinthiaFarina
Francesca Grassivaro, Gianvito Martino, , Cinthia Farina,
Abstract Microglia, the tissue resident macrophages of the brain, are increasingly recognized as key players for central nervous system development and homeostasis. They are long-lived cells deriving from a transient wave of yolk-sac derived erythro-myeloid progenitors early in development. Their unique ontology has prompted the search for specific markers to be used for their selective investigation and manipulation. The first generation of genomewide expression studies has provided a bundle of transcripts (such as Olfml3, Fcrls,Tmem119, P2ry12, Gpr34, and Siglech) useful to distinguish microglia from peripheral macrophages. However, more recent reports have revealed that microglial phenotype is constantly shaped by the microenvironment in a time-, and context-dependent manner.In this article, we review data that provide additional pieces to this complex scenario and show the existence of unexpected phenotypic convergence between microglia and peripheral macrophages at certain developmental stages and under pathological conditions. These observations suggest that the two cell types act synergically boosting their mutual activities depending on the microenvironment. This novel information about the biology of microglia and peripheral macrophages sheds new light about their therapeutic potential for neuroinflammatory and neurodegenerative diseases.
Key Words: cell reprogramming; cell therapy; macrophages; microglia; neuroinflammation;plasticity
Ontogeny of Central Nervous System Resident Myeloid Cells
Mononuclear phagocytes, including blood monocytes and tissue-resident macrophages, belong to the innate immune system and support direct fast immune responses to microbial or danger signals, while regulating the generation of more specific adaptive immune reactions. Microglia are the resident macrophages of the brain but, in addition to their role in immune surveillance, play vital functions for central nervous system (CNS) development and homeostasis (Salter and Stevens, 2017; Li and Barres, 2018). They are longlived cells deriving from a transient hematopoietic wave of erythro-myeloid progenitors emerging in the yolk sac during development (Ginhoux et al., 2010; Kierdorf et al., 2013). As CNS colonization by other types of myeloid cells from fetal liver or postnatal hematopoiesis does not occur (Hoeffel and Ginhoux, 2018), the myeloid cells of the CNS remain exclusively yolk sac-derived throughout life.
Search Strategy and Selection Criteria
We performed a PubMed literature search of articles with search terms including “microglia”, “peripheral macrophages”, “myeloid cells”, “ontogeny”, “specific markers”,“neuroinflammation”, “regeneration”, “plasticity”, “tissuereprogramming”, “M1 macrophages”, “M2 macrophages”,“M1/M2 paradigm”, and “myeloid cell-based therapy”.Selection criteria included recent articles (2011–2020)on microglia and peripheral macrophage phenotypes and putative specific markers. In addition, we also included important articles on M1/M2 paradigm published earlier (<2011), as well as a pertinent selection regarding myeloid cellbased therapies.
Phenotypic Convergence between Microglia and Peripheral Macrophages during Development
After 2010, when the unique ontology of microglia is unraveled (Ginhoux et al., 2010), several attempts are made to identify specific markers to be used for the selective investigation and manipulation of this cell type. The first generation studies compare genome-wide expression profiles of microglia to those of different tissue-resident macrophages and blood monocytes, and consistently identify a bundle of transcripts (including Olfml3, Fcrls, Tmem119, P2ry12,Gpr34, and Siglech), which are defined as highly specific microglia markers (Gautier et al., 2012; Hickman et al., 2013;Butovsky et al., 2014; Zhang et al., 2014). However, the focus on adult steady-state myeloid cells is a major limitation of these descriptions, as the second generation of transcriptome studies, based on the more recent technologies of RNA-Seq and single-Cell RNA-Seq, reveal that microglia phenotype is constantly shaped by the microenvironment in time,context- and region-dependent manner, as during acute CNS inflammation when they acquire the proinflammatory macrophage phenotype (Gosselin et al., 2014, 2017; Lavin et al., 2014; Mass et al., 2016; Matcovitch-Natan et al., 2016;Hammond et al., 2019; Masuda et al., 2019). A relevant issue is whether and to what extent the phenotypes of microglia and peripheral macrophages differ throughout development and under chronic CNS inflammation. A recent study of our group describes whole-genome transcriptomic profiles of brain microglia and peripheral liver macrophages obtained at two critical time windows, i.e., the mist of embryonic life(E14.5) and early postnatal life (P1), when microglia mostly display neuroprotective functions, such as the control of proper brain development and synaptic wiring maturation(Grassivaro et al., 2020). Large transcriptional differences exist between myeloid cells from the two distinct compartments(brain and liver), while minor time-related (E14.5vs. P1)intra-compartment changes occur (Grassivaro et al., 2020).Notably, some commonly referred microglia markers (Gpr84,Socs3, Tyrobp, Fcrls, P2ry12, Tmem119, Trem2, Itgb5 and Hexb) display expression in liver macrophages at E14 and/or P1, thus questioning their specificity for microglia. Further,transcripts exclusively detected in the brain or liver play a role in neurogenesis and neural development if expressed in microglia, or host defense and immune processes if derived from liver macrophages (Grassivaro et al., 2020).Altogether these observations unravel unexpected phenotypic convergence between brain and liver myeloid cells, despite their distinct ontology, for the most widely referred microgliaspecific markers. On the other hand, they highlight the distinct phenotypic and functional potentials of peripheral and brain myeloid cells during development and support the emerging concept that the microenvironment is the major force driving transcriptional reprogramming of tissue-resident myeloid cells early in life (Mass et al., 2016; Bennett et al., 2018).
Another key observation is that microglial phenotype changes from development to adult age under physiological conditions(Grassivaro et al., 2020), presumably as a reflection of the different functions exerted by microglia at this stage, which are mainly related to brain homeostasis (Salter and Stevens,2017; Wolf et al., 2017; Thion et al., 2018). In fact, while several hundred transcripts are conserved by microglia during embryonic and early postnatal life, only 94 are expressed by microglia from the development to adulthood. Among them,only 65 are not detected in adult macrophages at steady state or after differentiation towards the M1 or M2 phenotypes(Grassivaro et al., 2020), and thus can be considered truly stable microglia-specific genes. This list of 65 transcripts may,therefore, represent a valuable source of information for the development of more appropriate transgenic mice for microglia targeting during physiological processes.
Phenotypic Convergence between Microglia and Peripheral Macrophages during Neuroinflammation
The phenotypic convergence between central and peripheral myeloid cells is not an exclusive event for developmental processes but can also occur in adult life under neuroinflammatory stress. In fact, well known microglia-specific markers, such as Lag3, Tnfrsf17, and Siglech (Crotti and Ransohoff, 2016; Wolf et al., 2017), are expressed by CNS-infiltrating macrophages during the chronic phase of experimental multiple sclerosis, the experimental autoimmune encephalitis (EAE) model (Grassivaro et al., 2020). This phenomenon is strictly confined to CNS infiltrating macrophages since blood monocytes and spleen macrophages do not display it along the entire course of the disease (Grassivaro et al., 2020). Interestingly, these markers are strongly downregulated in microglia at the peak of disease(Butovsky et al., 2014; Buttgereit et al., 2016; Grassivaro et al., 2020) but regain their basal levels in the chronic phase(Grassivaro et al., 2020). This is in apparent contradiction with studies, showing that microglia and macrophages maintain their distinct expression profile during neuroinflammation(Butovsky et al., 2014; Buttgereit et al., 2016; Jordao et al.,2019). However, these descriptions have a major limitation in the analysis of the short time-window of the acute phase of the disease. The longer time-course of our expression study stretched until the chronic phase of EAE underlines how crucial time is for macrophage plasticity to become evident.Indeed, blood monocytes are known as highly plastic cells(Mantovani et al., 2013) and have been recently shown to differentiate into specialized tissue-resident macrophages in the lung, liver and also CNS, when genetic or pharmacological ablation of microglia makes the niche vacant (Scott et al.,2016; van de Laar et al., 2016; Guilliams and Scott, 2017).Microglia themselves have been recently shown to be highly dependent on their environment since they quickly lose their specific signature when cultured but regain it once transferred back to the brain (Bohlen et al., 2017; Gosselin et al., 2017).The convergence of CNS infiltrating macrophages towards the microglial phenotype in our model suggests the acquisition of an anti-inflammatory, protective function especially during the chronic phase of EAE. This scenario is supported by the finding of the detectable and increasing expression of Sall1 by macrophages along the course of EAE. The transcription factor Sall1 is crucial for the maintenance of microglial identity in the CNS, as microglial Sall1 deficiency leads to rapid loss of microglial signature and upregulation of genes associated with inflammatory macrophages, which results in impaired neurogenesis (Buttgereit et al., 2016; Gosselin et al., 2017).
Overall, these findings suggest the existence of bidirectional plasticity, according to which in the acute phase of EAE microglia shift to a “pro-inflammatory macrophage phenotype”whereas during the chronic phase macrophages turn down their pro-inflammatory signature to acquire, at least in part the neurosupportive “resting microglia phenotype” (Figure 1). In this scenario, microglia and infiltrating macrophages converge phenotypically, in a time-dependent and dynamic fashion,which suggests that they act as a functional unit and boost their mutual activities depending on the microenvironment.Following this reasoning, we can speculate that chronic neuroinflammatory diseases may emerge when this synergism fails and in particular when macrophage transition to the“resting microglia phenotype” is prevented or somehow defective.
Therapeutic Implications of Myeloid Cell Plasticity
Further investigations and additional data are required in support of our hypothesis, but, if confirmed, it would hold relevant implications for the treatment of CNS chronic neuroinflammatory diseases. In fact, identifying the inherited or acquired factors that prevent infiltrating macrophages from gaining the “resting microglia signature” could pave the way for the development of new invaluable therapeutic tools. Furthermore, discerning the epigenetic landscape that orchestrates phenotypic convergence between microglia and infiltrating macrophages is fundamental in view of the emerging use of monocytes and macrophages as therapeutic tools to treat a variety of human diseases. Indeed, these cells have been tested in recent clinical trials to treat a variety of diseases, including spinal cord injury and ischemic stroke (Chan and Viswanathan, 2019), but, despite being safe, have failed to show significant therapeutic efficacy. This may be due to the polarizing protocols which have been exploited so far, which are meanly grounded on the M1/M2 paradigm. According to this paradigm, defined by Mills in the early 2000s (Mills et al., 2000), macrophages can be polarized in-vitro towards two different dichotomic phenotypes: the M1 phenotype(induced by interferon-γ and typical of classically activated macrophages), which is pro-inflammatory and important for direct host defense against pathogens, and the M2 phenotype(induced by interleukin-4 and interleukin-13), which supports resolution of inflammation and tissue repair. Such dichotomy,which has been extensively used over the last twenty years,has been questioned by the numerous evidences about diversity and plasticity of myeloid cells (Mosser and Edwards,2008; Xue et al., 2014; Roszer, 2015), and does not take into account local changes to multiple combinations of stimuli(Gautier et al., 2012; Lavin et al., 2014). On the contrary, we propose a different polarizing approach, whereby ex-vivo macrophage manipulation is tailored to let them acquire the endogenous resting microglia signature, whose protective functions are widely known. To that goal, we need to gain a deeper understanding of the factors selectively expressed by the CNS milieu, which allow macrophages to acquire microglia signature and functionsin vitroand maintain itin vivowithin the CNS. We foresee that significant efforts addressing these issues hold the potential to mark the dawn of a new era in treating chronic CNS neuroinflammatory disorders.
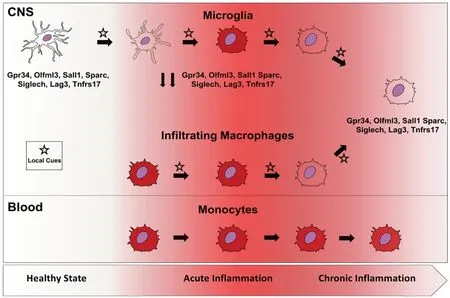
Figure 1 |Microglia and macrophage display phenotypic convergence during neuroinflammation.
Author contributions:Manuscript preparation and literature search: FG;manuscript writing and review: FG, CF, and GM. All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was supported by Merk-Serono, TargetBrain(EU Framework 7 Project, No. HEALTH-F2–2012-279017 to GM), Fondazione Italiana Sclerosi Multipla (FISM, No. grant 2016/R/14 to CF), and cofinanced with the 5 per mille public funding. The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- The use of hydrogel-delivered extracellular vesicles in recovery of motor function in stroke: a testable experimental hypothesis for clinical translation including behavioral and neuroimaging assessment approaches
- Advances in human stem cell therapies: pre-clinical studies and the outlook for central nervous system regeneration
- MicroRNAs in laser-induced choroidal neovascularization in mice and rats: their expression and potential therapeutic targets
- The emerging role of probiotics in neurodegenerative diseases: new hope for Parkinson’s disease?
- Modeling subcortical ischemic white matter injury in rodents: unmet need for a breakthrough in translational research
- Hippo signaling: bridging the gap between cancer and neurodegenerative disorders
